Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(4); 2012 > Article
-
Original Article
HDAC1 Expression in Invasive Ductal Carcinoma of the Breast and Its Value as a Good Prognostic Factor - Minseob Eom, Sung Soo Oh1, Sayamaa Lkhagvadorj, Airi Han2, Kwang Hwa Park
-
Korean Journal of Pathology 2012;46(4):311-317.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.4.311
Published online: August 23, 2012
Department of Pathology, Yonsei University Wonju College of Medicine, Wonju, Korea.
1Department of Occupational and Environmental Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
2Department of Surgery, Yonsei University Wonju College of Medicine, Wonju, Korea.
- Corresponding Author: Kwang Hwa Park, M.D. Department of Pathology, Yonsei University Wonju College of Medicine, 20 Ilsan-ro, Wonju 220-701, Korea. Tel: +82-33-741-1552, Fax: +82-33-731-6590, abba@yonsei.ac.kr
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Histone deacetylase 1 (HDAC1) is associated with the expression and function of estrogen receptors and the proliferation of tumor cells, and has been considered a very important factor in breast tumor progression and prognosis. Several studies have reported an association between HDAC1 expression and poorer prognosis in cancers including breast cancer, with a few exceptions. However, because of the dearth of studies on HDAC1 expression in breast cancer, its significance for breast cancer prognosis has not been well defined. Therefore, we examined HDAC1 expression in invasive ductal carcinoma (IDC), the most common breast cancer, and investigated its potential prognostic significance.
-
Methods
- We used 203 IDC tissue samples. Immunohistochemical stains for HDAC1 and real-time polymerase chain reaction for HDAC1 mRNA were performed and the results were compared to generally well-established prognostic factors in breast cancer and patient survival rates.
-
Results
- HDAC1 expression was significantly reduced in proportion to higher histologic grade, higher nuclear pleomorphism score, and higher mitotic counts, and with lower estrogen receptor expression. Furthermore, it was significantly associated with the survival rate.
-
Conclusions
- HDAC1 expression is a good prognostic indicator in IDC.
- Patients
- This study was approved by the Institutional Review Board of Yonsei University Wonju Christian Hospital (CR107064). The study subjects were 203 cases of IDC. All cases were tissue samples that had been surgically resected and pathologically diagnosed at the Yonsei University Wonju Christian Hospital from 1998 to 2009. All samples were formalin-fixed paraffin-embedded tissues. Fresh tissue was also available for 46 cases. The pathologic diagnoses were reconfirmed by examining hematoxylin and eosin (H&E)-stained slides and reviewing pathological reports and clinical records. The histologic grade was classified by two expert pathologists using the modified Bloom and Richardson grading method.7,8
- Immunohistochemistry
- Paraffin blocks from the 203 IDC cases were used. Using the H&E-stained slides, the representative tumor site was chosen and the corresponding site in the paraffin block was marked. Areas with necrosis, hemorrhage, and artifacts were excluded. The selected tumor area was harvested using a 5 mm Quick-ray tip-punch (Unitma, Seoul, Korea), placed on a TMA mold with 20 pores (Unitma), and re-embedded with paraffin. The TMA blocks were prepared as 4 µm-thick sections and stained with H&E. They were examined again when the appropriate tumor site was selected.
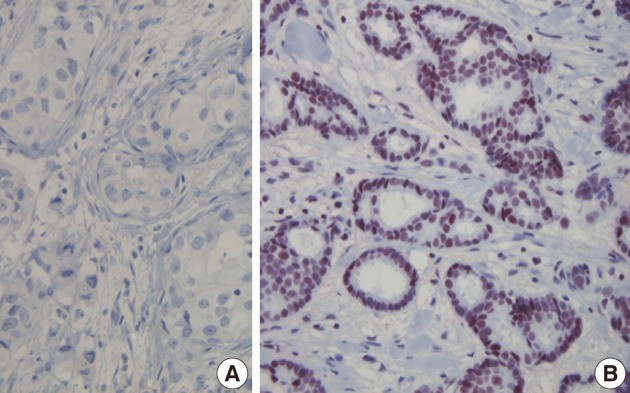
- Sections (4 µm) of TMA blocks were cut, attached onto coated slides, labeled, and then placed on the Ventana Benchmark XT (Roche Diagnostics, Basel, Switzerland). The sections were deparaffinized and subjected to pretreatment with CC1 (Roche Diagnostics) for 60 minutes at 42℃. The sections were then washed with reaction buffer followed by incubation with primary antibodies for 60 minutes at 42℃. The applied primary antibodies, all obtained from Thermo Fisher Scientific (Fremont, CA, USA), were HDAC1, ER, HER-2, and Ki-67, in dilutions of 1:3,200, 1:400, 1:300, and 1:3,000, respectively. An ultraView Universal DAB kit (Roche Diagnostics) was used in accordance with the manufacturer's recommendations to detect the location of the primary antibody followed by counterstaining with hematoxylin (Roche Diagnostics). A negative control stain without primary antibody was also performed (Fig. 1A).
- The tumor cells in which the nucleus was stained a dark brown color were read as positive. For the quantification of HDAC1, we evaluated slides broadly according to the staining intensity and the distribution of stained cells. To determine the staining intensity scores, cases without staining were given 0 points; those with weak staining, 1 point; a moderate level, 2 points; and strong staining, 3 points. Scores for the distribution of stained cells were assessed depending on the percentage of stained tumor cells: 0%, 0 points; less than 25%, 1 point; between 25% and 50%, 2 points; and more than 50%, 3 points. A staining score was obtained by adding the score of staining intensity and stained cell distribution scores. A staining score from 0 to 2 points was read as negative and a score from 3 to 6 points was read as positive.9 For nuclear ER staining, the staining intensity was characterized as 0 points (negative), 1 point (weak), 2 points (moderate), or 3 points (strong), and the distribution of positive-stained cells was assessed as 0 points (negative), 1 point (<1%), 2 points (1-10%), 3 points (10-33%), 4 points (33-67%), or 5 points (>67%). A staining score was obtained by adding the two estimated scores. A staining score from 0 to 2 points was read as negative and a score from 3 to 8 points was read as positive. The degree of HER-2 overexpression was estimated based on the membrane staining pattern and scored as 0, 1+, 2+, or 3+. Tumors with scores of 2+ or greater were considered to be positive for HER-2 expression. The Ki-67 proliferation index was defined as the percentage of positively stained cells in five randomly selected high-power fields.10
- Polymerase chain reaction (PCR)
- PCR was performed on 46 fresh IDC tissues. Each tissue sample was cut into 5 µm-thick slices and washed with phosphate-buffered saline buffer twice. RNA was extracted using the RNeasy plus mini kit (Qiagen, Hilden, Germany) and RNA concentration was measured at 260 nm and 280 nm using a spectrophotometer (Bio-Rad, Hercules, CA, USA). Applying the extracted RNA, cDNA was synthesized using the QuantiTect reverse transcription (RT) kit (Qiagen). RNA extracts less than 1 µg were mixed with 2 µL 7× gDNA wipeout buffer, kept at 42℃ for 2 minutes, and the following were added: 1 µL Quantiscript reverse transcriptase, 4 µL 5× Quantiscript RT buffer, and 1 µL RT primer mix. The mixture was reacted at 42℃ for 30 minutes and at 95℃ for 3 minutes. The synthesized cDNA was used as a PCR template.
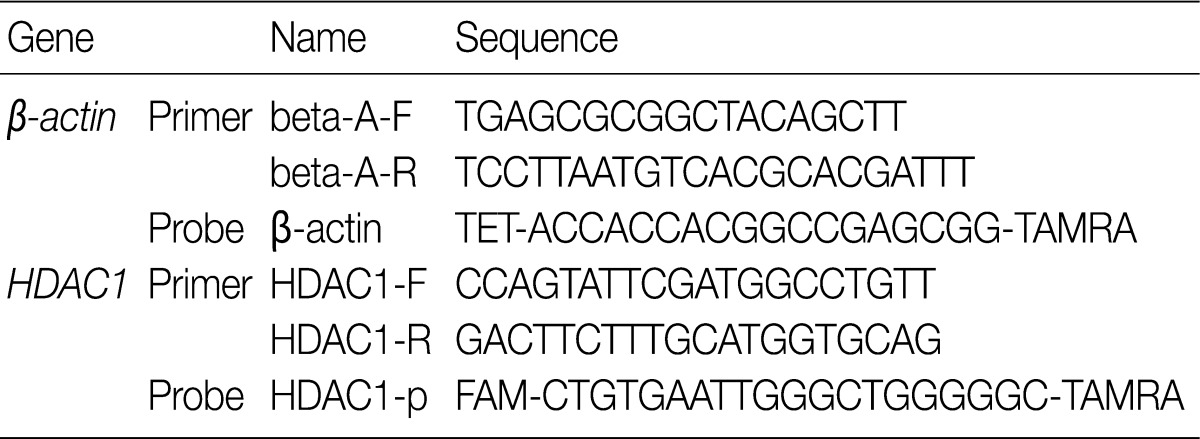
- The PCR reaction mixture was a mixture of 400 ng cDNA, 5 µL 2× QuantiTect probe PCR master mix (Qiagen), 10 pmol primer, and 30 pmol probe. PCR was performed using the RotorGene real-time Q-PCR system (Corbett, Sydney, Australia). The PCR conditions were as follows: HotStarTaq polymerase activation at 95℃ for 15 minutes, denaturation at 94℃ for 15 seconds, annealing at 60℃ for 1 minute, and extension at 72℃ for 30 seconds; the steps were repeated 50 times. Using β-actin as the internal control, the relative concentration of mRNA was obtained and used for comparison. The nucleic acid sequences of the primer and probe are shown in Table 1.
- Statistical analysis
- PASW statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used. Applying the χ2 test, the expression of HDAC1 was compared to the histologic grade, tubule formation, nuclear pleomorphism, mitotic counts, tumor size, lymph node status, ER status, and HER-2. Correlation between the HDAC1 expression and the Ki-67 proliferation index was analyzed by univariate logistic regression. Applying an independent Kruskal-Wallis analysis and the Mann-Whitney U test as non-parametric methods, the relative concentration of HDAC1 mRNA was compared to valuable prognostic factors, as confirmed by immunohistochemical stains. Survival rates were estimated using the Kaplan-Meier method and verified by the log-rank (Mantel-Cox) test. All results with a p-value less than 0.05 were considered to be statistically significant.
MATERIALS AND METHODS
Tissue microarray (TMA) block preparation
Staining methods
Evaluating method
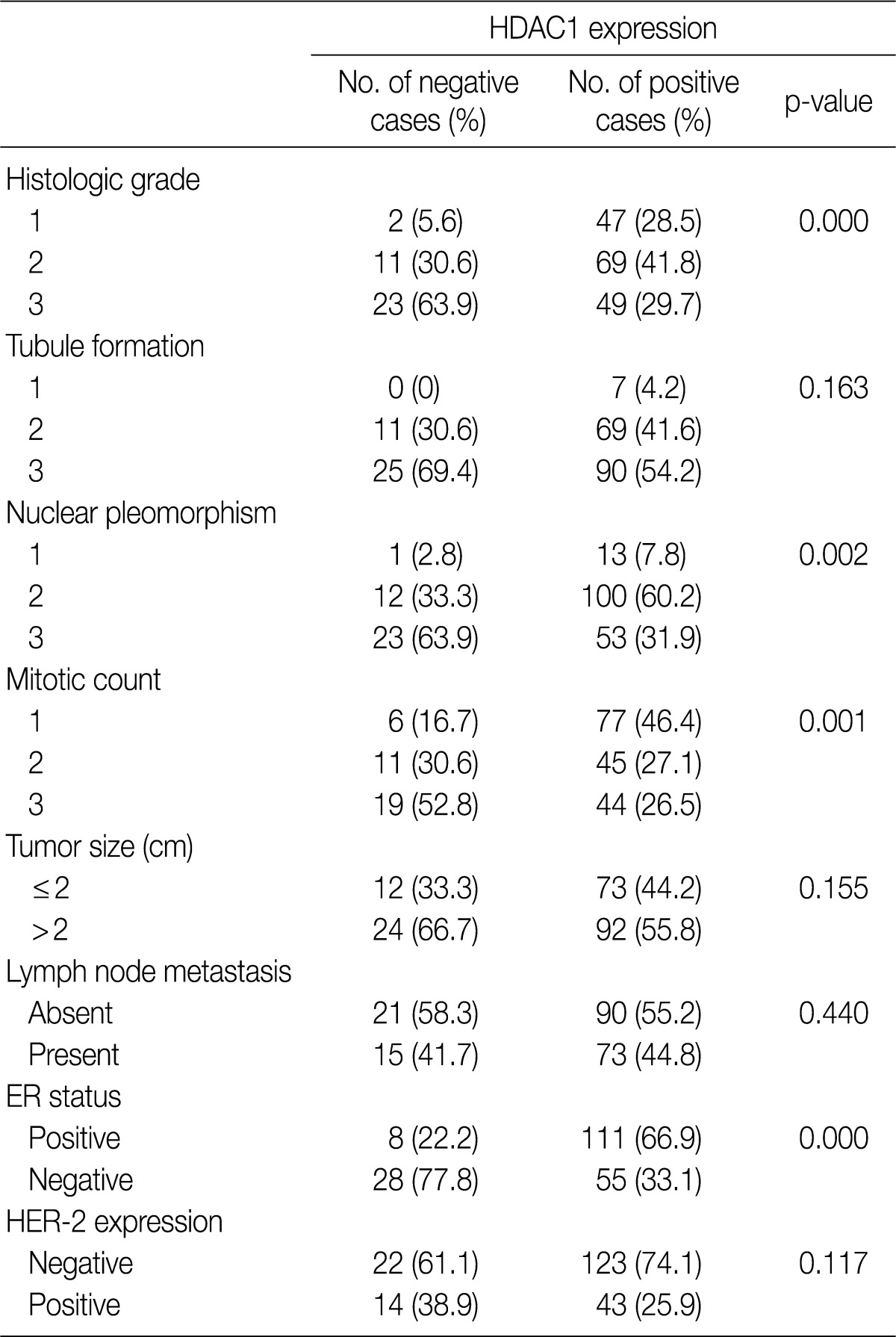
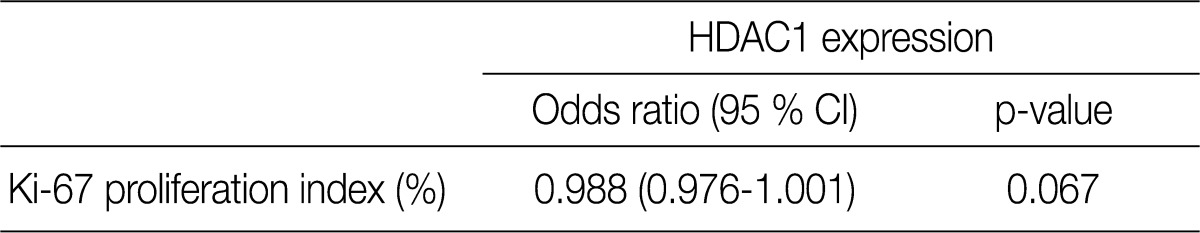
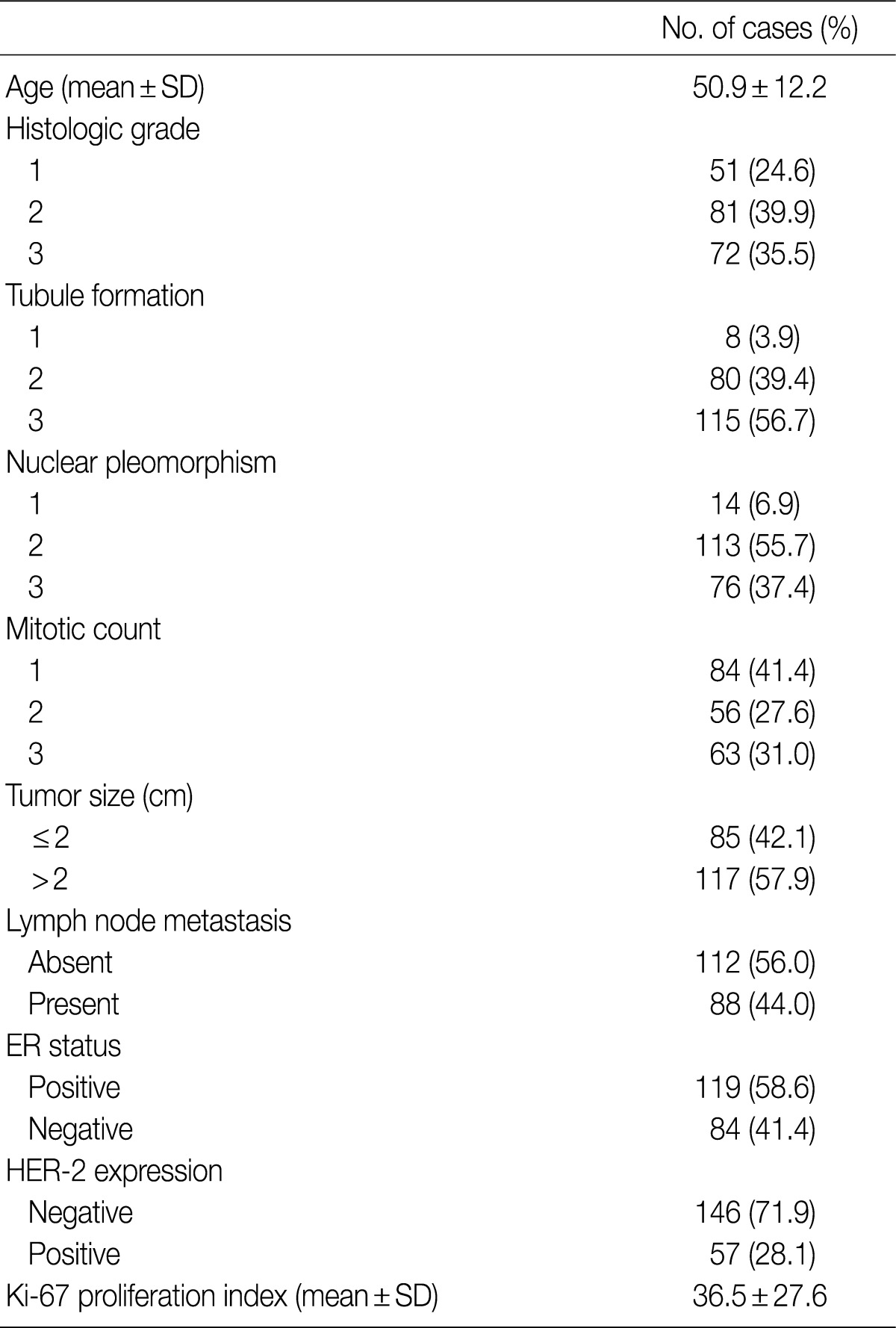
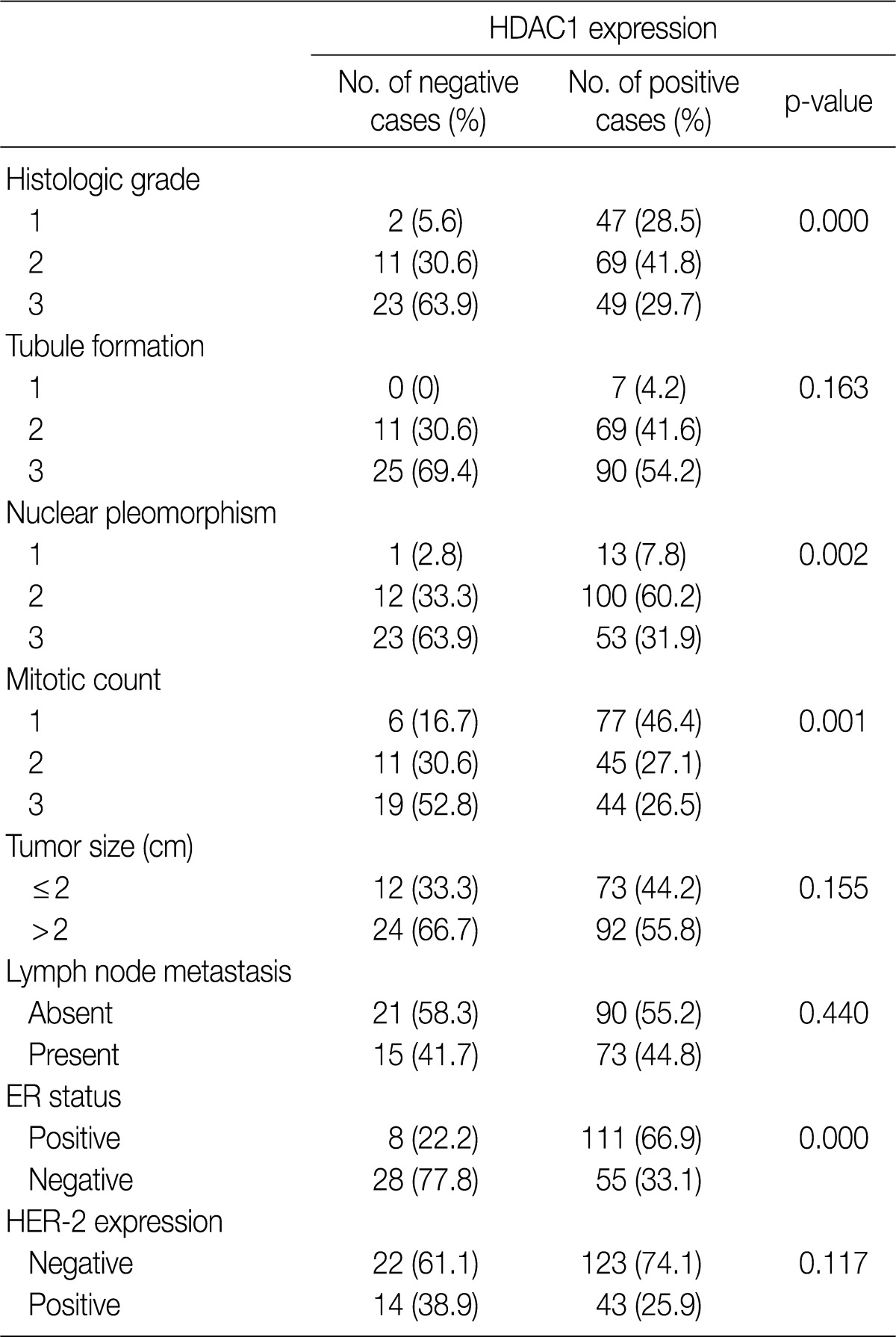
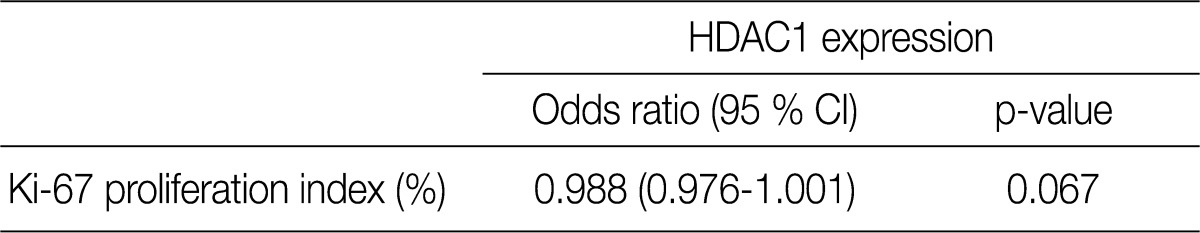
- All patients were female. The clinicopathologic characteristics of the patients and the results of immunohistochemical stains for ER, HER-2, and Ki-67 are summarized in Table 2. Immunohistochemical stains for HDAC1 were performed on 203 cases of IDC. The nuclei of the tumor cells were stained for HDAC1 (Fig. 1B). A higher HDAC1 expression rate was significantly related to a lower histologic grade (p=0.000). No relation between HDAC1 expression and the tubule formation score was observed. A higher HDAC1 expression was significantly related to a lower nuclear pleomorphism score (p=0.002). The HDAC1 expression rate decreased significantly as the mitotic count increased (p=0.001). No relation between HDAC1 expression and tumor size or lymph node status was shown. HDAC1 expression was significantly higher when ER expression was present (p=0.000). There was no significant correlation between HDAC1 expression and HER-2 expression (Table 3). A comparison of the Ki-67 proliferation index and HDAC1 expression using logistic regression showed that HDAC1 expression decreased as the proliferation index was elevated, although the result was not statistically significant (p= 0.067) (Table 4).
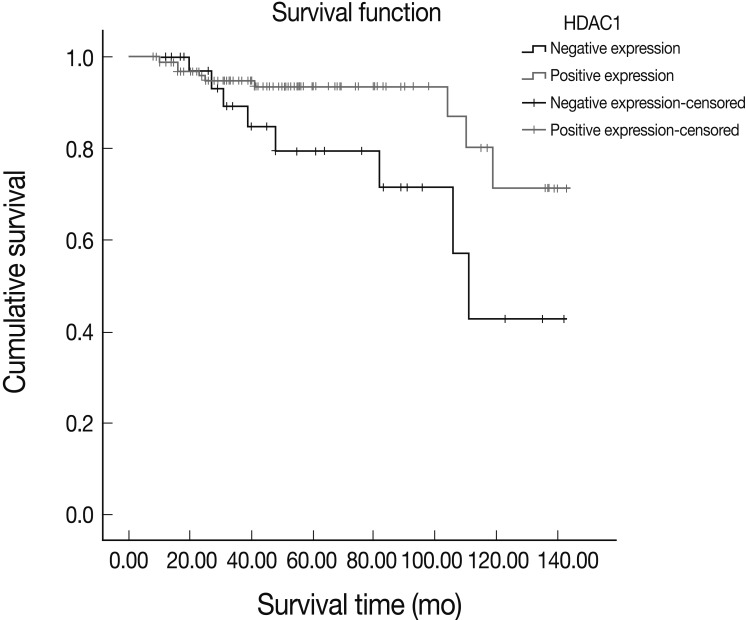
- A survival analysis using the Kaplan-Meier method showed a better survival rate in IDC patients with HDAC1 expression compared to those without HDAC1 expression; the result was statistically significant (p=0.033) (Fig. 2).
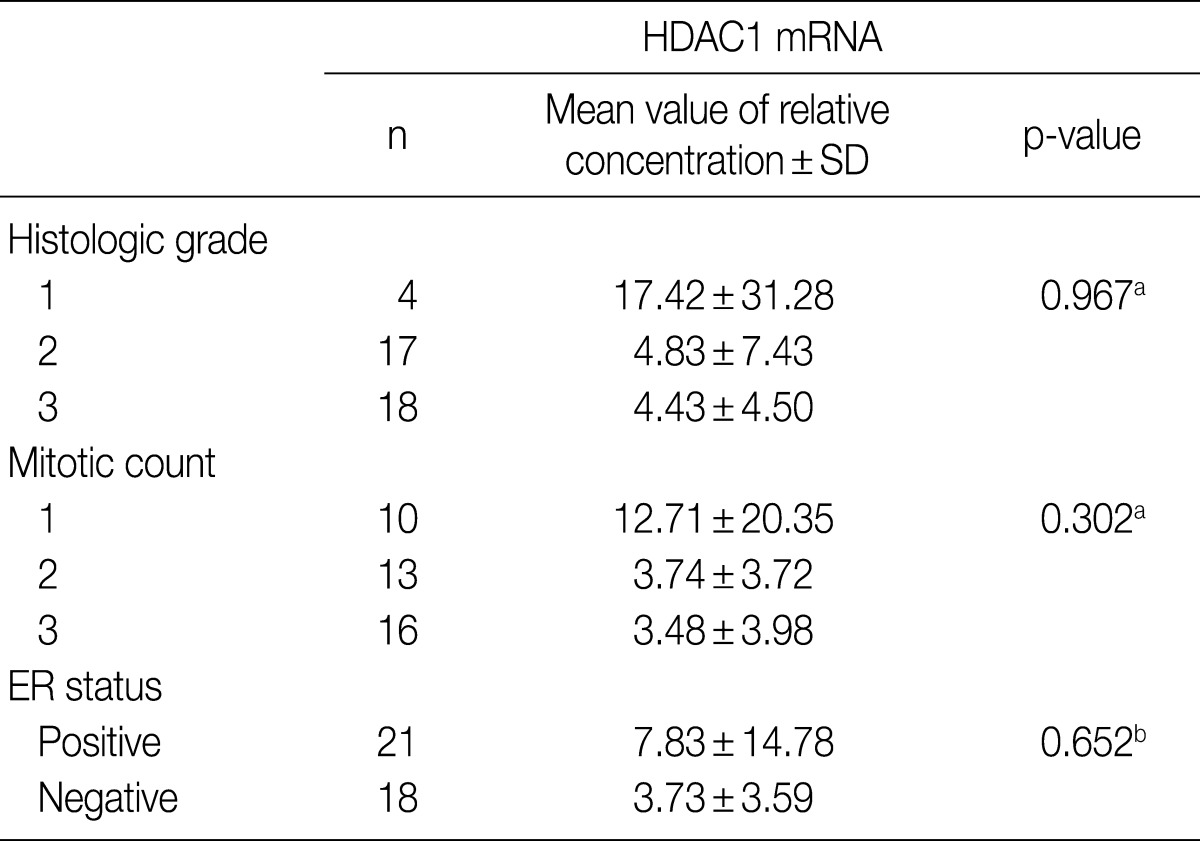
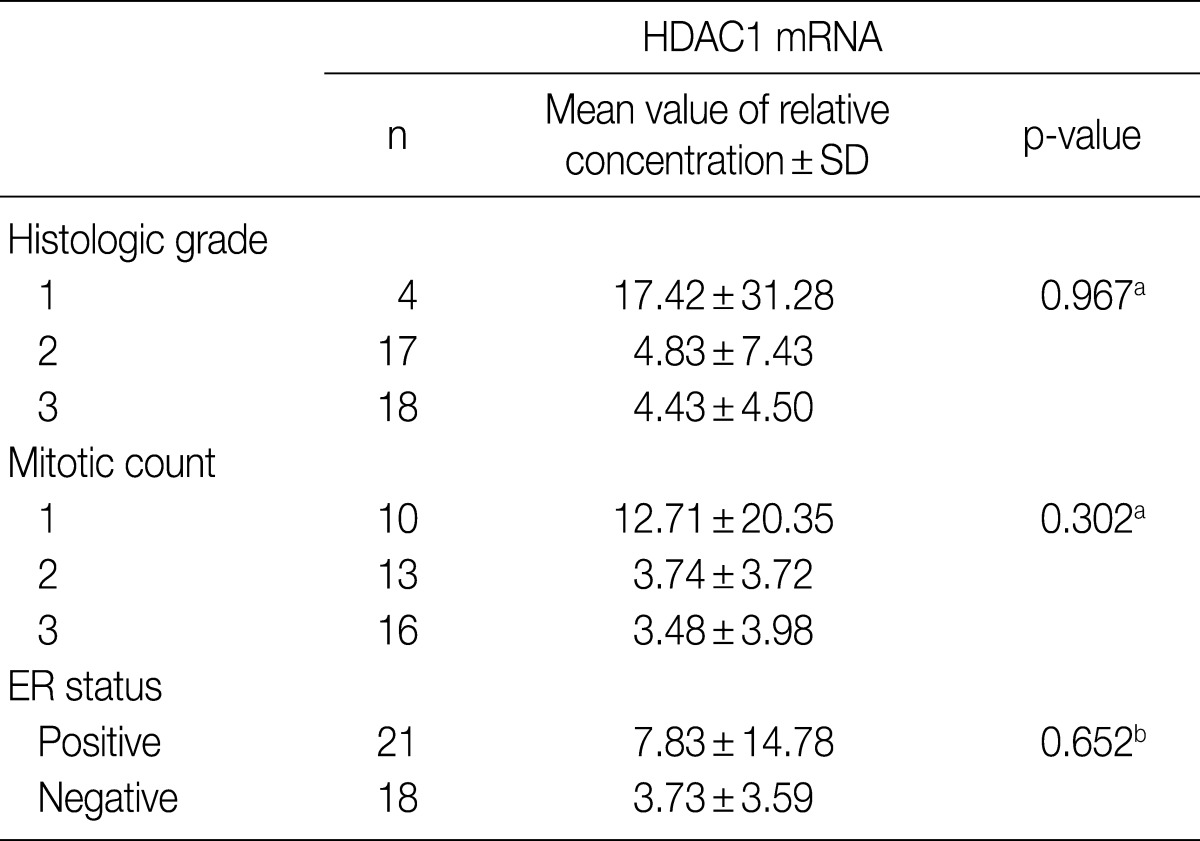
- PCR was performed on 46 cases of IDC for which fresh tissues were available. The relative concentration of HDAC1 mRNA was reduced as the histologic grade and mitosis increased, although neither correlation was statistically significant. The relative concentration of HDAC1 mRNA also was reduced in samples without ER, but again, without statistical significance (Table 5).
RESULTS
- Cancer has been considered to be the result of diverse genetic and genomic alteration. Epigenetic changes may also influence carcinogenesis. The main epigenetic modifications in humans are DNA methylation and the modification of histones, including histone acetylation. Histones are necessary for packaging DNA into chromatin.11 Histone acetylation in vivo is a dynamic reversible process regulated by histone acetyltransferases (HATs) and HDACs. The degree of histone acetylation plays a crucial role in chromatin remodeling and in translation regulation.12 Mutation, overexpression, and improper recruitment of HATs and HDACs can influence the development of malignant tumors.11 However, the mechanisms of HAT and HDAC activity are very complex and may be influenced by various factors. HDAC1, a member of the class I HDACs, is believed to play a key role in the development and progression of diverse malignant tumors of humans, including breast cancer.4-6,13-18 In addition, HDACs also participate in the regulation of gene expression by nuclear receptors. ERs are nuclear receptors that modulate gene expression to regulate the function and growth of the mammary gland. ER-α is a critical growth-regulatory gene in breast cancer and its expression status is closely linked to the prognosis and treatment outcome of breast cancer patients.5 HDAC1, therefore, is assumed to be closely related to the tumorigenesis of breast cancer. In this study, we compared HDAC1 expression in IDC to generally established risk factors for IDC and analyzed the survival rate of patients.
- Studies have shown HDAC1 expression in human ovarian cancer, endometrial cancer, pancreatic cancer, and hepatocellular carcinoma tumors; this expression increases in tumors with poor prognosis. In addition, HDAC1 expression is associated with the proliferation of tumor cells.13-15 Recently, the expression of HDAC1 in testicular germ cell tumors was examined and a significant difference between seminoma and non-seminomatous germ cell tumors was observed. However, a correlation with prognosis was not demonstrated.19 A recent study on lung adenocarcinoma reported that HDAC1 expression is an independent predictor of poor prognosis.16 HDAC1 expression in colon and gastric cancer is associated with aggressive behavior and poor prognosis.17,18 In addition, strong HDAC1 expression in the prostate cancer contributes to progression and poor prognosis.20 In 2003, Kawai et al.5 reported that HDAC1 expression was simultaneously associated with the loss of ER-α expression and the proliferation of tumor cells in breast cancer cell lines. Therefore, they inferred that HDAC1 expression is associated with poor prognostic factors in breast cancer. In addition, HDAC1 has been reported to be associated with the invasive growth of breast cancer.6 In 2011, Patani et al.21 analyzed histone-modifier gene expression profiles in human breast cancer and compared the expression with conventional pathologic and clinical parameters. In their study, HDAC1 was significantly more overexpressed in breast cancer than in normal or benign breast tissue and higher HDAC1 expression was associated with higher tumor grade. Until now, most studies of HDAC1 expression in malignant tumors have reported that HDAC1 expression may be associated with tumor growth and progression and might be a poor prognostic indicator in these tumors.
- In 2005, Zhang et al.4 reported that HDAC1 mRNA increased in breast cancer cases with no lymph node metastasis, a tumor size of less than 2 cm, decreased histologic grade, negative HER-2 oncogene, and positive ER and PR; because the survival rate increased with the expression level of HDAC1, they concluded that this expression is a useful prognostic factor. In this study, we showed that HDAC1 expression is significantly associated with histologic grade, especially nuclear pleomorphism and mitotic count. Using univariate logistic regression, we found that the HDAC1 expression decreased as the Ki-67 proliferation index increased; however, no statistical significance was observed. The histologic grade of breast cancer is now recognized as a powerful prognostic factor, and the World Health Organization's classification of tumors recommends that it be included in pathologic reports.8 Among the components of the histologic grade, the mitotic count is considered to be a very important independent prognostic factor; the cell proliferation rate, including the Ki-67 proliferation index, is the most significant prognostic factor in both resected specimens and small biopsy tissues.22-25 This study showed a close correlation between HDAC1 expression and these strong prognostic factors. ER expression, one important prognostic factor in IDC, was also elevated significantly as HDAC1 expression was enhanced. Real-time PCR on HDAC1 mRNA showed similar trends with the results of immunohistochemical stains. The relative concentration of HDAC1 mRNA decreased in cases with a higher histologic grade and mitotic count and decreased ER expression, although the results were not statistically significant. This may be due to the limited number of cases with fresh tissue samples. The survival analysis showed that the survival rate significantly increased in patients with HDAC1 expression compared to patients without HDAC1 expression. Therefore, HDAC1 expression was inferred to be an important prognostic factor in breast cancer.
- The results of our study were consistent with the results of Zhang et al.4 but contradict other studies,5,6,21 a discrepancy that may be partially attributable to the difference in cell types. Zhang et al.4 used HDAC1 mRNA from frozen tissue. We examined both HDAC1 protein and HDAC1 mRNA expression, with similar findings that may support their study. In addition, it has been reported that the expression of HDAC6, one of the class II HDACs, is correlated with better survival in breast cancer and the levels of HDAC6 mRNA may serve as a predictive indicator of responsiveness to endocrine treatment.26 The class of HDACs used in their study was different from that of the present study, making a direct comparison of the results difficult. In 2007, Ishihama et al.27 investigated HDAC1 and HAT-associated molecules such as CREB-binding proteins (CBP) and p300 in human colorectal carcinoma. There was no statistical significance between HDAC1 overexpression and prognosis. However, they reported that the high expression of CBP, a HAT-associated protein, was correlated with significantly better survival. These studies support the results of this study. Other studies of breast cancer with opposite results5,6 were performed with cell lines and did not evaluate survival data. Only two studies, including the present study, have analyzed HDAC1 expression using fixed or fresh samples from human breast cancer tissue, have compared survival data simultaneously, and have revealed that higher HDAC1 expression correlates with better survival.4 Although the results of several other studies are contradictory, the results of both protein and gene expression analyses were consistent with survival data in our study. Therefore, we conclude that HDAC1 may be a good prognostic factor in IDC. However, further studies with a larger number of cases and longer follow-up are needed to confirm the prognostic significance of HDAC1 in IDC.
- We observed that HDAC1 expression positively correlated with ER expression. However, another study showed reduced expression of ER-α in cells with HDAC1 overexpression.5 We cannot adequately explain this disparity. However, another investigator has postulated that HDAC1 expression and its relationship with ER-α may be influenced by other factors in vivo. In addition, it has been determined that HDACs cannot function independently and that HDACs do not interact directly with DNA.4 A complex of several proteins is necessary to modulate the deacetylase activity of HDACs and DNA binding, together with proteins that mediate the recruitment of HDACs to the promoter genes.28 The suppression of ER-α in human tissue can be influenced by other proteins, although HDACs affect ER expression. Further studies are warranted to validate the function of HDAC1 and the relationship between HDAC1 and ER in breast cancer.
- In this study, consistent data, including survival analysis, validated the association between HDAC1 expression and prognostic factors of breast cancer. HDAC1 expression may be closely associated with good prognostic factors in human IDC. The survival analysis showed that the survival rate increased when HDAC1 was expressed. Based on the results of this study, HDAC1 may be a valuable prognostic factor in IDC.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Osborne CK. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat 1998; 51: 227-238. ArticlePubMed
- 2. Hortobagyi GN. Treatment of breast cancer. N Engl J Med 1998; 339: 974-984. ArticlePubMed
- 3. Zhou Q, Agoston AT, Atadja P, Nelson WG, Davidson NE. Inhibition of histone deacetylases promotes ubiquitin-dependent proteasomal degradation of DNA methyltransferase 1 in human breast cancer cells. Mol Cancer Res 2008; 6: 873-883. ArticlePubMedPMCPDF
- 4. Zhang Z, Yamashita H, Toyama T, et al. Quantitation of HDAC1 mRNA expression in invasive carcinoma of the breast. Breast Cancer Res Treat 2005; 94: 11-16. PubMed
- 5. Kawai H, Li H, Avraham S, Jiang S, Avraham HK. Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor alpha. Int J Cancer 2003; 107: 353-358. PubMed
- 6. Park SY, Jun JA, Jeong KJ, et al. Histone deacetylases 1, 6 and 8 are critical for invasion in breast cancer. Oncol Rep 2011; 25: 1677-1681. ArticlePubMed
- 7. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991; 19: 403-410. ArticlePubMed
- 8. Tavassoli FA, Devilee P. World Health Organization classification of tumours: pathology and genetics of tumours of the breast and female genital organs. 2003; Lyon: IARC Press, 18-19.
- 9. Park KH, Choi SE, Eom M, Kang Y. Downregulation of the anaphase-promoting complex (APC)7 in invasive ductal carcinomas of the breast and its clinicopathologic relationships. Breast Cancer Res 2005; 7: R238-R247. PubMedPMC
- 10. Dabbs DJ. Diagnostic immunohistochemistry: theranostic and genomic applications. 2010; 3rd ed. Philadelphia: Saunders/Elsevier.
- 11. Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 2007; 1: 19-25. PubMedPMC
- 12. Strahl BD, Allis CD. The language of covalent histone modifications. Nature 2000; 403: 41-45. PubMed
- 13. Weichert W, Denkert C, Noske A, et al. Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia 2008; 10: 1021-1027. PubMedPMC
- 14. Wang W, Gao J, Man XH, Li ZS, Gong YF. Significance of DNA methyltransferase-1 and histone deacetylase-1 in pancreatic cancer. Oncol Rep 2009; 21: 1439-1447. PubMed
- 15. Rikimaru T, Taketomi A, Yamashita Y, et al. Clinical significance of histone deacetylase 1 expression in patients with hepatocellular carcinoma. Oncology 2007; 72: 69-74. PubMed
- 16. Minamiya Y, Ono T, Saito H, et al. Expression of histone deacetylase 1 correlates with a poor prognosis in patients with adenocarcinoma of the lung. Lung Cancer 2011; 74: 300-304. PubMed
- 17. Higashijima J, Kurita N, Miyatani T, et al. Expression of histone deacetylase 1 and metastasis-associated protein 1 as prognostic factors in colon cancer. Oncol Rep 2011; 26: 343-348. PubMed
- 18. Sudo T, Mimori K, Nishida N, et al. Histone deacetylase 1 expression in gastric cancer. Oncol Rep 2011; 26: 777-782. PubMed
- 19. Fritzsche FR, Hasler A, Bode PK, et al. Expression of histone deacetylases 1, 2 and 3 in histological subtypes of testicular germ cell tumours. Histol Histopathol 2011; 26: 1555-1561. PubMed
- 20. Song Y, Shiota M, Tamiya S, Kuroiwa K, Naito S, Tsuneyoshi M. The significance of strong histone deacetylase 1 expression in the progression of prostate cancer. Histopathology 2011; 58: 773-780. PubMed
- 21. Patani N, Jiang WG, Newbold RF, Mokbel K. Histone-modifier gene expression profiles are associated with pathological and clinical outcomes in human breast cancer. Anticancer Res 2011; 31: 4115-4125. PubMed
- 22. Meyer JS, Alvarez C, Milikowski C, et al. Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Mod Pathol 2005; 18: 1067-1078. PubMed
- 23. van Diest PJ, van der Wall E, Baak JP. Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol 2004; 57: 675-681. PubMedPMC
- 24. Miglietta L, Vanella P, Canobbio L, et al. Prognostic value of estrogen receptor and Ki-67 index after neoadjuvant chemotherapy in locally advanced breast cancer expressing high levels of proliferation at diagnosis. Oncology 2010; 79: 255-261. PubMed
- 25. Trihia H, Murray S, Price K, et al. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors: a surrogate marker? Cancer 2003; 97: 1321-1331. PubMed
- 26. Zhang Z, Yamashita H, Toyama T, et al. HDAC6 expression is correlated with better survival in breast cancer. Clin Cancer Res 2004; 10: 6962-6968. PubMed
- 27. Ishihama K, Yamakawa M, Semba S, et al. Expression of HDAC1 and CBP/p300 in human colorectal carcinomas. J Clin Pathol 2007; 60: 1205-1210. PubMedPMC
- 28. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 2003; 370(Pt 3):737-749. PubMedPMC
REFERENCES


Figure & Data
References
Citations

- SNP rs4971059 predisposes to breast carcinogenesis and chemoresistance via TRIM46‐mediated HDAC1 degradation
Zihan Zhang, Xiaoping Liu, Lei Li, Yang Yang, Jianguo Yang, Yue Wang, Jiajing Wu, Xiaodi Wu, Lin Shan, Fei Pei, Jianying Liu, Shu Wang, Wei Li, Luyang Sun, Jing Liang, Yongfeng Shang
The EMBO Journal.2021;[Epub] CrossRef - The Impact of Androgen Receptor and Histone Deacetylase 1 Expression on the Prognosis of Ductal Carcinoma In Situ
Choong Man Lee, Il Yong Chung, Yangsoon Park, Keong Won Yun, Hwi Gyeong Jo, Hye Jin Park, Hee Jin Lee, Sae Byul Lee, Hee Jeong Kim, Beom Seok Ko, Jong Won Lee, Byung Ho Son, Sei Hyun Ahn, Jisun Kim
Journal of Breast Cancer.2020; 23(6): 610. CrossRef - Prognostic and clinical significance of histone deacetylase 1 expression in breast cancer: A meta-analysis
Weiqiang Qiao, Heyang Liu, Ruidong Liu, Qipeng Liu, Ting Zhang, Wanying Guo, Peng Li, Miao Deng
Clinica Chimica Acta.2018; 483: 209. CrossRef - HDAC1 triggers the proliferation and migration of breast cancer cells via upregulation of interleukin-8
Zhaohui Tang, Sijuan Ding, Honglin Huang, Pengfei Luo, Bohua Qing, Siyuan Zhang, Ruoting Tang
Biological Chemistry.2017; 398(12): 1347. CrossRef - Identification of novel histone deacetylase 1 inhibitors by combined pharmacophore modeling, 3D-QSAR analysis, in silico screening and Density Functional Theory (DFT) approaches
Sanjay K. Choubey, Richard Mariadasse, Santhosh Rajendran, Jeyakanthan Jeyaraman
Journal of Molecular Structure.2016; 1125: 391. CrossRef - The Potential of Histone Deacetylase Inhibitors in Breast Cancer Therapy
Namita Chatterjee, Martin Tenniswood
Breast Cancer Management.2015; 4(2): 85. CrossRef


Fig. 1
Fig. 2
SD, standard deviation; ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2.
HDAC1, histone deacetylase 1; ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2.
HDAC1, histone deacetylase 1; CI, confidence interval.
HDAC1, histone deacetylase 1; SD, standard deviation; ER, estrogen receptor. aCalculated by Kruskal-Wallis analysis; bCalculated by Mann-Whitney U test.

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article