Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(4); 2012 > Article
-
Original Article
Loss of E-cadherin and Acquisition of Vimentin in Epithelial-Mesenchymal Transition are Noble Indicators of Uterine Cervix Cancer Progression - Na-Hye Myong
-
Korean Journal of Pathology 2012;46(4):341-348.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.4.341
Published online: August 23, 2012
Department of Pathology, Dankook University College of Medicine, Cheonan, Korea.
- Corresponding Author: Na-Hye Myong, M.D. Department of Pathology, Dankook University College of Medicine, 119 Dandae-ro, Dongnam-gu, Cheonan 330-714, Korea. Tel: +82-41-550-3891, Fax: +82-41-561-9127, myongnh@dankook.ac.kr
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Epithelial-mesenchymal transition (EMT) has been known to play a key role in the stromal invasion of carcinoma in situ (CIS) lesion. Loss of E-cadherin and acquisition of vimentin are two critical steps in EMT, that are induced by Snail-1 upregulation associated with overexpression of epidermal growth factor receptor (EGFR). However, roles of EMT-related proteins in human cervical tissues have not been fully elucidated. In this study, we investigated the immunoexpressions of EMT-related proteins in CIS, microinvasive squamous cell carcinoma (SCC), and invasive SCC to demonstrate their key roles in tumor progression.
-
Methods
- Eighty one CIS, 17 microinvasive, and 21 invasive SCC cases were immunostained with primary antibodies for Snail-1, EGFR, E-cadherin, and vimentin on paraffin-embedded tissue microarray blocks.
-
Results
- EGFR and Snail-1 proteins were highly expressed but the levels were not significantly different between the three groups. However, loss of E-cadherin and acquisition of vimentin were proven to occur significantly higher in microinvasive and invasive SCC cases than in CIS.
-
Conclusions
- E-cadherin and vimentin were found to be two useful indicators of EMT in evaluating stromal invasion of CIS. However, it was not demonstrated for Snail-1 and EGFR proteins to play any key role in the progression of cervix cancer.
- Patient characteristics
- Tissue specimens were obtained from 119 patients with cervical carcinoma who had undergone hysterectomy from 1999 to 2009 at Dankook University Hospital. The current study was approved by the Institutional Review Board (IRB) of Dankook University. Patients' age ranged from 31 to 88 years. They had a median age of 50 and 55 years at diagnosis of CIS and invasive SCC, respectively. They have not received irradiation and anticancer chemotherapy prior to surgery. Stages by the International Federation of Gynecology and Obstetrics (FIGO) classification system (2008) included 81 cases of 0 (CIS), 17 Ia (microinvasive), and 21 Ib-IIIb (invasive). Clinical follow-up periods of microinvasive and invasive groups ranged from 1 to 10 years, with each median period of 5.25 and 2 years. During the follow-up periods, 13 cases of the invasive group (62%) were treated with radiation only or concurrent chemoradiation, but only one of microinvasive group (6%) received an additional radiotherapy. Neither recurrence nor distant metastasis was found in microinvasive carcinoma group, while 5 (24%) out of 21 invasive carcinoma cases showed either recurrent cervix cancer or distant metastasis in lung, liver, kidney, and brain.
- Tissue sample preparation by tissue microarray (TMA) technique
- Among 119 cases with cervical carcinoma, 59 tumor specimens were paired with their own normal samples for TMA assay to evaluate the staining patterns of E-cadherin, vimentin, EGFR, and Snail-1. In cases with multiple paraffin blocks, the tumor block was matched with a normal one. In an individual case with no more than one available block, each separate area of the tumor and the normal was sampled within the same block. TMA blocks were constructed using pairs of the most representative tumor and normal tissue cores with a diameter of 2 mm, which were obtained from appropriate areas in formalin-fixed paraffin-embedded tissue blocks. These tissue cores were transferred and embedded into the recipient block that had 60 empty 2 mm-sized holes. Using a microtome, 4 µm thick serial sections were cut from the TMA blocks and were transferred to poly-L-lysine-coated slides.
- Immunohistochemical staining and its semiquantitative analysis
- The microarrayed tissue sections were deparaffinized with standard xylene, rehydrated using graded alcohols, and rinsed with water. The sections were microwaved in 10 mM citrated buffer at 90℃ for 10 minutes and then treated with 3% H2O2-phosphate buffered saline solution to reduce an endogenous peroxidase activity. They were incubated with normal bovine serum to reduce non-specific antibody binding and subsequently subjected to primary antibody reactions. Monoclonal or polyclonal antibodies against EGFR (clone31G7, Invitrogen, Carlsbad, CA, USA), Snail-1 (ab82846, Abcam, Cambridge, MA, USA), E-cadherin (HECD-1, Takara, Shiga, Japan), and vimentin (V9, BioGenex, Fremont, CA, USA) were reacted with the sections for 1 hour at room temperature using dilution ratio of 1:200, 1:800, 1:200, and 1:3,200, respectively. A negative control was incubated without the primary antibodies. Positive controls for E-cadherin and vimentin were matched samples of normal exocervical squamous epithelium and the stromal cells beneath it, respectively. Detection of the immunoreactive staining was carried out by following the avidin-biotin-peroxidase complex method and using the LSAB kit (Dako, Glostrup, Denmark). The sections were subjected to a color reaction with 3,3-diaminobenzidine tetrahydrochloride that contained 3% H2O2 in Tris buffer. They were lightly counterstained with Meyer's hematoxylin.
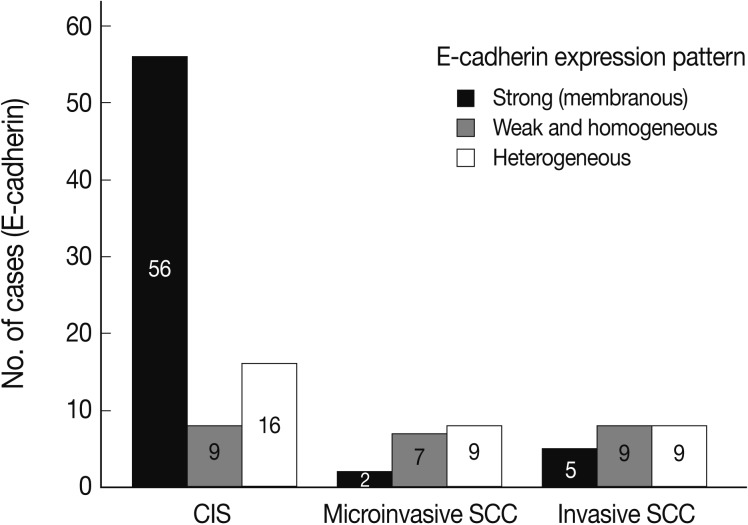
- The immunoexpressions for EGFR, Snail-1, E-cadherin, and vimentin were analyzed as follows: EGFR was interpreted as an overexpression when the tumor cells were immunostained distinctively either as a membranous or cytoplasmic pattern at the area greater than 10% or 50%, respectively.6,7 Upregulation of Snail-1 protein was proven by greater than 50% nuclear immunoreactivity seen at the tumor cells, because its overall immunoreactivity was found to be high with an average of about 50% even in the normal control tissue. Vimentin expression was interpreted as being acquired by the tumor cells, when they exhibited greater than 5% positive immunoreactivity in the tumor. Compared to the EGFR, Snail-1, and vimentin, the E-cadherin with either membranous or cytoplasmic immunoexpression was evaluated qualitatively rather than semiquantitatively as three patterns as follows: 1) strong (S) pattern: E-cadherin staining pattern in almost all tumor cells (>95%) is as strong as in the normal epithelial cells; 2) weak and homogeneous (W&H) pattern: All tumor cells are uniformly stained but more weakly expressed than in the normal squamous epithelium; 3) heterogeneous (HEG) pattern: The intensity of E-cadherin staining differs from cell to cell and the cells without immunostaining are included. W&H and HEG patterns are considered to show either a loss of or reduced E-cadherin expression.8
- Statistical analysis
- The comparison of each immunoreactivity for EGFR, Snail-1, E-cadherin, and vimentin between three uterine cervix tumor groups and their inter-relationships were analyzed by a Fisher's exact test using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). All tests were two-sided and p-values less than 0.05 were considered statistically significant.
MATERIALS AND METHODS
- Immunohistochemical staining patterns for four EMT-related proteins in normal and neoplastic squamous epithelial cells
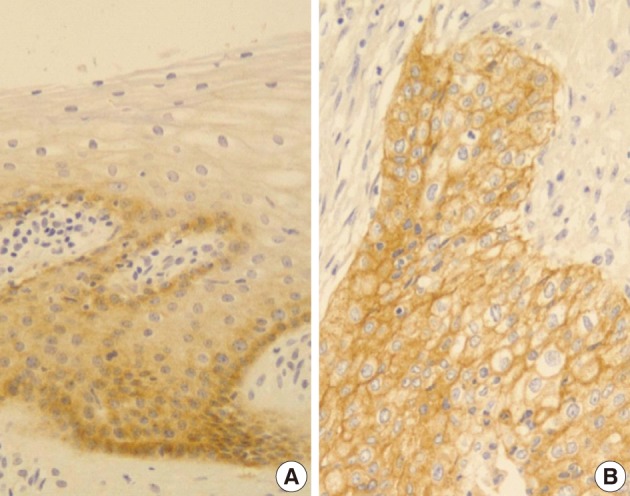
- Normal cervical mucosal cells expressed much less amounts of EGFR compared to the matched cervical carcinoma cells, mostly showing both membranous and cytoplasmic stainability that are mainly confined to the parabasal and basal layers of the cervix epithelium (Fig. 1A). Cervical carcinoma cells with EGFR overexpression showed diffuse immunoreactivity with honeycomb-like membranous and vaguely cytoplasmic patterns (Fig. 1B).
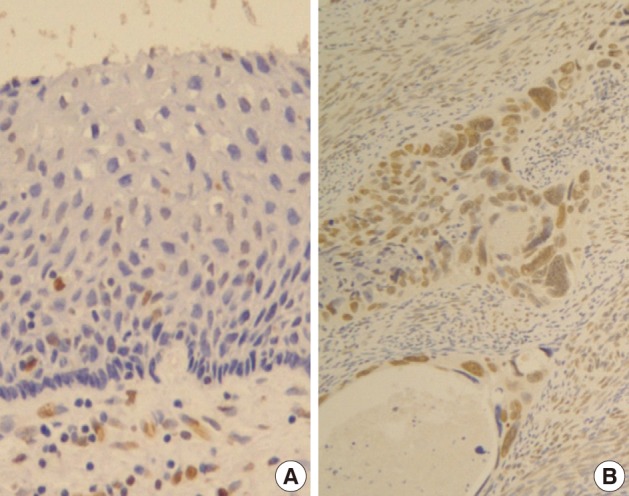
- Normal squamous epithelium showed no constant nuclear immunoreactivity (Fig. 2A), whereas invasive squamous carcinoma cells were stained in their nuclei much more than the normal by Snail-1 antibody (Fig. 2B).
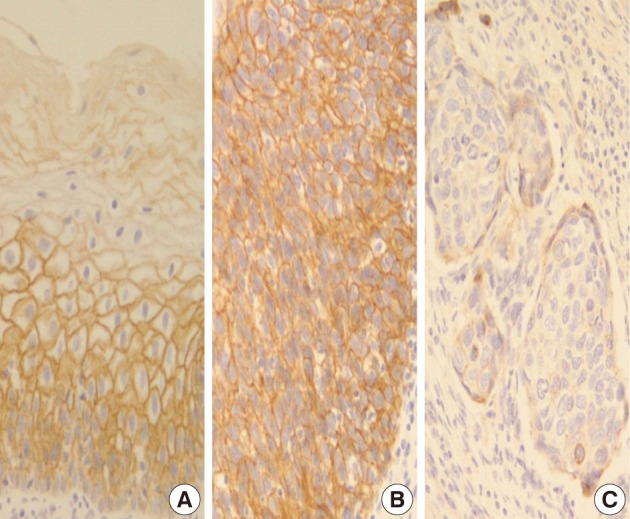
- In normal squamous epithelium, the staining had a fine granular appearance and was limited to the cell surface membrane. The basal layer presented membranous staining with occasional cytoplasmic staining. In the parabasal and intermediate cell layers, E-cadherin immunoreactivity was found circumferentially at the cell membrane, but the staining was lost at the superficial layer (Fig. 3A). This gradually decreasing pattern of membranous staining in the normal cervix was not found in the CIS and invasive SCC cases, which showed weak but diffuse cytoplasmic or heterogeneously reduced immunoreactivity (Fig. 3C) in addition to the strong membranous staining (Fig. 3B). The staining pattern of either reduced or strong membranous immunoreactivity could be found in both CIS and microinvasive carcinoma groups whether the foci was invasive or not.
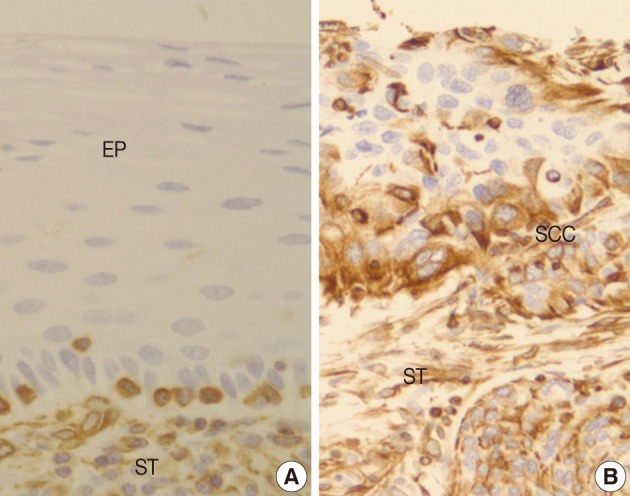
- Normal squamous mucosa showed no immunoreactivity for vimentin and there were only some scattered mesenchymal cells within it, which were considered to be entrapped (Fig. 4A). However, squamous carcinoma cells revealed a relatively dense cytoplasmic expression for vimentin, showing polygonal or multipolar appearance and heterogeneously scattered pattern (Fig. 4B). Additionally, there was no big difference in the staining pattern between non-invasive and invasive foci of the CIS and microinvasive (or invasive) carcinoma groups.
- Comparison of immunoexpressions for EGFR, Snail-1, E-cadherin, and vimentin between CIS, microinvasive, and invasive SCC groups
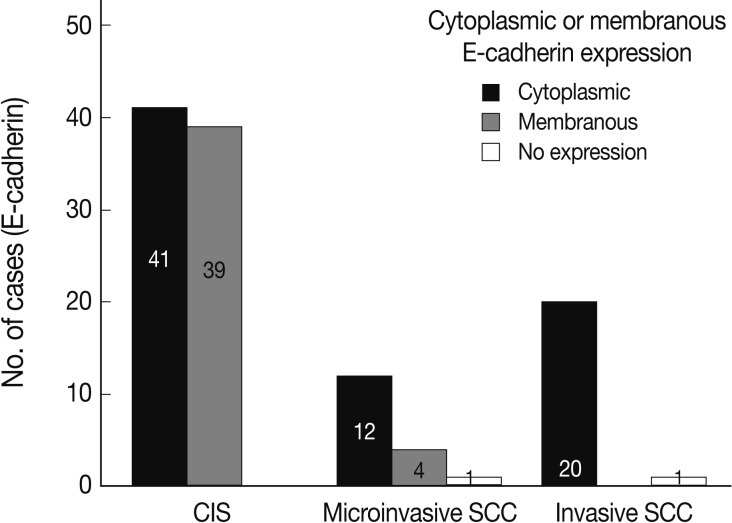
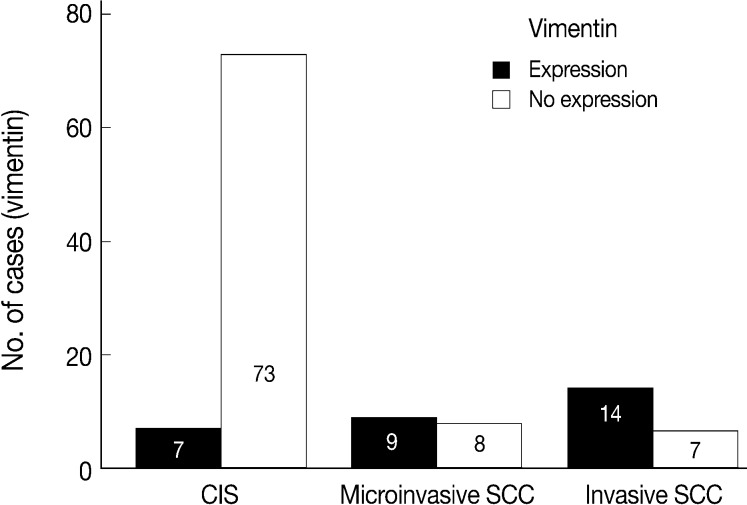
- EGFR overexpression was found in 70.4%, 82.4%, and 71.4% of CIS, microinvasive, and invasive SCC groups, respectively. There was no difference in EGFR expression rates between three groups (Fig. 5). Snail-1 was expressed more frequently in the nuclei of the tumor cells and in the underlying stromal tissue than in the normal control. Snail-1 was expressed in 82.7%, 82.4%, and 81.0% of CIS, microinvasive and invasive groups, respectively. Thus, no significant difference in Snail-1 expression was found between them (data not shown). When E-cadherin expression was classified as three patterns such as S, W&H, and HEG ones, W&H and HEG patterns were considered to show either a loss of or reduced E-cadherin expression. CIS group predominantly revealed 70% cases of strong or memebranous pattern of E-cadherin expression, whereas the invasive and microinvasive SCC groups only revealed 11.8% and 23.8%, respectively. The correlation between the loss of E-cadherin and stromal invasion was statistically significant (p<0.05) (Fig. 6). Moreover, when E-cadherin expression was analyzed by the pattern of either membranous or cytoplasmic staining, increased cytoplasmic immunoreactivity or loss of membranous immunoreactivity was found significantly higher in invasive SCC cases than in CIS group (p<0.001) (Fig. 7). Vimentin, a representative mesenchymal cell marker, was immunoexpressed aberrantly (>5% of entire cells) in 60.5% and 8.8% of the invasive and CIS cases, respectively. Meanwhile, normal and metaplastic squamous epithelium revealed none or rare (<5%) immunoreactive cells in the normal control tissues. Thus, microinvasive and invasive SCC cases showed a much higher vimentin expression than the CIS group (p<0.001) (Fig. 8).
- Inter-relationship between immunoexpressions for EGFR, Snail-1, E-cadherin, and vimentin as EMT markers in CIS and invasive SCC groups
- In CIS group alone, there was a significant correlation between EGFR overexpression and Snail-1 upregulation. EGFR-positive cases showed a significantly higher Snail-1 expression (89%) than the EGFR-negative ones (67%; p=0.022) (Table 1).
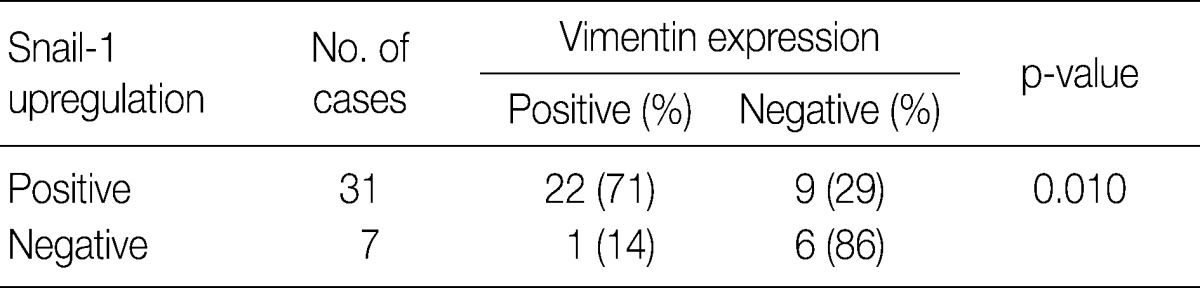
- In invasive SCC group including microinvasive carcinoma cases, there were statistically significant interrelationships between E-cadherin and Snail-1 and between vimentin and Snail-1. Reduced expression patterns (W&H and HEG) of E-cadherin were more frequent in cases showing Snail-1 upregulation than in cases without Snail-1 upregulation (p=0.026) (Table 2). Furthermore, the invasive carcinomas with Snail-1 upregulation showed much higher vimentin expression (71%) than those without Snail-1 upregulation (14%; p=0.010) (Table 3).
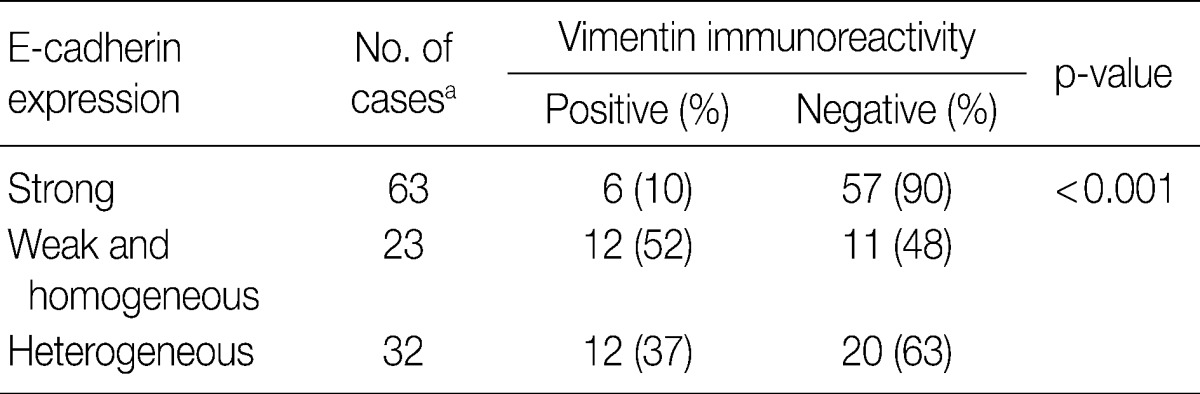
- In overall 118 cases including 80 CIS and 38 invasive SCC cases, there was an inverse relationship between E-cadherin and vimentin expressions, because the overall cases with reduced E-cadherin expression disclosed a significantly higher vimentin expression (45%) than the cases with strong membranous E-cadherin expression (10%; p<0.001) (Table 4).
RESULTS
EGFR
Snail-1
E-cadherin
Vimentin
- Stromal invasion of the tumor cells has been known to be an important criterion for malignancy and is also applied frequently to distinguish the invasive cervix cancer from squamous cell CIS. It is thought to be associated with EMT, which is characterized by the change of the phenotypes from epithelial cells to mesenchymal cells, disassembly of intercellular junctions, and increased cell motility. A recent study has concluded that EGF is a novel EMT inducer in cervical cancer cells based on several in vivo and in vitro results.1 Their conclusion was supported by the following results: a) Chronic EGF treatment induced elongation of cell shape, increased cell scattering and enhanced cancer cell invasion; b) EGF caused decreases in epithelial markers such as E-cadherin and β-catenin; c) EGF caused increases in mesenchymal markers such as vimentin, α-smooth muscle actin, and fibronectin. Their study has also demonstrated that cervical carcinoma progression is accompanied by overexpressed EGFR in parallel with downregulated E-cadherin and upregulated vimentin. In general, downregulation of E-cadherin has been known as the key step for the invasive phase of carcinoma, and its dominant transcriptional repression has been reported to be largely responsible for the loss of E-cadherin expression.9,10 Recent evidences have shown that Snail-1 plays a fundamental role in EMT through its suppression of E-cadherin, and it also induces upregulation and redistribution of mesenchymal markers such as vimentin and fibronectin.5,11 In normal cervical mucosa, EGFR is found to be in the cytoplasm and the membrane of the cells within the basal layer and its expression gets increased in the cytoplasm by HPV infection and associated with the increasing grade of intraepithelial neoplasia.7,12 In our study, both membranous and cytoplasmic immunostainability for EGFR was found to be increased in the tumor tissues compared to the internal control tissue, but there was no significant difference between the CIS and the SCC groups. However, 81 cases of CIS group showed a positive correlation between EGFR and Snail-1 immunoexpressions (p=0.022). Snail-1, which is another potent EMT inducer, suppresses E-cadherin expression and further controls the proteolytic activity of the matrix metalloproteinases that contribute to the phenotypic changes associated with EMT and stromal invasion.13,14 With EGFR activation, Snail-1 is then upregulated, accumulates in the nuclei, and performs its transcriptional function on EMT markers.1 In the present study, there was no significant difference in Snail-1 immunoexpressions between CIS and the invasive SCC groups, although invasive SCC cases revealed higher Snail-1 immunoexpression in the vimentin-positive and E-cadherin-negative cases than in the vimentin-negative and E-cadherin-positive ones (p=0.010 and p=0.026, respectively). These results might suggest that the upregulated Snail-1 protein in the invasive SCC plays a key role in stromal invasion by increasing the discohesiveness of tumor cells and acquisition of vimentin expression.
- In a similar-sized study (121 cases), high-grade squamous intraepithelial lesion (HSIL) and invasive cases showed significantly reduced E-cadherin expression and increased cytoplasmic immunoreactivity compared to the low-grade SIL (LSIL), atypical squamous cell of undetermined significance, and normal groups.15 They concluded that decreased E-cadherin expression appears to be a useful parameter of the malignant potential of cervical lesions. However, they didn't compare it between CIS and microinvasive SCC, although CIS is an important lesion with a potential of impending stromal invasion. The present study is, therefore, thought to be the first to demonstrate that E-cadherin might be the best indicator of stromal invasion of the CIS lesions, in that microinvasive and invasive SCC cases showed a significantly reduced or loss of E-cadherin expression compared to the CIS group (p<0.001). A loss of membranous staining and a progressive increase in cytoplasmic staining of E-cadherin have been reported in several studies to be observed from LSILs to HSILs to invasive SCC.15-17 In our study, this tendency for E-cadherin immunoreactivity to be increased in the cytoplasm or lost in the cell membranes was also observed from CIS, microinvasive, and invasive SCC by either cytoplasmic (51%, 71%, and 95%, respectively) or membranous (49%, 23%, and 0%, respectively) immunoreactivities. The differences in the immunoreactivities were statistically significant between CIS and microinvasive or invasive carcinomas (p<0.001). The cytoplasmic staining and loss of membranous E-cadherin observed in the HSIL and invasive SCC is likely to reflect a nonfunctional protein form of E-cadherin since it should be present at the cell membrane in order to perform its integral roles for intercellular cohesion and epithelial tissue integrity. The nonfunctional cytoplasmic E-cadherin may indicate an increased rate of protein synthesis and cell proliferation and consequently the molecule en route from the endoplasmic reticulum to the cell surface membrane, or failure of the protein to translocate or attach to the cell membrane due to the absence of other associated proteins.18-20 According to our results, higher cytoplasmic E-cadherin immunopositivity and more marked loss of membranous staining pattern in the microinvasive and invasive carcinomas than in CIS lesions appeared to be the indicator of the progression of CIS into microinvasive or invasive carcinoma, especially when there are suspicious foci of stromal invasion in any CIS lesions (Fig. 3).
- There is few data about vimentin expression as an EMT marker in cervical cancer progression. A recent study on EMT in cervical cancer tissue and cultured cells demonstrated vimentin expression by an immunofluorescent staining technique in the invasive or metastatic tumor cell nests, with no or rare expression in the normal or superficial (noninvasive) tumor cell nests.1 They have concluded that the progression of cervical carcinoma is accompanied by an upregulation of vimentin. However, data on the comparison of the vimentin expression profiles between squamous intraepithelial lesions and invasive carcinomas were not available and thus its role in the EMT process could not be elucidated in their study. In the present study, normal control tissues immunohistochemically presented almost no vimentin expression and the CIS lesions showed less than 10% of immunoreactivity (8.8%). Meanwhile, microinvasive and invasive carcinomas showed a much higher vimentin expression with 53% and 67% cases, respectively. Thus, it is considered that the significantly increased vimentin expression could be used as an important EMT marker in the progression of CIS into microinvasive or invasive SCC in the human cervical tissues.
- In summary, we obtained the necessary results for elucidating the important EMT markers playing in the stromal invasion of CIS as follows:
-
Of the four EMT markers, reduced E-cadherin expression and acquisition of vimentin expression were found to be significantly higher in the invasive than in the CIS group.
CIS group revealed a positive correlation between EGFR and Snail-1 expressions.
In the invasive carcinoma group, upregulated Snail-1 protein was correlated with loss of E-cadherin expression and gain of vimentin expression.
Overall group (119 cases) exhibited a statistically significant inverse correlation between E-cadherin and vimentin immunoexpressions.
- Based on these summarized results, we could arrive at a conclusion that loss of E-cadherin and acquisition of vimentin expression in the CIS of human uterine cervix might be noble indicators of the progression of CIS into microinvasive and subsequently into invasive SCC via EMT. However, in the present study, no significant difference in EGFR or Snail-1 protein expression between CIS and invasive SCC was demonstrated during the EMT process. Thus, further studies are needed to elucidate control mechanisms, other than EGFR overexpression and Snail-1 upregulation, in the cervix cancer progression.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res 2008; 14: 4743-4750. ArticlePubMed
- 2. Pignatelli M. E-cadherin: a biological marker of tumour differentiation. J Pathol 1993; 171: 81-82. ArticlePubMed
- 3. Frixen UH, Behrens J, Sachs M, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991; 113: 173-185. ArticlePubMedPMC
- 4. Yokoyama K, Kamata N, Hayashi E, et al. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol 2001; 37: 65-71. ArticlePubMed
- 5. Batlle E, Sancho E, Francí C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2: 84-89. ArticlePubMed
- 6. Kristensen GB, Holm R, Abeler VM, Tropé CG. Evaluation of the prognostic significance of cathepsin D, epidermal growth factor receptor, and c-erbB-2 in early cervical squamous cell carcinoma: an immunohistochemical study. Cancer 1996; 78: 433-440. ArticlePubMed
- 7. Soonthornthum T, Arias-Pulido H, Joste N, et al. Epidermal growth factor receptor as a biomarker for cervical cancer. Ann Oncol 2011; 22: 2166-2178. ArticlePubMed
- 8. Murakami A, Nakagawa T, Fukushima C, et al. Relationship between decreased expression of squamous cell carcinoma antigen 2 and E-cadherin in primary cervical cancer lesions and lymph node metastasis. Oncol Rep 2008; 19: 99-104. ArticlePubMed
- 9. Zhou BP, Deng J, Xia W, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 2004; 6: 931-940. ArticlePubMed
- 10. Birchmeier W, Behrens J, Weidner KM, Frixen UH, Schipper J. Dominant and recessive genes involved in tumor cell invasion. Curr Opin Cell Biol 1991; 3: 832-840. ArticlePubMed
- 11. Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 2002; 3: 155-166. ArticlePubMed
- 12. Brustmann H, Hinterholzer S, Brunner A. Expression of phosphorylated histone H2AX (gamma-H2AX) in normal and neoplastic squamous epithelia of the uterine cervix: an immunohistochemical study with epidermal growth factor receptor. Int J Gynecol Pathol 2011; 30: 76-83. ArticlePubMed
- 13. Jin H, Yu Y, Zhang T, et al. Snail is critical for tumor growth and metastasis of ovarian carcinoma. Int J Cancer 2010; 126: 2102-2111. ArticlePubMed
- 14. Cano A, Pérez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000; 2: 76-83. ArticlePubMed
- 15. Kaplanis K, Kiziridou A, Liberis V, Destouni C, Galazios G. E-cadherin expression during progression of squamous intraepithelial lesions in the uterine cervix. Eur J Gynaecol Oncol 2005; 26: 608-610. ArticlePubMed
- 16. Vessey CJ, Wilding J, Folarin N, et al. Altered expression and function of E-cadherin in cervical intraepithelial neoplasia and invasive squamous cell carcinoma. J Pathol 1995; 176: 151-159. ArticlePubMed
- 17. Faleiro-Rodrigues C, Lopes C. E-cadherin, CD44 and CD44v6 in squamous intraepithelial lesions and invasive carcinomas of the uterine cervix: an immunohistochemical study. Pathobiology 2004; 71: 329-336. ArticlePubMed
- 18. Kawanishi J, Kato J, Sasaki K, Fujii S, Watanabe N, Niitsu Y. Loss of E-cadherin-dependent cell-cell adhesion due to mutation of the beta-catenin gene in a human cancer cell line, HSC-39. Mol Cell Biol 1995; 15: 1175-1181. ArticlePubMedPMCPDF
- 19. Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin: a possible mechanism for regulating cadherin turnover. J Biol Chem 2001; 276: 12301-12309. ArticlePubMed
- 20. Chen YT, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol 1999; 144: 687-699. ArticlePubMedPMC
REFERENCES
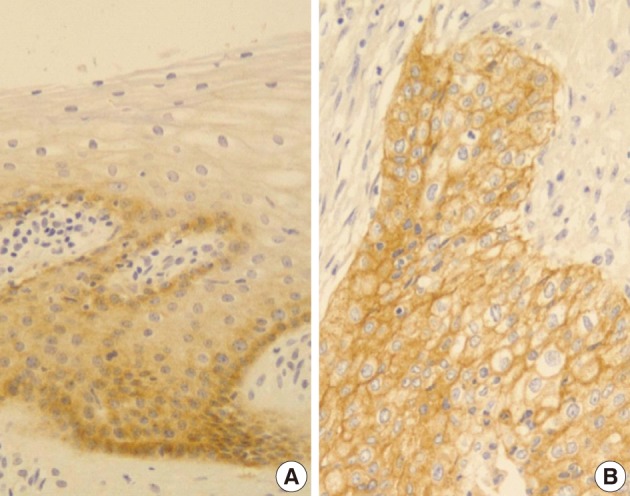
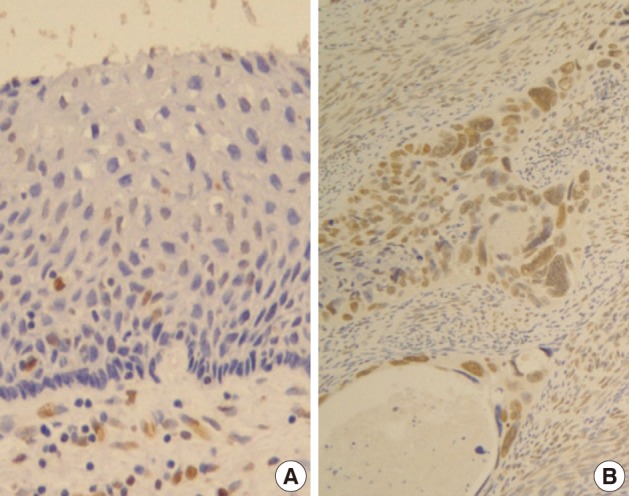
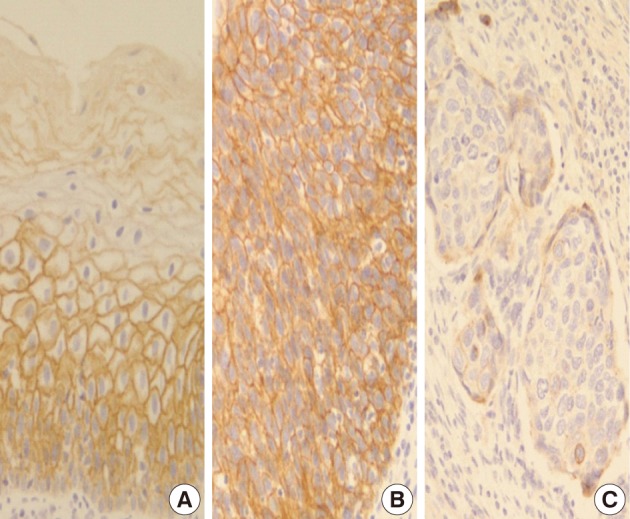
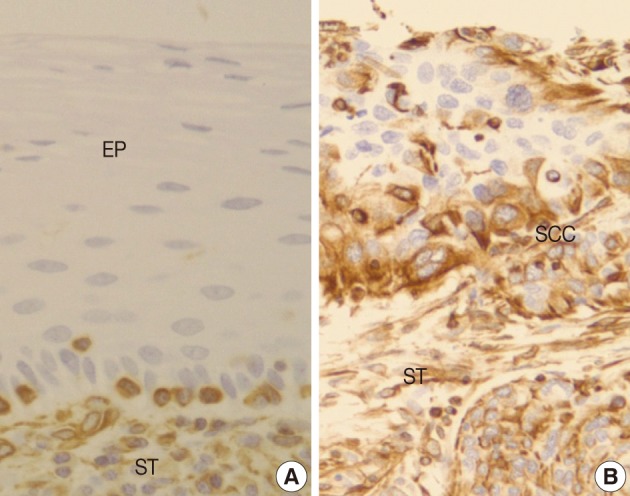
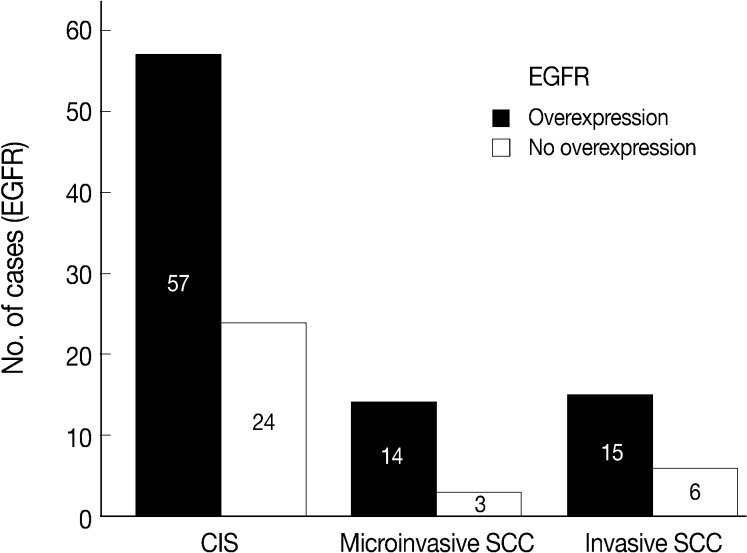
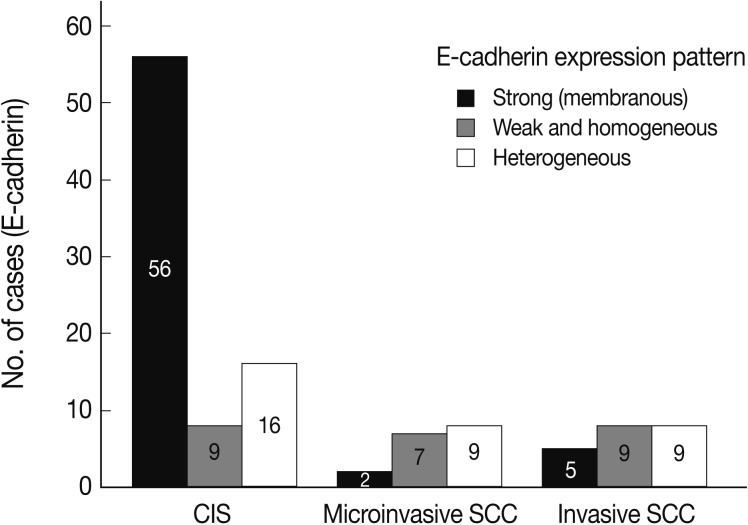
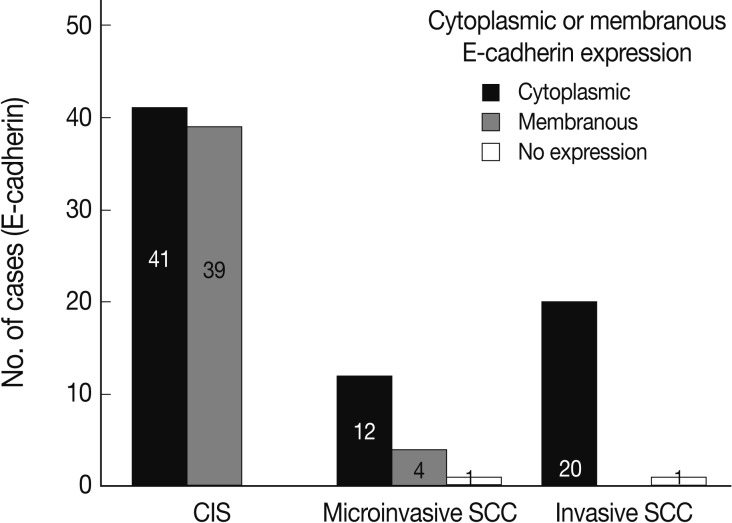
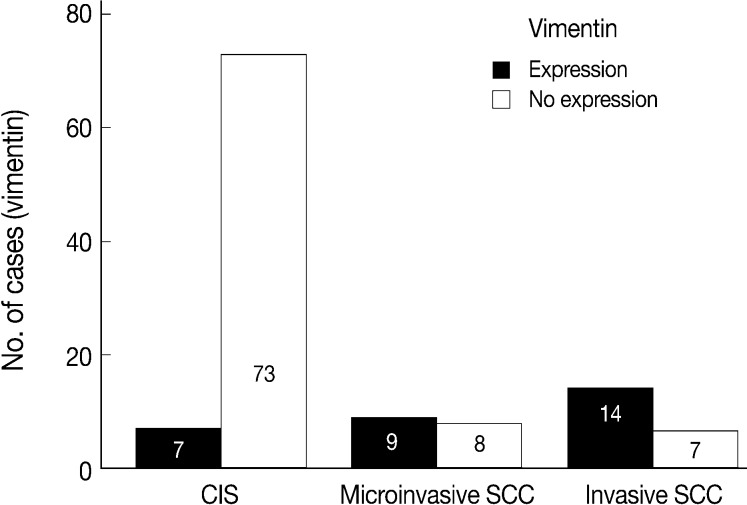
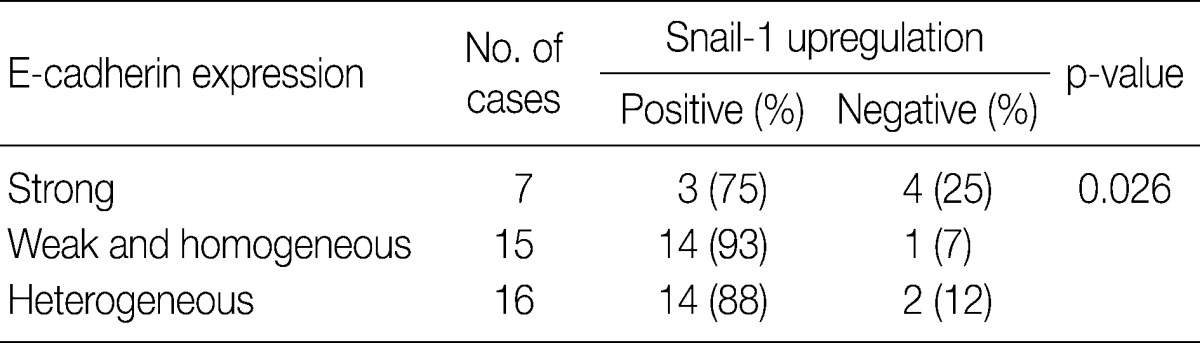
Figure & Data
References
Citations

- The effects of low-dose sorafenib on epithelial-mesenchymal transition and multidrug resistance markers in HepG2 cell line
Yaprak DÖNMEZ ÇAKIL, Zeynep AKBULUT, Gamze DEMİREL, Ranan GÜLHAN, Zeynep OZUNAL
The European Research Journal.2023; 9(2): 367. CrossRef - Oral Submucous Fibrosis: Etiological Mechanism, Malignant Transformation, Therapeutic Approaches and Targets
Xiaofeng Qin, Yujie Ning, Liming Zhou, Youming Zhu
International Journal of Molecular Sciences.2023; 24(5): 4992. CrossRef - Comparative Assessment of E-cadherin’s Expression between the Metastatic and Non-metastatic Oral Squamous Cell Carcinoma: An Immunohistochemical Study
Sufia Khan, Veda Hegde, Deepti Shrivastava, Mohammed Azamulla, Mohammad Khursheed Alam, Kumar Chandan Srivastava
Pesquisa Brasileira em Odontopediatria e Clínica Integrada.2023;[Epub] CrossRef - Role of Vimentin and E-cadherin Expression in Premalignant and Malignant Lesions of Oral Cavity
Sandesh Kumar Gupta, Sunita Agarwal, Shashank Nath Singh, Ritu Sehra, Prem Singh Jat, Pawan Singhal
Indian Journal of Otolaryngology and Head & Neck Surgery.2022; 74(3): 350. CrossRef - Increased Sphingosine Kinase 1 Expression Is Associated with Poor Prognosis in Human Solid Tumors: A Meta-Analysis
Chuanmeng Zhang, Chenglin Zhou, Jie Xu, Shanshan Xue, Alexander G Mathioudakis
Disease Markers.2022; 2022: 1. CrossRef - Current Progress of EMT: A New Direction of Targeted Therapy for Colorectal Cancer with Invasion and Metastasis
Zhuomin Tan, Wenyan Sun, Ya Li, Xingmeng Jiao, Mingliang Zhu, Junfei Zhang, Chen Qing, Yinnong Jia
Biomolecules.2022; 12(12): 1723. CrossRef - Study of Expression of Epithelial Cadherin in Benign and Malignant Epithelial Lesions of Uterine Cervix
Pooja Gupta, Pooja Agarwal, Lalit Kumar, Shikha Prakash, Poonam Yadav
Journal of Datta Meghe Institute of Medical Sciences University.2022; 17(3): 662. CrossRef - Evaluation of e-cadherin and vimentin expression for different grades of oral epithelial dysplasia and oral squamous cell carcinoma – An immunohistochemical study
Nagiredla Puneeta, Tummidi Santosh, Isha Mishra, Pravin Gaikwad, Anshuta Sahu
Journal of Oral and Maxillofacial Pathology.2022; 26(2): 285. CrossRef - Morphological and molecular characteristics of spheroid formation in HT-29 and Caco-2 colorectal cancer cell lines
Elmira Gheytanchi, Marzieh Naseri, Feridoun Karimi-Busheri, Fatemeh Atyabi, Ensie Sadat Mirsharif, Mahmood Bozorgmehr, Roya Ghods, Zahra Madjd
Cancer Cell International.2021;[Epub] CrossRef - MACC1 Is Associated With Epithelial–Mesenchymal Transition and Can Predict Poor Prognosis in Nasopharyngeal Carcinoma
Hao Cheng, Linxiang Zhou, Yalan Long, Juanjuan Xiang, Longhua Chen
Frontiers in Oncology.2021;[Epub] CrossRef - Cellular and biomolecular detection based on suspended microchannel resonators
Juhee Ko, Jaewoo Jeong, Sukbom Son, Jungchul Lee
Biomedical Engineering Letters.2021; 11(4): 367. CrossRef - Anticancer Properties of Eugenol: A Review
Ali T. Zari, Talal A. Zari, Khalid Rehman Hakeem
Molecules.2021; 26(23): 7407. CrossRef - Up-regulated microRNA-33b inhibits epithelial–mesenchymal transition in gallbladder cancer through down-regulating CROCC
Guohui Xu, Xiaoyong Wei, Qiang Tu, Cuncai Zhou
Bioscience Reports.2020;[Epub] CrossRef - Independent Validation of Tumor Budding Activity and Cell Nest Size as Determinants of Patient Outcome in Squamous Cell Carcinoma of the Uterine Cervix
Somaye Y. Zare, Omonigho Aisagbonhi, Farnaz Hasteh, Oluwole Fadare
American Journal of Surgical Pathology.2020; 44(9): 1151. CrossRef - Study of long non-coding RNA highly upregulated in liver cancer (HULC) in breast cancer: A clinical & in vitro investigation
ReyhanehRavanbakhsh Gavgani, Esmaeil Babaei, MohammadAli Hosseinpourfeizi, Ashraf Fakhrjou, Vahid Montazeri
Indian Journal of Medical Research.2020; 152(3): 244. CrossRef - Resveratrol suppresses the growth and metastatic potential of cervical cancer by inhibiting STAT3Tyr705 phosphorylation
Xiaodong Sun, Qianqian Xu, Lian Zeng, Lixia Xie, Qiang Zhao, Hongxia Xu, Xuanbin Wang, Nan Jiang, Pan Fu, Ming Sang
Cancer Medicine.2020; 9(22): 8685. CrossRef - microRNA-877 contributes to decreased non-small cell lung cancer cell growth via the PI3K/AKT pathway by targeting tartrate resistant acid phosphatase 5 activity
Xue Bai, Changjun He, Bicheng Fu, Xianglong Kong, Jianlong Bu, Kaibin Zhu, Wei Zheng, Fucheng Zhou, Boxiong Ni
Cell Cycle.2020; 19(23): 3260. CrossRef - TRPM4 channel is involved in regulating epithelial to mesenchymal transition, migration, and invasion of prostate cancer cell lines
Alfredo I. Sagredo, Eduardo A. Sagredo, Victor Pola, César Echeverría, Rodrigo Andaur, Luis Michea, Andrés Stutzin, Felipe Simon, Katherine Marcelain, Ricardo Armisén
Journal of Cellular Physiology.2019; 234(3): 2037. CrossRef - Overexpression of microRNA‐190 inhibits migration, invasion, epithelial‐mesenchymal transition, and angiogenesis through suppression of protein kinase B‐extracellular signal‐regulated kinase signaling pathway via binding to stanniocalicin 2 in breast canc
Guiming Sun, Meirong Liu, Hui Han
Journal of Cellular Physiology.2019; 234(10): 17824. CrossRef - Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review
Barbora Peltanova, Martina Raudenska, Michal Masarik
Molecular Cancer.2019;[Epub] CrossRef - YY1: A novel therapeutic target for diabetic nephropathy orchestrated renal fibrosis
Tingting Yang, Fanglin Shu, Hao Yang, Cai Heng, Yi Zhou, Yibing Chen, Xuan Qian, Lei Du, Xia Zhu, Qian Lu, Xiaoxing Yin
Metabolism.2019; 96: 33. CrossRef - Malignant ascites-derived exosomes promote peritoneal tumor cell dissemination and reveal a distinct miRNA signature in advanced gastric cancer
Yanting Hu, Changsong Qi, Xiang Liu, Cheng Zhang, Jing Gao, Yi Wu, Jing Yang, Qian Zhao, Jian Li, Xiaojuan Wang, Lin Shen
Cancer Letters.2019; 457: 142. CrossRef - Silencing of lysyl oxidase‑like 2 inhibits the migration, invasion and epithelial‑to‑mesenchymal transition of renal cell carcinoma cells through the Src/FAK signaling pathway
Xi Hong, Jian‑Jun Yu
International Journal of Oncology.2019;[Epub] CrossRef - YY1 Complex Promotes Quaking Expression via Super-Enhancer Binding during EMT of Hepatocellular Carcinoma
Jingxia Han, Jing Meng, Shuang Chen, Xiaorui Wang, Shan Yin, Qiang Zhang, Huijuan Liu, Rong Qin, Zhongwei Li, Weilong Zhong, Chao Zhang, Heng Zhang, Yuanhao Tang, Tingting Lin, Wanfeng Gao, Xiaoyun Zhang, Lan Yang, Yanrong Liu, Hong-gang Zhou, Tao Sun, Ch
Cancer Research.2019; 79(7): 1451. CrossRef - E-cadherin: Its dysregulation in carcinogenesis and clinical implications
Sonia How Ming Wong, Chee Mun Fang, Lay-Hong Chuah, Chee Onn Leong, Siew Ching Ngai
Critical Reviews in Oncology/Hematology.2018; 121: 11. CrossRef - NDRG2 suppresses proliferation, migration, invasion and epithelial-mesenchymal transition of esophageal cancer cells through regulating the AKT/XIAP signaling pathway
Cheng-Liang Yang, Xiao-Li Zheng, Ke Ye, Hong Ge, Ya-Nan Sun, Yu-Fei Lu, Qing-Xia Fan
The International Journal of Biochemistry & Cell Biology.2018; 99: 43. CrossRef - NUSAP1 gene silencing inhibits cell proliferation, migration and invasion through inhibiting DNMT1 gene expression in human colorectal cancer
Guoda Han, Zhijiang Wei, Haibin Cui, Wei Zhang, Xiaonan Wei, Zhiliang Lu, Xiyong Bai
Experimental Cell Research.2018; 367(2): 216. CrossRef - Human papillomavirus type 16 E5-mediated upregulation of Met in human keratinocytes
Matthew L. Scott, David T. Coleman, Kinsey C. Kelly, Jennifer L. Carroll, Brittany Woodby, William K. Songock, James A. Cardelli, Jason M. Bodily
Virology.2018; 519: 1. CrossRef - Lamin A/C might be involved in the EMT signalling pathway
Lingkun Zuo, Huanying Zhao, Ronghui Yang, Liyong Wang, Hui Ma, Xiaoxue Xu, Ping Zhou, Lu Kong
Gene.2018; 663: 51. CrossRef - Potential role of periodontal pathogens in compromising epithelial barrier function by inducing epithelial‐mesenchymal transition
A. A. Abdulkareem, R. M. Shelton, G. Landini, P. R. Cooper, M. R. Milward
Journal of Periodontal Research.2018; 53(4): 565. CrossRef - Loss of CAMSAP3 promotes EMT via the modification of microtubule–Akt machinery
Varisa Pongrakhananon, Onsurang Wattanathamsan, Masatoshi Takeichi, Paninee Chetprayoon, Pithi Chanvorachote
Journal of Cell Science.2018;[Epub] CrossRef - A Role for βA3/A1-Crystallin in Type 2 EMT of RPE Cells Occurring in Dry Age-Related Macular Degeneration
Sayan Ghosh, Peng Shang, Hiroto Terasaki, Nadezda Stepicheva, Stacey Hose, Meysam Yazdankhah, Joseph Weiss, Taiji Sakamoto, Imran A. Bhutto, Shuli Xia, J. Samuel Zigler, Ram Kannan, Jiang Qian, James T. Handa, Debasish Sinha
Investigative Opthalmology & Visual Science.2018; 59(4): AMD104. CrossRef - Nlrp3 Activation Induces Il-18 Synthesis and Affects the Epithelial Barrier Function in Reactive Cholangiocytes
Luca Maroni, Laura Agostinelli, Stefania Saccomanno, Claudio Pinto, Debora M. Giordano, Chiara Rychlicki, Samuele De Minicis, Luciano Trozzi, Jesus M. Banales, Espen Melum, Tom H. Karlsen, Antonio Benedetti, Gianluca Svegliati Baroni, Marco Marzioni
The American Journal of Pathology.2017; 187(2): 366. CrossRef - Corepressor metastasis-associated protein 3 modulates epithelial-to-mesenchymal transition and metastasis
Liang Du, Zhifeng Ning, Fuxing Liu, Hao Zhang
Chinese Journal of Cancer.2017;[Epub] CrossRef - JNK-associated scattered growth of YD-10B oral squamous carcinoma cells while maintaining the epithelial phenotype
Gayoung Lee, Hyun-Man Kim
Biochemical and Biophysical Research Communications.2017; 487(4): 862. CrossRef - Invasive Front Grading and Epithelial-Mesenchymal Transition in Canine Oral and Cutaneous Squamous Cell Carcinomas
E. Nagamine, K. Hirayama, K. Matsuda, M. Okamoto, T. Ohmachi, K. Uchida, T. Kadosawa, H. Taniyama
Veterinary Pathology.2017; 54(5): 783. CrossRef - Protein kinase B
Bhumika Wadhwa, Ubaid Makhdoomi, Ram Vishwakarma, Fayaz Malik
Anti-Cancer Drugs.2017; 28(6): 569. CrossRef - Correlation analysis between the parameters of contrast-enhanced ultrasonography in evaluating cervical cancer metastasis and expression of E-cadherin
Xiaolan Lv, Min Hou, Xiaojing Duan
Oncology Letters.2017; 14(4): 4641. CrossRef - Distribution of telocytes in the corpus and cervix of human uterus: an immunohistochemical study
Martin Klein, Ladislav Urban, Ivan Deckov, Lubos Danisovic, Stefan Polak, Ludovit Danihel, Ivan Varga
Biologia.2017; 72(10): 1217. CrossRef - Epithelial–mesenchymal transition, proliferation, and angiogenesis in locally advanced cervical cancer treated with chemoradiotherapy
Leonardo Rojas‐Puentes, Andrés F. Cardona, Hernán Carranza, Carlos Vargas, Luis F. Jaramillo, Delma Zea, Lucely Cetina, Beatriz Wills, Erika Ruiz‐Garcia, Oscar Arrieta
Cancer Medicine.2016; 5(8): 1989. CrossRef - Tumor Necrosis Factor-Like Weak Inducer of Apoptosis Accelerates the Progression of Renal Fibrosis in Lupus Nephritis by Activating SMAD and p38 MAPK in TGF-β1 Signaling Pathway
Zhiqin Liu, Leixi Xue, Zhichun Liu, Jun Huang, Jian Wen, Ji Hu, Lin Bo, Ru Yang
Mediators of Inflammation.2016; 2016: 1. CrossRef - Identification of SEC62 as a potential marker for 3q amplification and cellular migration in dysplastic cervical lesions
Maximilian Linxweiler, Florian Bochen, Bernhard Schick, Silke Wemmert, Basel Al Kadah, Markus Greiner, Andrea Hasenfus, Rainer-Maria Bohle, Ingolf Juhasz-Böss, Erich-Franz Solomayer, Zoltan Ferenc Takacs
BMC Cancer.2016;[Epub] CrossRef - The indicative function of Twist2 and E-cadherin in HPV oncogene-induced epithelial-mesenchymal transition of cervical cancer cells
YUAN LIU, WENYAN QIAN, JIAWEN ZHANG, YU DONG, CAN SHI, ZHIQIANG LIU, SUFANG WU
Oncology Reports.2015; 33(2): 639. CrossRef - Prognostic significance of YY1 protein expression and mRNA levels by bioinformatics analysis in human cancers: A therapeutic target
Benjamin Bonavida, Samantha Kaufhold
Pharmacology & Therapeutics.2015; 150: 149. CrossRef - Expression of podoplanin and vimentin is correlated with prognosis in esophageal squamous cell carcinoma
MAKIKO TANAKA, HIROSHI KIJIMA, HIDEO SHIMADA, HIROYASU MAKUUCHI, SOJI OZAWA, SADAKI INOKUCHI
Molecular Medicine Reports.2015; 12(3): 4029. CrossRef - Podoplanin expression is correlated with the prognosis of lung squamous cell carcinoma
Yoichiro IKOMA, Hiroshi KIJIMA, Ryota MASUDA, Makiko TANAKA, Sadaki INOKUCHI, Masayuki IWAZAKI
Biomedical Research.2015; 36(6): 393. CrossRef - Abnormal expression of EMT-related proteins, S100A4, vimentin and E-cadherin, is correlated with clinicopathological features and prognosis in HCC
Xiaolu Zhai, Huijun Zhu, Wei Wang, Shu Zhang, Yixin Zhang, Guoxin Mao
Medical Oncology.2014;[Epub] CrossRef - The Clinicopathological Significance of Epithelial Mesenchymal Transition Associated Protein Expression in Head and Neck Squamous Cell Carcinoma
Kyu Ho Kim, Lucia Kim, Suk Jin Choi, Jee Young Han, Joon Mee Kim, Young Chae Chu, Young-Mo Kim, In Suh Park, Joo Han Lim
Korean Journal of Pathology.2014; 48(4): 263. CrossRef - Thrombomodulin mediates the migration of cervical cancer cells through the regulation of epithelial–mesenchymal transition biomarkers
Cheng-Jeng Tai, Chao-Wen Cheng, Hou-Yu Su, Wei-Yu Chen, Chun-Te Wu, Feng-Yen Lin, Chien-Kai Wang, Chen-Jei Tai, Po-Li Wei
Tumor Biology.2014; 35(1): 47. CrossRef - Role of epithelial to mesenchymal transition proteins in gynecological cancers: pathological and therapeutic perspectives
Xiao-mei Zhou, Hai Zhang, Xia Han
Tumor Biology.2014; 35(10): 9523. CrossRef - Normal Fibroblasts Induce E-Cadherin Loss and Increase Lymph Node Metastasis in Gastric Cancer
Wen Xu, Xinlei Hu, Zhongting Chen, Xiaoping Zheng, Chenjing Zhang, Gang Wang, Yu Chen, Xinglu Zhou, Xiaoxiao Tang, Laisheng Luo, Xiang Xu, Wensheng Pan, Elad Katz
PLoS ONE.2014; 9(5): e97306. CrossRef - The Culture of Cancer Cell Lines as Tumorspheres Does Not Systematically Result in Cancer Stem Cell Enrichment
Christophe Y. Calvet, Franck M. André, Lluis M. Mir, Anita B. Hjelmeland
PLoS ONE.2014; 9(2): e89644. CrossRef - Role of YY1 in the pathogenesis of prostate cancer and correlation with bioinformatic data sets of gene expression
Vaishali Kashyap, Benjamin Bonavida
Genes & Cancer.2014; 5(3-4): 71. CrossRef - Expression and Significance of Twist and E-cadherin in Ovarian Cancer Tissues
Wen-Shuang Wang, Shou-Li Yu, Xing-Sheng Yang, Shu-De Chang, Jian-Qing Hou
Asian Pacific Journal of Cancer Prevention.2013; 14(2): 669. CrossRef - The glioma-associated oncogene homolog 1 promotes epithelial–mesenchymal transition in human esophageal squamous cell cancer by inhibiting E-cadherin via Snail
S Min, X Xiaoyan, P Fanghui, W Yamei, Y Xiaoli, W Feng
Cancer Gene Therapy.2013; 20(7): 379. CrossRef - TACC3 Is Essential for EGF-Mediated EMT in Cervical Cancer
Geun-Hyoung Ha, Jung-Lye Kim, Eun-Kyoung Yim Breuer, Antimo Migliaccio
PLoS ONE.2013; 8(8): e70353. CrossRef - Overexpression of E2F1 Promotes Tumor Malignancy And Correlates with TNM Stages in Clear Cell Renal Cell Carcinoma
Xin Ma, Yu Gao, Yang Fan, Dong Ni, Yu Zhang, Weihao Chen, Peng Zhang, Erlin Song, Qingbo Huang, Qing Ai, Hongzhao Li, Baojun Wang, Tao Zheng, Taoping Shi, Xu Zhang, Hiromu Suzuki
PLoS ONE.2013; 8(9): e73436. CrossRef - Transforming growth factor-β1 induces bronchial epithelial cells to mesenchymal transition by activating the Snail pathway and promotes airway remodeling in asthma
ZHAO-CHUAN YANG, MING-JI YI, NI RAN, CHONG WANG, PENG FU, XUE-YING FENG, LEI XU, ZHENG-HAI QU
Molecular Medicine Reports.2013; 8(6): 1663. CrossRef








Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
Fig. 8
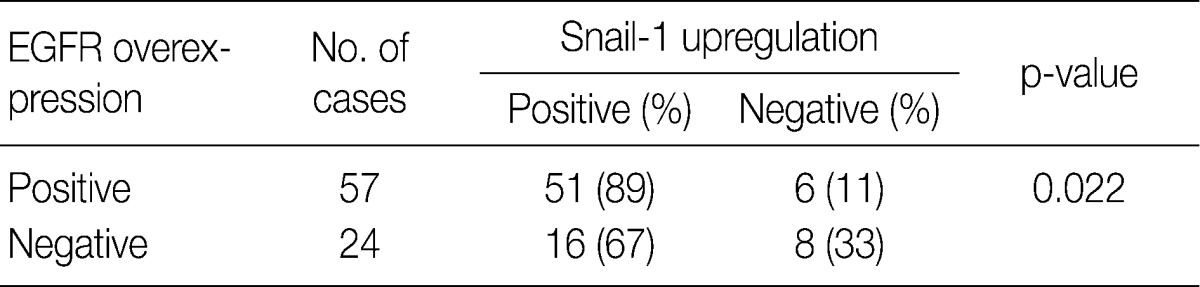
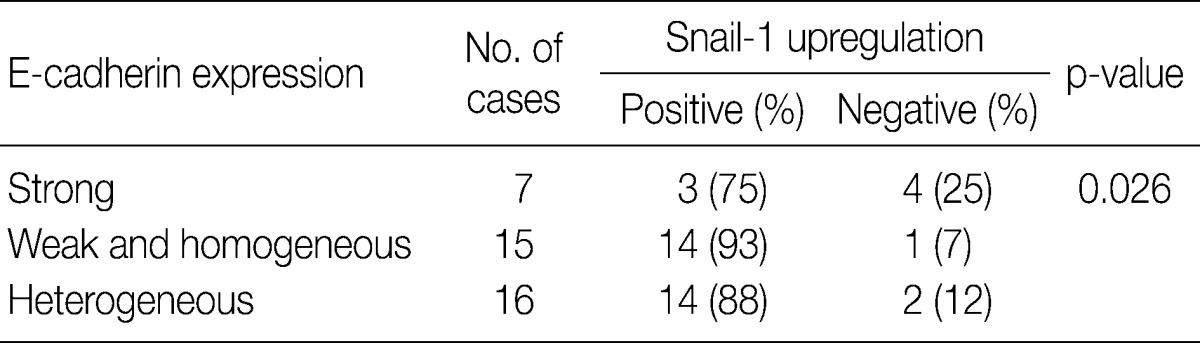
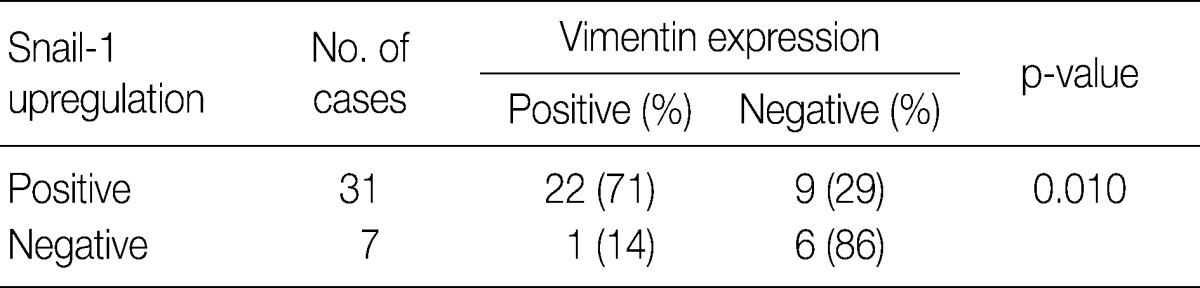

EGFR, epidermal growth factor receptor.
aOne CIS case is excluded in the analysis because of no immunostaining results for E-cadherin and vimentin.

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article