Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(3); 2012 > Article
-
Original Article
Proposal for Creating a Guideline for Cancer Registration of Microinvasive Tumors of the Breast and Ovary (II) - Jin Hee Sohn1,2, Gyungyub Gong3,4, Kyu Rae Kim3,5, Chang Suk Kang2,6, Youn Soo Lee2,6, Jin Man Kim2,7, Woo Hee Jung4,8, Kwang Sun Suh5,7
-
Korean Journal of Pathology 2012;46(3):226-232.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.226
Published online: June 22, 2012
1Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
2The Cancer Registration Committee of the Korean Society of Pathologists, Seoul, Korea.
3Department of Pathology, Asan Medical Center, Ulsan University College of Medicine, Seoul, Korea.
4The Breast Pathology Study Group, Seoul, Korea.
5The Gynecological Pathology Study Group, Seoul, Korea.
6Department of Pathology, The Catholic University of Korea School of Medicine, Seoul, Korea.
7Department of Pathology, Chungnam National University School of Medicine, Daejeon, Korea.
8Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- Corresponding Author: Jin Hee Sohn, M.D. Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 110-746, Korea. Tel: +82-2-2001-2391, Fax: +82-2-2001-2398, jhpath.sohn@samsung.com
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Cancer registration in Korea has a longer than 30-years of history, during which time cancer registration has improved and become well-organized. Cancer registries are fundamental for cancer control and multi-center collaborative research. However, there have been discrepancies in assigning behavior codes. Thus, we intend to propose appropriate behavior codes for the International Classification of Disease Oncology, 3rd edition (ICD-O-3) for microinvasive tumors of the ovary and breast not only to improve the quality of the cancer registry but also to prevent conflicts.
-
Methods
- As in series I, two pathology study groups and the Cancer Registration Committee of the Korean Society of Pathologists (KSP) participated. To prepare a questionnaire on provisional behavior code, the relevant subjects were discussed in the workshop, and consensus was obtained by convergence of opinion from members of KSP.
-
Results
- Microinvasive tumor of the breast should be designated as a microinvasive carcinoma which was proposed as malignant tumor (/3). Serous borderline tumor with microinvasion of the ovary was proposed as borderline tumor (/1), and mucinous borderline tumor with microinvasion of the ovary as either borderline (/1) or carcinoma (/3) according to the tumor cell nature.
-
Conclusions
- Some issues should be elucidated with the accumulation of more experience and knowledge. Here, however, we present our second proposal.
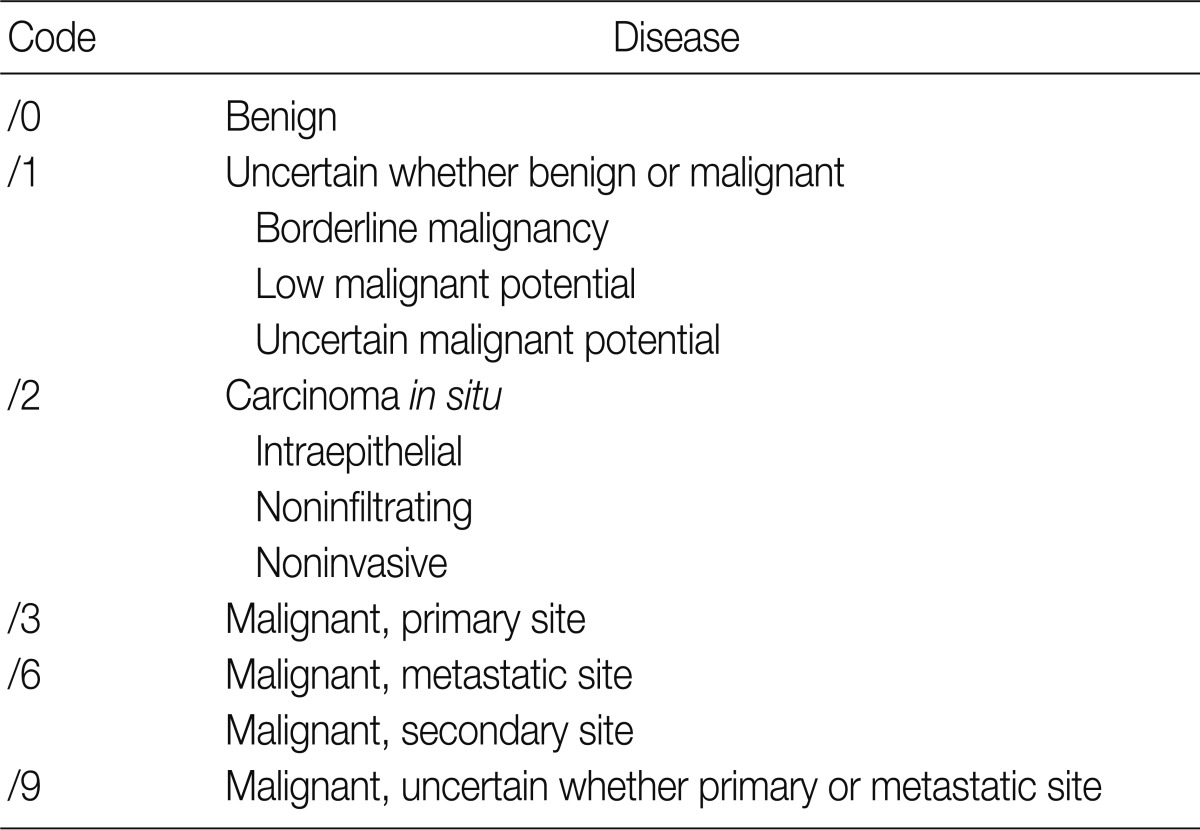
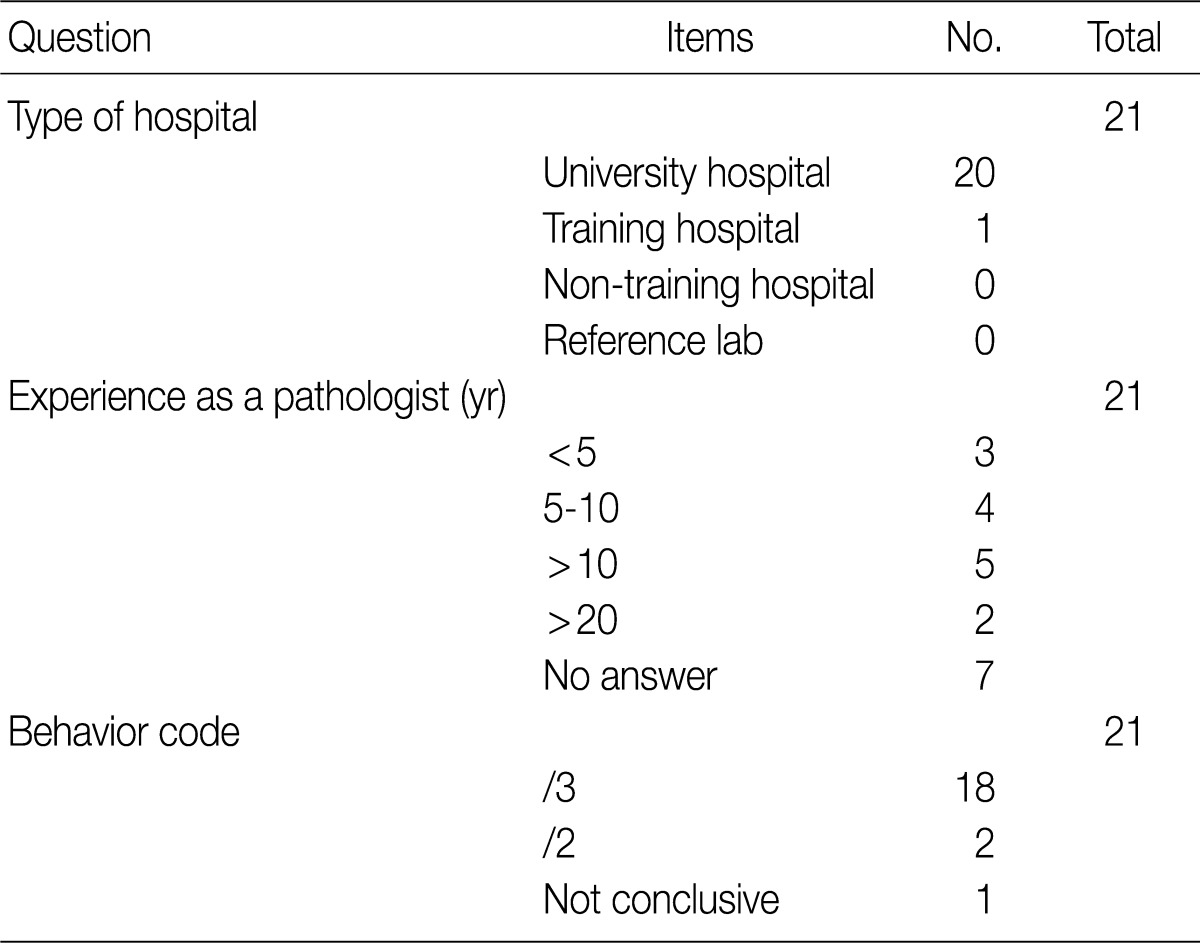
- The ICD-O is composed of five digits; the first four digits represent the morphology code and the last one does biological behavior code ranging from benignity to malignancy (Table 1). To create a guideline for behavior codes, we selected issuable tumors, such as microinvasive tumors of the breast and ovary. To select topics, two members of the Breast Pathology Study Group and the Gynecological Pathology Study Group of the KSP presented their opinions regarding the behavior code with a list of references and a rationale. This was followed by the discussion and a convergence of opinion in the workshop held within the conference of the Breast Pathology Study Group, the Gynecological Pathology Study Group and the Cancer Registration Committee of the KSP. At the 2008 Autumn Congress of the KSP, each KSP member was asked to complete a questionnaire sheet comprising ICD-behavior codes (Table 2). Finally, provisional behavior codes were proposed for the selected tumors.
MATERIALS AND METHODS
- Microinvasive tumors of the breast
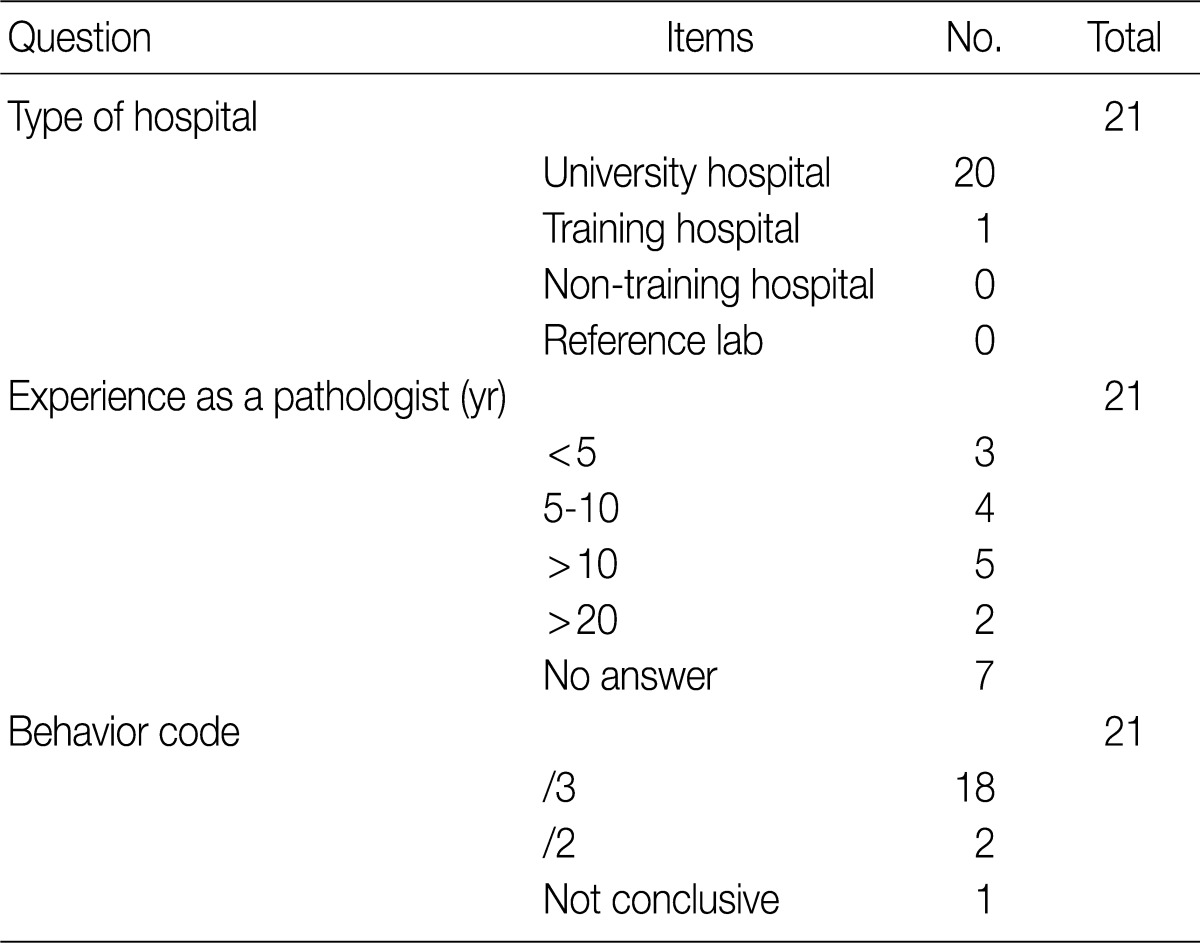
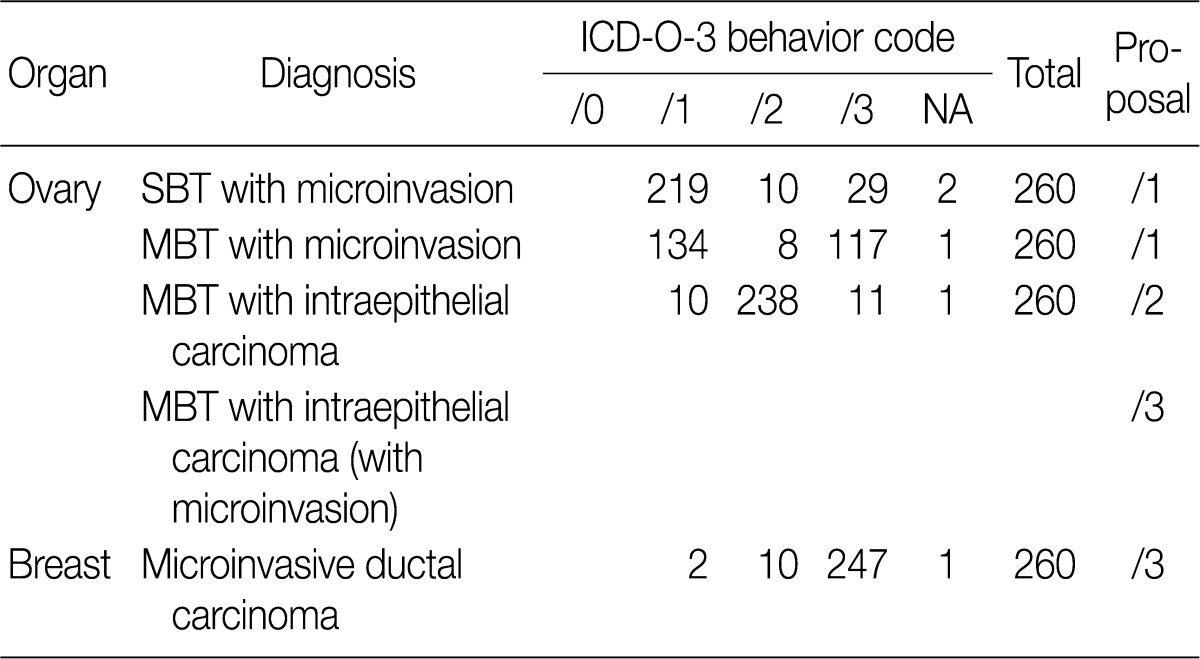
- Based on workshop and a questionnaire survey, members of the Breast Pathology Study Group and those of the KSP drew a conclusions that microinvasive tumors or DCISM of the breast are an invasive form of carcinoma, which should be termed as MIC, and they should be differentiated from ductal carcinoma in situ and then be registered as an invasive carcinoma (/3) with the ICD-O code M8500/3 (study group members, 18/20; KSP members, 247/260) (Tables 3, 4).
- Microinvasive tumors of the ovary
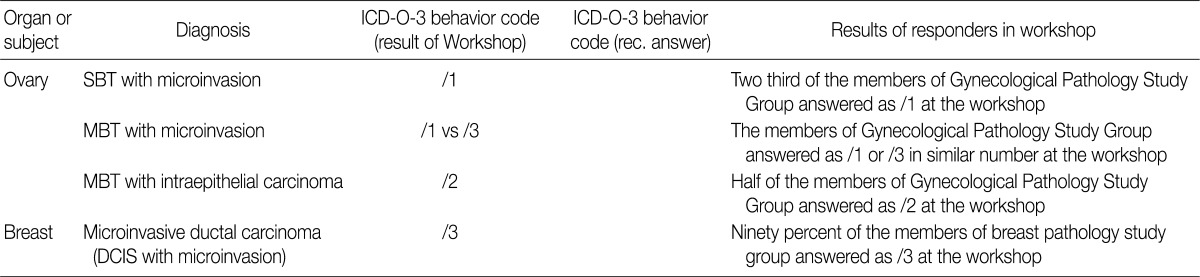
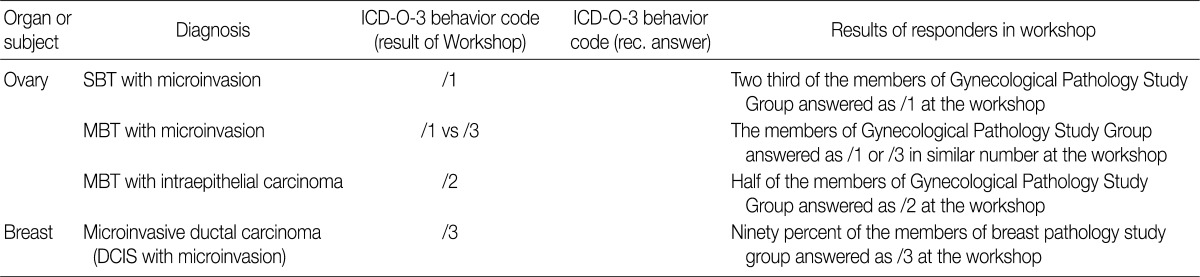
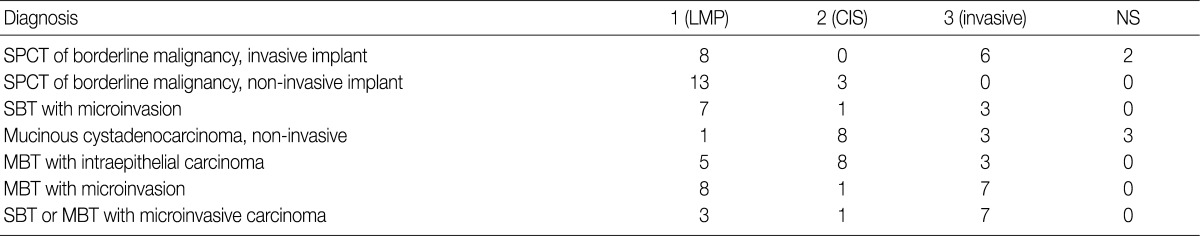
- In the workshop, there was a diversity of the responses on behavior code of microinvasive tumor between members of Gynecological Pathology Study Group (Table 5). According to a questionnaire survey based on the workshop, however, serous borderline tumors (SBT) with microinvasion were regarded as borderline tumors (/1) with the ICD-O code M8442/1. The mucinous borderline tumors (MBT) with microinvasion, where the cellular components were not carcinomatous, were registered as borderline tumors (/1) with the ICD-O code M8472/1. In addition, the MBT with intraepithelial carcinoma were registered as carcinoma in situ (/2). Therefore, the MBT with microinvasion, composed of carcinomatous cells were registered as invasive carcinoma (/3) with the ICD-O code M8470/3 (Tables 4, 5).
RESULTS
- Microinvasive tumors of the breast
- Concerning the breast cancer registration program, whether microinvasive tumors should be treated as carcinoma in situ or invasive carcinoma is an important issue. In addition, diagnostic terms of the microinvasive tumors are used as DCISM or MIC. When the cancer registrars encounter with DCISM, they might confuse the disease entity with its behavior. The definition of microinvasion in ductal and lobular carcinoma and their behavior are not clearly defined and varies between the authors.6-10 For example, microinvasion is defined in several different way, such as: 1) invasive focus is too small to grade; 2) invasive focus <10%; 3) invasive focus <0.2 cm; and 4) invasive focus <0.1 cm. The rate of axillary lymph node metastasis was varied from 0% to 20% and follow-up data was also varied from no further recurrence during 11 years to death.6-10
- This poses a diagnostic challenge to pathologists. But Silver and Tavassoli10 performed a review of lieratures and thereby reported that MIC should be treated differently from carcinoma in situ based on its clinical behavior including the lymph node metastasis. But this has not been confirmed but remains controversial, which is due to a variability in the definition of microinvasion between the authors. Since the publication of the 5th edition of American Joint Committee on Cancer (AJCC) cancer staging manual in 1997,22 the AJCC defined MIC as follows:
-
Microinvasion is the extension of tumor cells beyond the basement membrane into the adjacent tissues with no focus >0.1 cm in the greatest dimension
When there are multiple foci of microinvasion, the size of the largest focus is used to classify the microinvasion (do not use the sum of all individual foci)
Presence of multiple foci should be noted and/or quantified, as with multiple larger invasive carcinomas.
- These criteria for definition of MIC resolved the diagnostic problems. Thus, most of the pathologists now follow the AJCC cancer staging manual guidelines. The behaviors of microinvasive tumors remain inconclusive, for which many controversial opinions exist. De Mascarel et al.11 showed that MIC exhibits distant metastases (2.8%) and death due to breast cancer (1.4%; same as DCIS) within ten years. Padmore et al.12 showed that only one of 11 patients suffered local recurrence of MIC and these patients had no further recurrence eight years later although there was a lung metastasis in one of them who had DCIS with the greatest dimension of 8 cm and 17 microinvasive foci. Yang et al.13 reported that all of their patients they studied had negative lymph nodes, 3.5% of whom did a family history of breast cancer. In addition, these authors also noted that 54% of the cases were human epidermal growth factor (HER2)/neu-positive and 40% were estrogen receptor- and progesterone receptor-negative. These authors showed that the behavior of MIC was similar to the DCIS. Zavagno et al.14 reported that axillary involvement was found in 7.5% of patients with MIC who were treated with axillary lymph node dissection and 14.3% of those who were treated with a sentinel node biopsy. These results indicate that there is a difference in the clinical behavior between MIC and DCIS. In AJCC cancer staging manual22 MIC was categorized as invasive carcinoma (T1). However, the World Helath Organization (WHO) tumor classification did not have an ICD-O code for MIC, on the ground that MIC is not generally accepted as a tumor entity and more evidence and follow-up data are needed for adjusting the code.23 Furthermore, in the Rosen's textbook for breast pathology,24 MIC is included in the chapter of intraductal carcinoma. We have therefore speculated not only that it would be clinically meaningful to collect opinions about the biological behavior of MIC but also that it would be of help for statistics to share common concepts. This is why we consider an microinvasive tumor as an issue although MIC was considered more as an invasive carcinoma according to the AJCC guidelines. Based on these results, members of the Breast Pathology Study Group and members of the KSP drew the proposal as follows:
-
Microinvasive tumors (MIC or DCISM) of the breast are an invasive form of carcinoma
They should be designated as MIC
They should be differentiated from ductal carcinoma in situ
They should be registered as an invasive carcinoma (/3) with the ICD-O code M8500/3 (study group members, 18/20; KSP members, 247/260) (Tables 3, 4).
- Microinvasive tumors of the ovary
- Every carcinoma has its own definition depending on the histological type. The behavior of the microinvasive tumors of the ovary also varies depending on the histological type. Moreover, the classification of malignant ovarian tumors has undergone changes with the advances in understanding of molecular pathogenetic mechanisms. In the current proposal, we focused on the two most common ovarian tumors; serous and mucinous types.
- In 1973, International Federation of Gynecology and Obstetrics (FIGO) and WHO defined the diganostic criteria of ovarian carcinoma as a tumor with a destructive stromal invasion within the primary ovarian tumor and a borderline tumor as a tumor without a destructive stromal invasion.25 However, this general definition cannot be commonly applied to all histological types of ovarian carcinoma. Therefore, each type of ovarian carcinoma has its own diagnostic criteria.
- Ovarian serous tumor is generally classified as a benign serous tumor, SBT and serous carcinoma. SBT is defined as a tumor showing one or more cysts with polypoid excrescences, glands and closely packed papillae that are lined by stratified cuboidal to columnar epithelial cells without a frank stromal invasion. The degree of nuclear atypia of the epithlelial cells varies from mild to moderate, which is the different feature from carcinoma in situ.25,26
- Ovarian serous carcinoma is defined as a tumor having a destructive stromal invasion regardless of the presence of extraovarian tumor implants or lymph node metastasis.16 A tumor showing a stromal invasion of less than 3-5 mm without a desmoplastic reaction has been designated as a microinvasive tumor.15 Microinvasion is described in the borderline tumor category as one of the associated features while dealing with SBT.26 Microinvasion can be found in otherwise typical SBT and these microinvasive tumors have similar biologic behaviors and prognosis to those of tumors lacking this feature.15-18,26 Accordingly, SBTs with microinvasion should be distinguished from serous carcinoma. The latter means that serous tumors exhibit definite destructive stromal invasion.
- SBTs with micropapillary features are known to be frequently associated with extraovarian implants, tumor recurrences, and tumor-related deaths. It has also been described that 50-60% of patients with non-invasive low-grade micropapillary serous carcinoma have cancer-associated deaths after a long-term follow-up.27 Therefore, there is an increasing consensus that it should be regarded as a carcinoma despite a lack of evidence of destructive stromal invasion.27 However, this is not a well-accepted viewpoint by most investigators.26 Therefore, further investigation is warranted to resolve this issue.
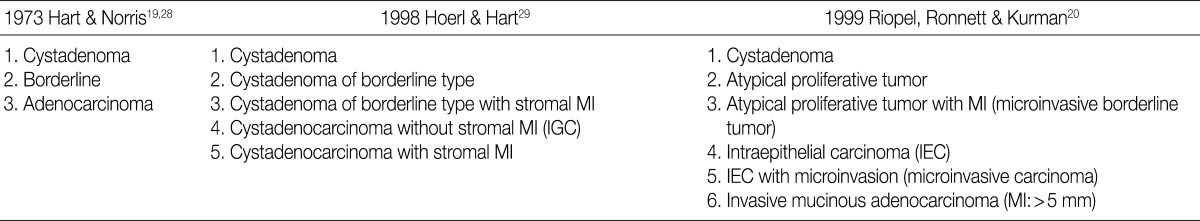
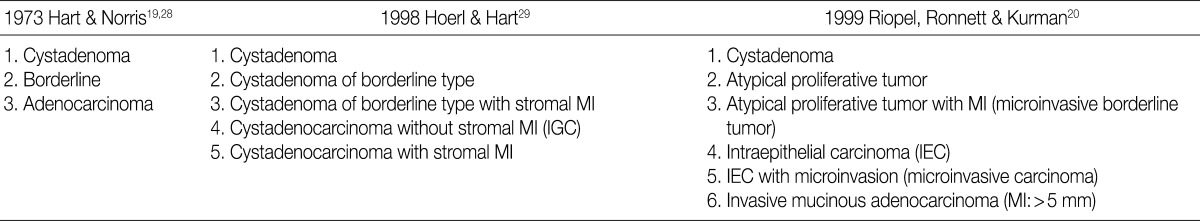
- There is a difference in the definition between the carcinoma in ovarian mucinous tumors and the ovarian serous tumors. In addition, there is also a difference in the clinical significance between the microinvasive tumor in ovarian mucinous tumors and the ovarian serous tumors. Although FIGO and WHO defined carcinoma as a tumor having destructive invasion,25 Hart and Norris added several histologic criteria for the mucinous type, and these include, more than four layers of epithelium, presence of back-to-back arrangement or cribriform pattern, and the presence of severe nuclear atypia, thus termed as Hart and Norris criteria.19,28 However, it is well known that significant interobserver disagreement exists using these diagnostic criteria. Subsequently, new diagostic criteria have been proposed by Hoerl and Hart29 and Riopel et al.,20 which are most commonly used in place of WHO and FIGO classification (Table 6). A cribriform pattern or back-to-back arrangements have not been mentioned as diagnostic criteria of carcinomas. It is quite confusing that these classification systems use the term "microinvasion" even in the category of borderline tumors. Thus, many tumors that have been classified as carcinomas have been revised to borderline tumors, while some carcinomas have been re-classified into the MBT with microinvasion or intraepithelial carcinomas.20,29 The most important change was in the concept of stromal microinvasion in the mucinous tumor.29,30 The definition of microinvasion in mucinous tumors was the presence of one or several tumor cell clusters or that of invasive irregular glands with an ill-defined margin. But there is no consensus existed regarding the degree of cytologic atypia and the size of the microinvasive lesion. Despite an incomplete consensus, many authors suggest that significant cellular atypia as well as the size of the invasive foci are required for the diagnosis of MIC.21,26 We have a consensus on many authors' opinion that the presence of significant cellular atypia is a requisite for the diagnosis of MIC. This is because the presence of microinvasive foci composed of only low grade nuclear atypia (MBT with microinvasion) does not alter the favorable prognosis of the otherwise typical MBT.20,26 In addition, irregularly marginated cellular clusters can also be seen in the stroma in association with mucin granulomas, which are frequently formed in MBT. This explains why MBT with microinvasion should be distinguished from MIC. MBT with intraepithelial carcinoma can be defined as MBT exhibiting areas of four or more epithelial cell layers, scattered foci of cribriform or stroma-free papillary architecture, and most importantly, moderate or severe atypical nuclei.26 Moreover, the presence of microinvasive component and the adjacent glands containing severe atypical nuclei, should result in the diagnosis of an MIC.26 This led to a conclusion that MBT with intraepithelial carcinoma with microinvasion by severely atypical carcinoma cells should be diagnosed as MBT with MIC. But this was not confirmed on a questionnaire survey. Based on these tentative conclusions, we can propose that not only MBT with microinvasion, whose cellular constituents are not severely atypical, should be regarded as borderline tumors (/1), but also that those whose cellular constituents are severely atypical (carcinomatous), should be regarded as MBT with MIC (/3).
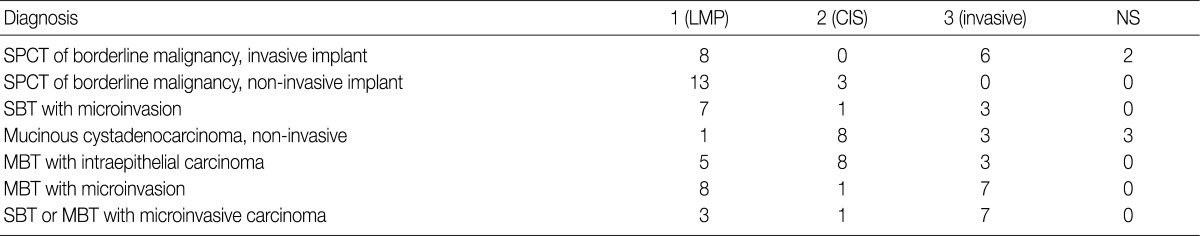
- In the workshop, there was a diversity of the responses on behavior code of microinvasive tumor between the members of Gynecological Pathology Study Group. According to a questionnaire survey based on the KSP workshop, however, the responses were more integrative and conclusive. Thus, based on these results, members of the Gynecological Pathology Study Group and the KSP drew the proposal as follows:
-
SBT with microinvasion should be regarded as borderline tumors (M8442/1)
MBT with microinvasion should be regarded as borderline tumors with behavior code /1 (M8472/1)
MBT with intraepithelial carcinoma should be regarded as carcinoma in situ (/2)
MBT with microinvasion by carcinomatous cells (MBT with MIC) should be regarded as invasive carcinoma with behavior code /3 (M8470/3) (Tables 4, 5).
- We have not reached conclusive and precise definitions for the diagnostic criteria and behavior codes, but the above proposal will be used as another reference that is subject to alterations upon further analysis and feedback in the future. Our results demonstrate great merit in improving the quality of cancer registration and cancer control programs. In addition, we'll also continue to make efforts to develop and improve guidelines for more tumors in the future.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Shin HR, Won YJ, Jung KW, et al. Nationwide cancer incidence in Korea, 1999~2001: first result using the national cancer incidence database. Cancer Res Treat 2005; 37: 325-331. ArticlePubMedPMC
- 2. Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat 2011; 43: 1-11. ArticlePubMedPMC
- 3. Fritz A, Percy C, Jack A. International classification of diseases for oncology. 2000; 3rd ed. Geneva: World Health Organization.
- 4. World Health Organization. Manual of the international statistical classification of diseases, injuries, and causes of death. 1992; 10th rev. Geneva: World Health Organization.
- 5. Cho MY, Kang YK, Kim KM, et al. Porposal of creating a guideline for cancer registration of the gastrointestinal tumors (I). Korean J Pathol 2008; 42: 140-150.
- 6. Cavaliere A, Scheibel M, Bellezza G, et al. Ductal carcinoma in situ with microinvasion: clinicopathologic study and biopathologic profile. Pathol Res Pract 2006; 202: 131-135. ArticlePubMed
- 7. Schwartz GF, Patchefsky AS, Finklestein SD, et al. Nonpalpable in situ ductal carcinoma of the breast: predictors of multicentricity and microinvasion and implications for treatment. Arch Surg 1989; 124: 29-32. ArticlePubMed
- 8. Solin LJ, Fowble BL, Yeh IT, et al. Microinvasive ductal carcinoma of the breast treated with breast-conserving surgery and definitive irradiation. Int J Radiat Oncol Biol Phys 1992; 23: 961-968. ArticlePubMed
- 9. Silverstein MJ, Gierson ED, Colburn WJ, Rosser RJ, Waisman JR, Gamagami P. Axillary lymphadenectomy for intraductal carcinoma of the breast. Surg Gynecol Obstet 1991; 172: 211-214. PubMed
- 10. Silver SA, Tavassoli FA. Mammary ductal carcinoma in situ with microinvasion. Cancer 1998; 82: 2382-2390. ArticlePubMed
- 11. de Mascarel I, MacGrogan G, Mathoulin-Pélissier S, Soubeyran I, Picot V, Coindre JM. Breast ductal carcinoma in situ with microinvasion: a definition supported by a long-term study of 1248 serially sectioned ductal carcinomas. Cancer 2002; 94: 2134-2142. ArticlePubMed
- 12. Padmore RF, Fowble B, Hoffman J, Rosser C, Hanlon A, Patchefsky AS. Microinvasive breast carcinoma: clinicopathologic analysis of a single institution experience. Cancer 2000; 88: 1403-1409. ArticlePubMed
- 13. Yang M, Moriya T, Oguma M, et al. Microinvasive ductal carcinoma (T1mic) of the breast: the clinicopathological profile and immunohistochemical features of 28 cases. Pathol Int 2003; 53: 422-428. ArticlePubMedPDF
- 14. Zavagno G, Belardinelli V, Marconato R, et al. Sentinel lymph node metastasis from mammary ductal carcinoma in situ with microinvasion. Breast 2007; 16: 146-151. ArticlePubMed
- 15. McKenney JK, Balzer BL, Longacre TA. Patterns of stromal invasion in ovarian serous tumors of low malignant potential (borderline tumors): a reevaluation of the concept of stromal microinvasion. Am J Surg Pathol 2006; 30: 1209-1221. ArticlePubMed
- 16. Bell DA, Scully RE. Ovarian serous borderline tumors with stromal microinvasion: a report of 21 cases. Hum Pathol 1990; 21: 397-403. ArticlePubMed
- 17. Bell DA, Weinstock MA, Scully RE. Peritoneal implants of ovarian serous borderline tumors: histologic features and prognosis. Cancer 1988; 62: 2212-2222. ArticlePubMed
- 18. Bell KA, Smith Sehdev AE, Kurman RJ. Refined diagnostic criteria for implants associated with ovarian atypical proliferative serous tumors (borderline) and micropapillary serous carcinomas. Am J Surg Pathol 2001; 25: 419-432. ArticlePubMed
- 19. Hart WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol 2005; 24: 4-25. PubMed
- 20. Riopel MA, Ronnett BM, Kurman RJ. Evaluation of diagnostic criteria and behavior of ovarian intestinal-type mucinous tumors: atypical proliferative (borderline) tumors and intraepithelial, microinvasive, invasive, and metastatic carcinomas. Am J Surg Pathol 1999; 23: 617-635. PubMed
- 21. Kim KR, Lee HI, Lee SK, Ro JY, Robboy SJ. Is stromal microinvasion in primary mucinous ovarian tumors with "mucin granuloma" true invasion? Am J Surg Pathol 2007; 31: 546-554. ArticlePubMed
- 22. Fleming ID, Cooper JS, Henson DE, et al. AJCC cancer staging manual. 1997; 5th ed. New York: Springer-Verlag.
- 23. Ellis IO, Tavassoli FA. In: Tavassoli FA, Devilee P, eds. Microinvasive carcinoma. World Health Organization classification of tumors: pathology and genetics of tumours of the breast and female genital organs. 2003; Lyon: IARC press, 74-75.
- 24. Rosen PP. Rosen's breast pathology. 2009; 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 285-357.
- 25. Serov SF, Scully RE, Sobin LH. International histological classification of tumours: histologic typing of ovarian tumors. 1973; Geneva: World Health Organization, 17-54.
- 26. Robboy SJ, Mutter GL, Prat J, Bentley RC, Russell P, Anderson MC. Robboy's pathology of the female reproductive tract. 2009; 2nd ed. Edinburgh: Churchill Livingstone/Elsevier, 611-654.
- 27. Shih IeM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 2004; 164: 1511-1518. PubMedPMC
- 28. Hart WR. Ovarian epithelial tumors of borderline malignancy (carcinomas of low malignant potential). Hum Pathol 1977; 8: 541-549. ArticlePubMed
- 29. Hoerl HD, Hart WR. Primary ovarian mucinous cystadenocarcinomas: a clinicopathologic study of 49 cases with long-term follow-up. Am J Surg Pathol 1998; 22: 1449-1462. PubMed
- 30. Caduff RF, Svoboda-Newman SM, Ferguson AW, Johnston CM, Frank TS. Comparison of mutations of Ki-RAS and p53 immunoreactivity in borderline and malignant epithelial ovarian tumors. Am J Surg Pathol 1999; 23: 323-328. ArticlePubMed
REFERENCES
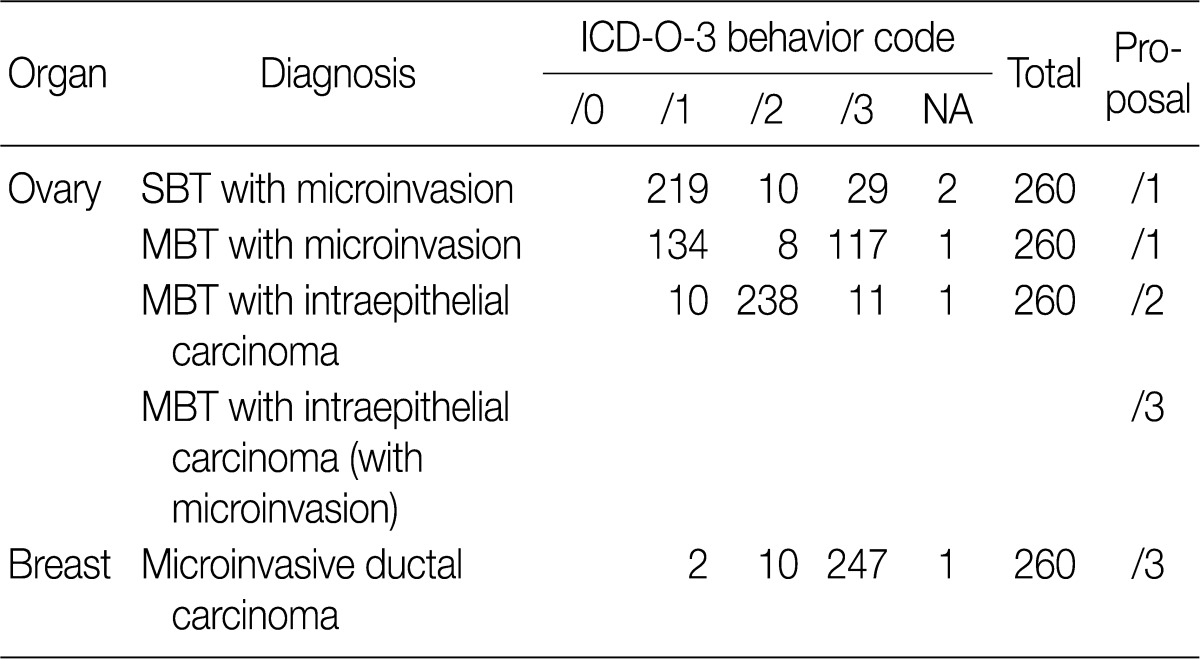
Figure & Data
References
Citations

- Update on the Proposal for Creating a Guideline for Cancer Registration of the Gastrointestinal Tumors (I-2)
Eun Sun Jung, Yun Kyung Kang, Mee-Yon Cho, Joon Mee Kim, Won Ae Lee, Hee Eun Lee, Sunhoo Park, Jin Hee Sohn, So-Young Jin
Korean Journal of Pathology.2012; 46(5): 443. CrossRef - A Proposal for Creating a Guideline for Cancer Registration of the Fibromatosis, PEComa Group, Malignant LymphomaIn Situand Dendritic Cell Tumors (III)
Changyoung Yoo, Chang Suk Kang, Yoon La Choi, Hye Yoon Kang, Jin Man Kim, Young Hye Koh, Joo Hee Lee, Seung Sook Lee, In Sun Kim, Dong Hoon Kim, Yong Ku Park, Jin Hee Sohn
Korean Journal of Pathology.2012; 46(5): 436. CrossRef
ICD-O, International Classification of Diseases for Oncology.
KSP, Korean Society of Pathologists; ICD-O-3, International Classification of Disease Oncology, 3rd edition; SBT, serous borderline tumor; MBT, mucinous borderline tumor; DCIS, ductal carcinoma
KSP, Korean Society of Pathologists; ICD-O-3, International Classification of Disease Oncology, 3rd edition; NA, no answer; SBT, serous borderline tumor; MBT, mucinous borderline tumor.
LMP, low malignant potential; CIS, carcinoma
MI, microinvasion; IGC, intraglandular carcinoma.

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article

