Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(3); 2012 > Article
-
Case Report
Plexiform Angiomyxoid Myofibroblastic Tumor of the Stomach: Report of Two Cases and Review of the Literature - Youngran Kang, Wonkyung Jung, In-Gu Do1, Eui Jin Lee1, Min Hyeong Lee2, Kyoung-Mee Kim1, Jongsang Choi
-
Korean Journal of Pathology 2012;46(3):292-296.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.292
Published online: June 22, 2012
Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Department of Biology, Cornell University, Ithaca, NY, USA.
- Corresponding Author: Kyoung-Mee Kim, M.D. Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-2800, Fax: +82-2-3410-0025, kkmkys@skku.edu
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Plexiform angiomyxoid myofibroblastic tumor (PAMT) of the stomach is a recently recognized entity. Because of its rarity, only 22 cases have been reported in the English-language literature and most of these are single case reports. We report two cases of gastric PAMT. The tumor cells were bland and plexiform arranged in a myxoid stroma, which was positive for alcian blue. Immunohistochemically, the tumor cells were positive for smooth muscle actin, but negative for c-kit, CD34, desmin, S-100 protein, epithelial membrane antigen, neurofilament, and protein kinase C-theta. Mutation analyses for exon 9, 11, 13, and 17 of KIT genes and 12, 14, and 18 of the platelet-derived growth factor receptor alpha (PDGFRA) genes were performed and the tumors were wild-type for mutation.
- Case 1
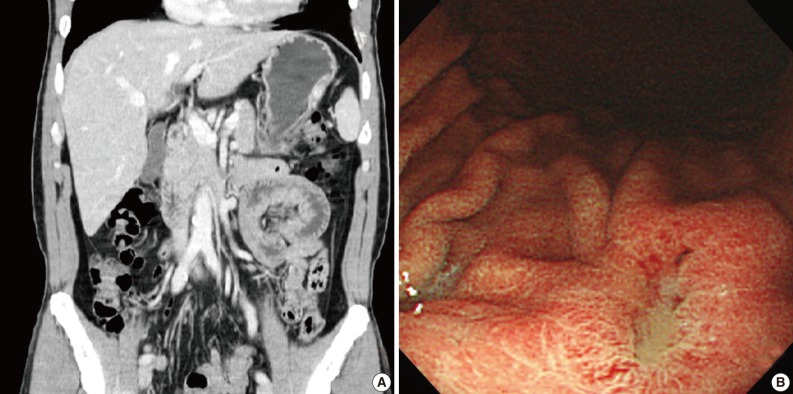
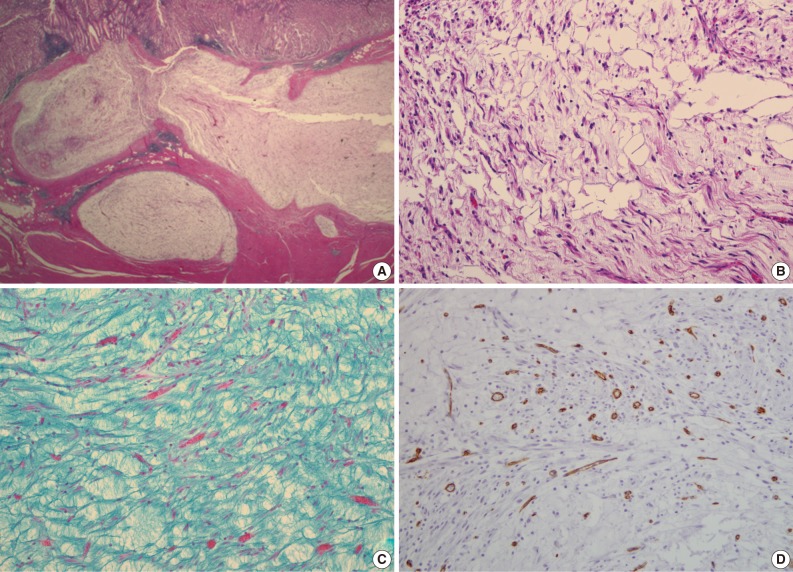
- A 47-year-old man with a clinical impression of a submucoal tumor of the stomach detected during the health screening examination visited the Samsung Medical Center for further evaluation of the lesion. A computed tomography scan demonstrated a 2 cm-sized mass with prominent contrast enhancement (Fig. 1A). There was no enlargement of any lymph node at the peritoneum or retroperitoneum. On endoscopy, a 3×2 cm-sized flat and submucosal tumor-like elevated lesion with mucosal ulceration was found at the mid body, posterior wall and greater curvature side of the stomach (Fig. 1B). Given a sufficient margin, wedge resection was performed. The resected specimen contained mucinous material within the mass. Microscopically, the tumor demonstrated a multinodular plexiform growth pattern (Fig. 2A). The tumor cells were composed of spindle and epithelioid cells with oval nuclei and an abundant myxoid stroma rich in small blood vessels (Fig. 2B). The myxoid matrix was alcian blue positive (Fig. 2C). Blood vessels were dilated and arborizing, but thrombus was not found. Some c-kit positive mast cells were scattered in the myxoid stroma. There was no evidence of necrosis or significant cytological atypia in the tumor cells. Three mitotic figures were found per 50 high powered fields. In this case, differential diagnoses included neurofibroma, schwannoma, extracardiac myxoma, and GIST. On immunohistochemistry, the tumor cells were positive for smooth muscle actin (SMA) and negative for c-kit, desmin, CD34 (Fig. 2D), S-100 protein, protein kinase C (PKC)-theta, epithelial membrane antigen (EMA), and neurofilament. In the differential diagnoses, neurofibroma was excluded because the tumor cells were negative for S-100 protein and neurofilament. Schwannoma was excluded by SMA expression and immunonegativity for S-100 protein. Extracardiac myxoma was also excluded by SMA immunoreactivity and was negative for CD34. GIST was excluded due to their multinodular plexiform growth pattern, less eosinophilic cytoplasm, and complete negativity for c-kit, CD34 and PKC-theta. The Ki67 labeling index was around 6%. The patient was alive without any recurrence or metastasis of the tumor after being followed up for 6 years. Genetic analyses for KIT and platelet-derived growth factor receptor alpha (PDGFRA) genes were wild-type for mutation.
- Case 2
- A 63-year-old woman with no associated clinical symptom visited Korea University Guro Hospital for a health screening examination. Endoscopic examination showed a sessile polypoid mass at the lower body and greater curvature. The size of the mass was 2.2×1.6 cm. The surface of the polyp showed edematous, ulcerated mucosa and thin vasculature. Endoscopic resection was performed. The tissue was processed to formalin fixed and paraffin embedded block and we obtained hematoxylin and eosin stained 4 µm sections. Microscopically, the tumor was located at the submucosal layer with mucosal ulceration and was relatively well demarcated. In the microscopic examinations, the tumor was composed of loosely arranged spindle cells, myxoid stroma and was rich in small vessels (Fig. 3A). The spindle cells were admixed with myxoid or fibromyxoid stroma. The tumor cells showed a bland looking, oval to slightly elongated nuclei without atypia and a faintly eosinophilic cytoplasm. The stroma was abundant in variable shaped small vessels and was positive for alcian blue stain (Fig. 3B). On immunohistochemical staining, the tumor cells were immunoreactive for SMA (Fig. 3D), but negative for c-kit, CD34, desmin, S-100 protein (Fig. 3C), and EMA (Table 1). Mitosis was not detected in 50 high powered fields. Although some mast cells were noted in the stroma, other inflammatory cells including lymphocytes, neutrophils, plasma cells and eosinophils were inconspicuous. Mutation analyses for exon 9, 11, 13, and 17 of the KIT genes and exon 12, 14, and 18 of the PDGFRA genes were performed and the tumor was wild-type for mutation.
CASE REPORTS
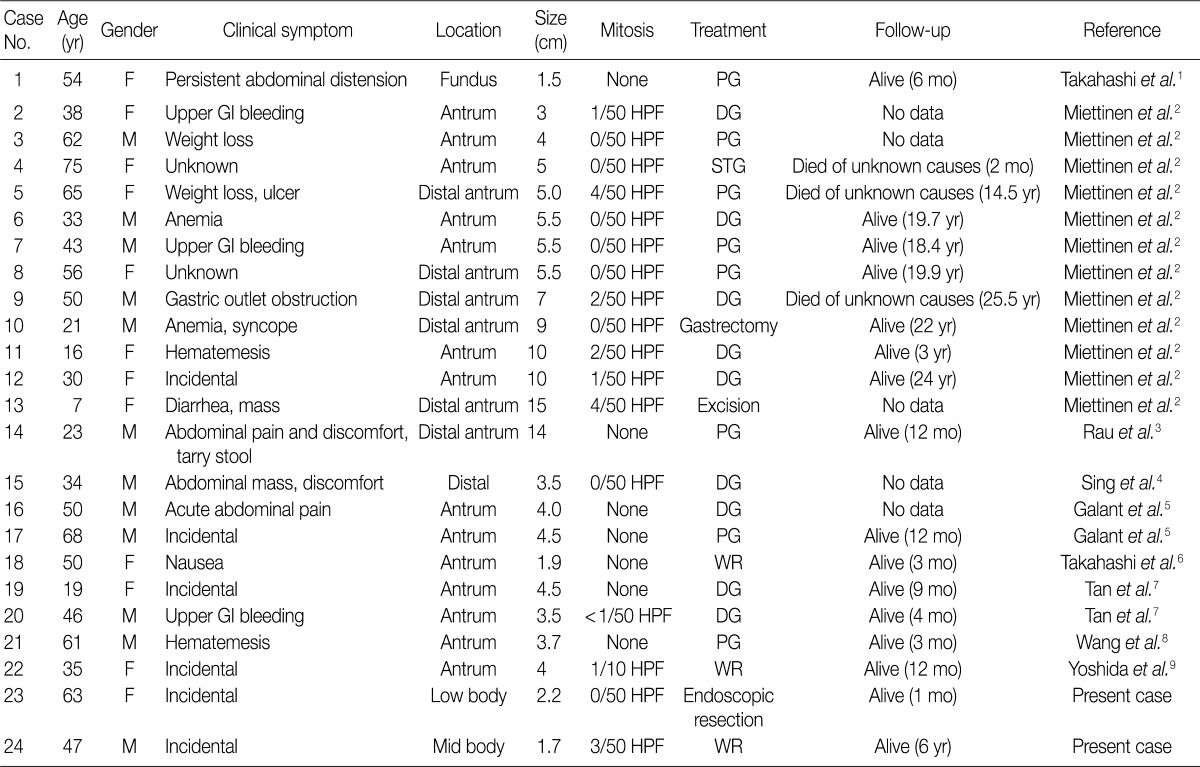
- PAMT of the stomach is a recently described unique gastric mesenchymal tumor that is myofibroblastic in origin with the potential to differentiate toward smooth muscle cells. The tumor cells were bland and plexiform arranged in a myxoid stroma. The diagnosis of PAMT is based on the histologic feature, plexiform architecture, an abundant myxoid stroma rich in small blood vessels, and immunohistochemical findings. Reported cases reveal features of smooth muscle differentiation and a spectrum of fibrous, fibromyxoid and myxoid stromal features by immunohistochemistry and electron microscopy. To date, only 22 cases have been reported worldwide as "plexiform fibromyxoma,"2 "plexiform angiomyxoid myofibroblastic tumor,"1 and "plexiform angiomyxoid tumor."9 Recently, Takahashi et al.6 reported that this tumor is not a pure myofibroblastic tumor and may contain tumor cells with a fibroblastic or smooth muscle nature. Table 1 summarizes the clinicopathological features of the previously reported cases with an unpublished case and our two PAMT cases. To date, there is no sex predilection. Most tumors occurred in the gastric antrum,6 but they can occur anywhere in the stomach including the fundus8 and body, similarly to our cases. Distal or partial gastrectomy, wedge resection and polypectomy were performed for treatment. In all reported 22 cases to date, there was no recurrence or metastasis of disease, although the tumor reached a large size of up to 15 cm in its greatest dimension.8 However, in some cases, the follow-up duration was too short to precisely predict prognosis. Although the number of reported PAMT cases is small, malignant change, metastasis to adjacent organs, and recurrence or death from the disease have not yet been reported. Based on favorable prognoses, absence of nuclear atypia, and low mitotic index, we suggest that PAMT can be categorized as a benign lesion.
- Pathologic differential diagnosis of this new disease entity includes GIST, inflammatory fibroid polyp, leiomyoma, neurofibroma, schwannoma, extracardiac myxoma, desmoids (mesenteric fibromatosis), solitary fibrous tumor and inflammatory myofibroblastic tumor. Histologic characteristics of PAMT include a multinodular plexiform growth pattern, bland looking spindle cells, and an alcian blue positive myxoid stromal matrix rich in variable sized small vessels. On immunohistochemistry, the tumor cells of PAMT are positive for SMA and negative for c-kit, CD34, S-100 protein, EMA, and desmin. These histologic and immunophenotypic features are helpful in diagnosing this rare disease. In our cases, as the tumor cells were ovoid, showed no atypia, and were located in the submucosa and proper muscle layer, the most important differential diagnosis was GIST. Histopathologically, this tumor shows less eosinophilic cytoplasm than GIST. Moreover, compared to tumor cell populations found in GIST, the stromal component is prominent and the immunohistochemisty of PAMT is clearly differentiated from GIST.
- Here, we report two cases of PAMT, which has not yet been reported in Korea. Our report may help increase the awareness of this rare but important new disease entity.
DISCUSSION
- 1. Takahashi Y, Shimizu S, Ishida T, et al. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol 2007; 31: 724-728. ArticlePubMed
- 2. Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol 2009; 33: 1624-1632. PubMed
- 3. Rau TT, Hartmann A, Dietmaier W, et al. Plexiform angiomyxoid myofibroblastic tumour: differential diagnosis of gastrointestinal stromal tumour in the stomach. J Clin Pathol 2008; 61: 1136-1137. ArticlePubMed
- 4. Sing Y, Subrayan S, Mqadi B, et al. Gastric plexiform angiomyxoid myofibroblastic tumor. Pathol Int 2010; 60: 621-625. ArticlePubMed
- 5. Galant C, Rousseau E, Ho Minh Duc DK, Pauwels P. Re: Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol 2008; 32: 1910.
- 6. Takahashi Y, Suzuki M, Fukusato T. Plexiform angiomyxoid myofibroblastic tumor of the stomach. World J Gastroenterol 2010; 16: 2835-2840. ArticlePubMedPMC
- 7. Tan CY, Santos LD, Biankin A. Plexiform angiomyxoid myofibroblastic tumour of the stomach: a case report. Pathology 2010; 42: 581-583. ArticlePubMed
- 8. Wang WY, Li JN, Li GD. Plexiform angiomyxoid myofibroblastic tumour of the gastric fundus: successful diagnosis and treatment by endoscopy. J Clin Pathol 2010; 63: 569-570. ArticlePubMed
- 9. Yoshida A, Klimstra DS, Antonescu CR. Plexiform angiomyxoid tumor of the stomach. Am J Surg Pathol 2008; 32: 1910-1912. Article
REFERENCES
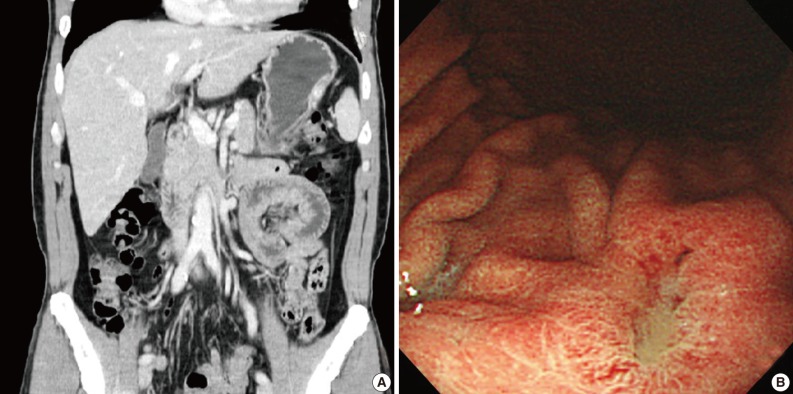
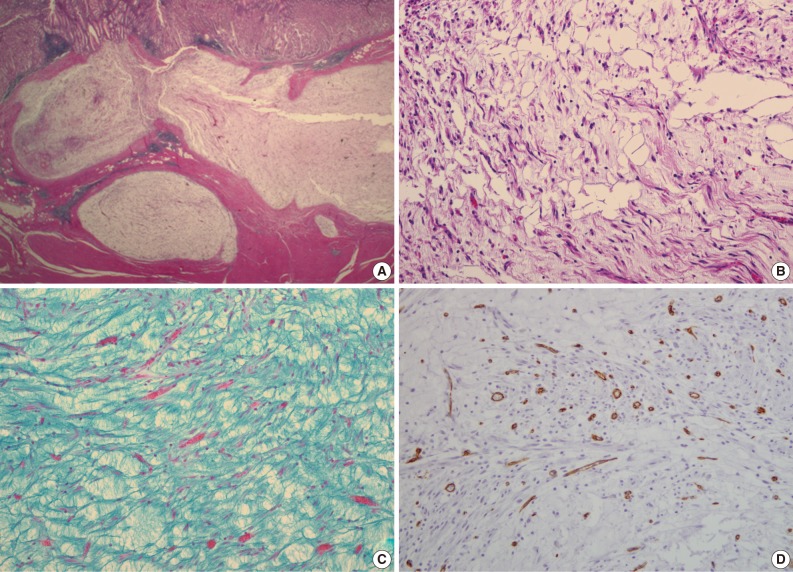
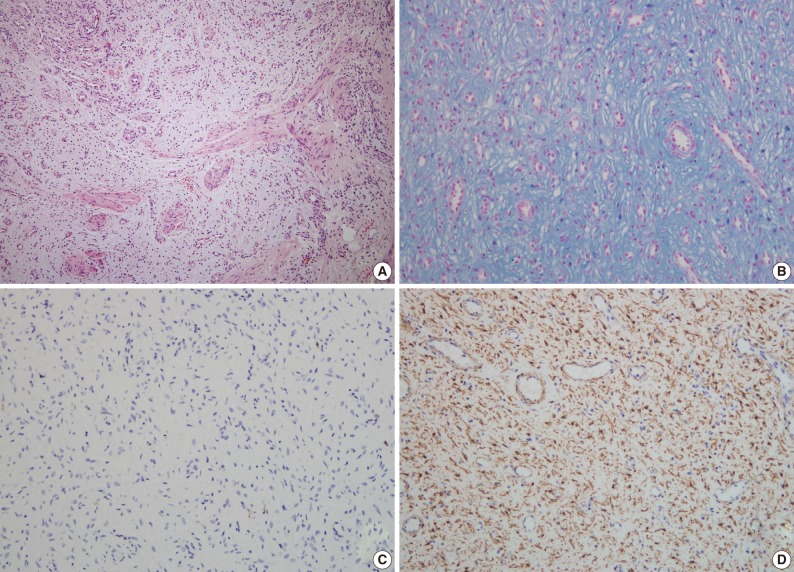
Figure & Data
References
Citations

- Plexiform Fibromyxoma in the Stomach: Immunohistochemical Profile and Comprehensive Genetic Characterization
Annabella Di Mauro, Rosalia Anna Rega, Maddalena Leongito, Vittorio Albino, Raffaele Palaia, Alberto Gualandi, Andrea Belli, Imma D’Arbitrio, Pasquale Moccia, Salvatore Tafuto, Annarosaria De Chiara, Alessandro Ottaiano, Gerardo Ferrara
International Journal of Molecular Sciences.2024; 25(9): 4847. CrossRef - Endoscopic submucosal excavation for gastric plexiform fibromyxoma: A case report and systematic review of literature
Ziqin Xia, Zhidai Zhou, Wei Guo, Hongling Wang, Fan Wang, Feng Zhou
Frontiers in Oncology.2023;[Epub] CrossRef - Recurrent plexiform angiomyxoid myofibroblastic tumour (PAMT) of the stomach with aggressive behaviour
Pavithra Ayyanar, Hemanta Kumar Nayak, Subash Chandra Samal, Madhabananda Kar, Pritinanda Mishra, Susama Patra
Pathology.2022; 54(5): 650. CrossRef - An Unusual Stomach Tumour: Plexiform Angiomyxoid Fibroma Stomach—A Case Report
Sharath K. Krishnan, Ravindran Chirukandath, Togy Zachariah, Rajiv Sajan Thomas
Indian Journal of Surgical Oncology.2022; 13(4): 691. CrossRef - Gastric Plexiform Fibromyxoma: A Case Report and Literature Review
路 张
Advances in Clinical Medicine.2022; 12(12): 12033. CrossRef - Plexiform angiomyxoid myofibroblastic tumor treated by endoscopic submucosal dissection: A case report and review of the literature
Jian-Di Wu, Yi-Xiong Chen, Chang Luo, Feng-Hua Xu, Lei Zhang, Xiao-Hua Hou, Jun Song
World Journal of Gastroenterology.2021; 27(31): 5288. CrossRef - Gastric Plexiform Fibromyxoma with Two Different Growth Patterns on Histological Images: a Case Report
Zhenyu Li, Qingming Jiang, Dongfang Guo, Yangling Peng, Jing Zhang, Xinyu Chen
Journal of Gastric Cancer.2021; 21(2): 213. CrossRef - Gastric plexiform fibromyxoma resected by endoscopic submucosal dissection: A case report and review of literature
XiaoBo Zhao, XinLou Li, Xin Huang, Le Shang, JianZhong Zhang, JiHua Wu
Human Pathology: Case Reports.2021; 23: 200468. CrossRef - Plexiform fibromyxoma: a clinicopathological and immunohistochemical analysis of two cases with a literature review
Shaofei Ma, Jing Wang, Zhanjun Lu, Chaoying Shi, Daohua Yang, Jun Lin
Journal of International Medical Research.2021;[Epub] CrossRef - A rare case of plexiform fibromyxoma in stomach: FNA diagnosis with histological correlation and differential diagnoses
Yujun Gan, Ghassan Hammoud, Magda Esebua
Annals of Diagnostic Pathology.2020; 44: 151453. CrossRef - Gastric plexiform fibromyxoma: A case report
Jin-Yu Pei, Bin Tan, Peng Liu, Guang-Hua Cao, Zu-Sen Wang, Lin-Lin Qu
World Journal of Clinical Cases.2020; 8(22): 5639. CrossRef - GASTRIC PLEXIFORM FIBROMYXOMA, AN UNCOMMON MESENCHYMAL TUMOR
Cristina Magadán Álvarez, Jose M. Olmos-Martínez, M Soledad Trugeda Carrera, María José Fernandez Diaz, Enrique Toledo Martínez, Remigio Mazorra Horts, Marta M Mayorga Fernández, Ruben Darío Arias Pacheco, Berta Martín Rivas
Revista Española de Enfermedades Digestivas.2020;[Epub] CrossRef - Pediatric plexiform fibromyxoma
Mitsuharu Fukazawa, Hiroshi Koga, Shoji Hiroshige, Toshifumi Matsumoto, Yuichi Nakazono, Yasuji Yoshikawa
Medicine.2019; 98(3): e14186. CrossRef - An Update on Clinicopathological and Molecular Features of Plexiform Fibromyxoma
Hsuan-An Su, Hsu-Heng Yen, Chih-Jung Chen
Canadian Journal of Gastroenterology and Hepatology.2019; 2019: 1. CrossRef - A rare case of plexiform angiomyxoid myofibroblastic tumor in the stomach which was diagnosed at the earliest stage in the literature
Xi Li, Shuangqing Li, Shenghua Xiong, Zhujun Wang, Hu Zhang
Gastroenterology Report.2018; 6(4): 313. CrossRef - Plexiform fibromyxoma of the small bowel: A case report
Wei-Guang Zhang, Liang-Bi Xu, Yi-Ning Xiang, Chen-Hong Duan
World Journal of Clinical Cases.2018; 6(15): 1067. CrossRef - Plexiform angiomyxoid myofibroblastic tumor of the stomach: A case report
Li Liang, Lin Fanzong, Zhang Peixi, Han Cuihong
Diagnostic Cytopathology.2017; 45(1): 55. CrossRef - Duodenal plexiform fibromyxoma as a cause of obscure upper gastrointestinal bleeding
Demetrios Moris, Evangelia Spanou, Stavros Sougioultzis, Nikolaos Dimitrokallis, Polyxeni Kalisperati, Ioanna Delladetsima, Evangelos Felekouras
Medicine.2017; 96(1): e5883. CrossRef - Computed tomography and magnetic resonance imaging of a plexiform angiomyxoid myofibroblastic tumor: a case report
Hiroyuki Akai, Shigeru Kiryu, Masaru Shinozaki, Yasunori Ohta, Yoshiyasu Nakano, Koichiro Yasaka, Kuni Ohtomo
BMC Medical Imaging.2017;[Epub] CrossRef - Plexiform Angiomyxoid Myofibroblastic Tumor of the Stomach: a Rare Case
Su Mi Kim, Ji Yeong An, Min-Gew Choi, Jun Ho Lee, Tae Sung Sohn, Kyung-Mee Kim, Sung Kim, Jae Moon Bae
Journal of Gastric Cancer.2017; 17(3): 277. CrossRef - Imaging findings of gastric plexiform fibromyxoma with a cystic change
Min-Xia Yang, Zhen-Hua Zhao, Jian-Feng Yang, Bing Chen, Xun-Ze Shen, Jian-Guo Wei, Bo-Yin Wang
Medicine.2017; 96(52): e8967. CrossRef - Gastrointestinal stromal tumor with a PDGFRA mutation masquerading as gastric plexiform fibromyxoma: A comparative clinicopathological study of two cases
Jun Zhou, Jingjing Xu, Guozhong Jiang, Yihui Ma, Jingwen Qi, Wencai Li, Dandan Zhang
Oncology Letters.2017; 13(2): 887. CrossRef - Gastric plexiform fibromyxoma tumor in a child – Case report and review of the literature
Michael W. Morris, Lisa Sullivan, David E. Sawaya, Michael A. Steiner, Michael J. Nowicki
Journal of Pediatric Surgery Case Reports.2016; 4: 38. CrossRef - Unusual focal keratin expression in plexiform angiomyxoid myofibroblastic tumor
Giuseppe Quero, Teresa Musarra, Alfredo Carrato, Michelangelo Fici, Maurizio Martini, Angelo Paolo Dei Tos, Sergio Alfieri, Riccardo Ricci
Medicine.2016; 95(28): e4207. CrossRef - Laparoscopy endoscopy cooperative surgery for gastric plexiform fibromyxoma: a case report
Yoshikage Inoue, Shutaro Gunji, Kazutaka Obama, Hiroshi Okabe, Yoshiharu Sakai
Surgical Case Reports.2016;[Epub] CrossRef - Plexiform fibromyxoma with cotyledon-like serosal growth: A case report of a rare gastric tumor and review of the literature
JOSHUA ROBERT KANE, NATASHA LEWIS, REBECCA LIN, CELINA VILLA, ALEXANDRA LARSON, JEFFREY D. WAYNE, ANJANA V. YELDANDI, WILLIAM B. LASKIN
Oncology Letters.2016; 11(3): 2189. CrossRef - Plexiform angiomyxoid myofibroblastic tumour of the duodenum: a rare entity
Niladri Banerjee, Shahana Gupta, Suvashis Dash, Shibajyoti Ghosh
BMJ Case Reports.2015; : bcr2015210004. CrossRef - A case of gastric plexiform fibromyxoma: radiological and pathological findings
Katsumi Sakamoto, Masakazu Hirakawa, Kazushige Atsumi, Koshi Mimori, Kohei Shibata, Taro Tobo, Hidetaka Yamamoto, Hiroshi Honda
Japanese Journal of Radiology.2014; 32(7): 431. CrossRef - Plexiform Angiomyxoid Myofibroblastic Tumor of the Stomach: Report of a Case and Review of the Literature
Soo-Heui Baek, Jung-Hee Yoon, Ji-Yeon Kim
Journal of the Korean Society of Radiology.2014; 70(1): 47. CrossRef - Plexiform Fibromyxoma: Report of Two Pediatric Cases and Review of the Literature
Lizette Vila Duckworth, Raul S. Gonzalez, Matthew Martelli, Chen Liu, Cheryl M. Coffin, John D. Reith
Pediatric and Developmental Pathology.2014; 17(1): 21. CrossRef



Fig. 1
Fig. 2
Fig. 3
F, female; PG, partial gastrectomy; GI, gastrointestinal; HPF, high power field; DG, distal gastrectomy; M, male; STG, subtotal gastrectomy; WR, wedge resection.

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article