Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(1); 2014 > Article
-
Original Article
Effects of Fixation and Storage of Human Tissue Samples on Nucleic Acid Preservation - Soo Kyung Nam, Joon Im, Yoonjin Kwak1, Nayoung Han1, Kyung Han Nam, An Na Seo, Hye Seung Lee
-
Korean Journal of Pathology 2014;48(1):36-42.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.1.36
Published online: February 25, 2014
Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- Corresponding Author: Hye Seung Lee, M.D. Department of Pathology, Seoul National University Bundang Hospital, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 463-707, Korea. Tel: +82-31-787-7714, Fax: +82-31-787-4012, hye2@snu.ac.kr
• Received: November 4, 2013 • Revised: January 22, 2014 • Accepted: January 27, 2014
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Because of recent advances in the molecular diagnosis of cancer patients, tissue quality has become more important in daily practice.
-
Methods
- To evaluate the effects of fixative, duration of fixation, decalcification, and storage periods on nucleic acid integrity, DNA and RNA were extracted from gastrointestinal cancer tissue. The yield and purity were analyzed, and polymerase chain reaction (PCR) for glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 60 bp), β-actin (148 bp), and human growth hormone (hGH; 434 bp) and real-time reverse transcription-PCR for β-actin (97 bp) were performed.
-
Results
- All formalin-fixed paraffin-embedded (FFPE) and methacarn-fixed paraffin-embedded (MFPE) samples tested positive for GAPDH and β-actin by PCR. hGH was successfully detected in all MFPE samples, but in only 46.7% of the FFPE samples. Prolonged formalin fixation resulted in fewer GAPDH and β-actin PCR products, and amplification of hGH was not successful. The PCR and reverse transcription-PCR results were significantly affected by the duration of decalcification. The yield, purity, and integrity of mRNA progressively decreased with increased storage periods of paraffin blocks.
-
Conclusions
- Fixation and storage should therefore be standardized in order to improve the quality of molecular pathologic diagnosis.
- Samples and preparations
- We selected 30 FFPE-fixed tissue samples from 2003 to 2011 and 15 methacarn-fixed paraffin-embedded (MFPE) tissue samples from 2009 to 2011 from the Department of Pathology at Seoul National University Bundang Hospital. All samples were from surgical resections of gastric or colorectal cancer. We used 10% neutral buffered formaldehyde (formalin; Samchun Chemicals, Pyeongtaek, Korea). The methacarn solution consisted of 60% methanol (Duksan Pure Chemicals, Ansan, Korea), 30% chloroform (Duksan Pure Chemicals), and 10% acetic acid (Duksan Pure Chemicals).
- To investigate the effects of decalcification on the quantity and quality of nucleic acids, five samples of gastrointestinal cancer tissue were treated with decalcification solution for 0, 10, 30, 60, 120, and 180 minutes. To evaluate the effects of duration of fixation, we fixed the tissue in formalin for 3, 7, 30, 90, and 180 days at room temperature. In order to compare the influence of formalin, we fixed the tissue in formalin for one day and then stored them in 70% ethanol at 4℃ for 2, 6, 29, 89, and 179 days.
- DNA and RNA extraction
- Six 8-µm-thick FFPE or MFPE tissue sections were used for DNA extraction, and 11 FFPE or MFPE tissue sections were used for RNA. We microscopically dissected a 1×1 cm area, which consisted of more than 60% tumor cells. Tissue sections were deparaffinized by the boiling method14 with incubation at 70℃ for 10 minutes and centrifugation for 10 minutes at maximum speed. DNA was extracted using a chelating ion-exchange resin (InstaGene Matrix, Bio-Rad, Hercules, CA, USA), and RNA was extracted using the High Pure RNA Paraffin Kit (Roche, Penzberg, Germany). After nucleic acid extraction, the purity and quantity of the nucleic acids were measured using a NanoDrop UV spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA).
- Polymerase chain reaction
- To investigate DNA integrity, PCR was performed on glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 60 bp), β-actin (148 bp), and human growth hormone (hGH; 434 bp). The primer sequences were as follows: GAPDH forward, 5'-ACACCCACTCCTCCACCTTT-3'; GAPDH reverse, 3'-TGACAAAGTGGTCGTTGAGG-3'; ACTB forward, 5'-CCGCCAGCTCACCATGGAT-3'; ACTB reverse, 5'-CACCATCACGCCCTGGTGC-3'; hGH forward, 5'-TGCCTTCCCAACCATTCCCTTA-3'; and hGH reverse, 5'-CCACTCACGGATTTCTGTTGTGTTTC-3'. All reactions were performed using the same cycling conditions: 95℃ for 30 seconds, 60℃ for 30 seconds, and 72℃ for 1 minute, for 35 amplification cycles. The PCR products were analyzed by electrophoresis on a 2% agarose gel.
- Real-time reverse transcription-PCR
- Real-time reverse transcription (RT)-PCR was performed to assess the integrity of the RNA. First, cDNA was synthesized using 1 µg total RNA, the Transcriptor First Strand cDNA Synthesis Kit, and oligo(dT) primer (Roche). Real-time TaqMan PCR for β-actin (97 bp) was then performed on each sample using the Universal ProbeLibrary (UPL) probe (Roche) and the Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The UPL probe sequences were as follows: left, CCAACCGCGAGAAGATGA; right, CCAGAGGCGTACAGGGATAG. Cycle parameters were 95℃ for 10 minutes and 50 cycles of 10 seconds at 95℃ and 60 seconds at 58℃.
- Statistical analyses
- The Mann-Whitney test or Kruskal-Wallis test was used to compare non-parametric continuous variables. Either the χ2 test or Fisher's exact test (two-sided) was performed to compare categorical variables. The trends of DNA PCR results according to the duration of decalcification were tested by linear-by-linear association. Results were considered significant when p-values were less than .05. All statistical analyses were conducted using the PASW ver. 19.0 (IBM Co., Armonk, NY, USA).
MATERIALS AND METHODS
- Comparison between formalin and methacarn
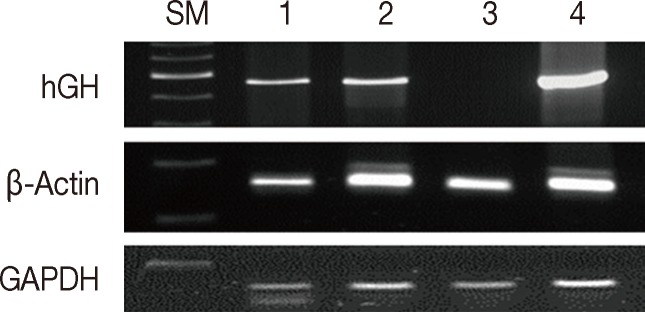
- We compared the effects of formalin and methacarn fixation on DNA preservation by investigating 15 FFPE human cancer samples and 15 corresponding MFPE samples. For FFPE samples, surgical specimens were fixed with formalin for 24 to 48 hours. When GAPDH (60 bp), β-actin (148 bp), and hGH (434 bp) DNA fragments were amplified via PCR, all FFPE and MFPE DNA samples were positive for GAPDH and β-actin. Amplification of hGH was successful in all 15 MFPE samples but only in seven FFPE samples (p=.002). In most cases, a greater amount of PCR products was obtained from MFPE DNA than from FFPE DNA (Fig. 1).
- Both RNA extraction and real-time RT-PCR of β-actin were performed using 12 FFPE and matched 12 MFPE human cancer samples, which were stored for less than 1 year. The yield was greater for FFPE samples than MFPE samples (637.87±312.17 ng/µL vs 149.43±84.75 ng/µL, p<.001). The purity, as measured by 260/280-nm ratios, was also higher for the FFPE samples than for the MFPE samples (1.97±0.06 ng/µL vs 1.85±0.18 ng/µL, p<.001). However, real-time RT-PCR was possible in all 12 MFPE samples but in nine of the 12 FFPE samples, which was statistically insignificant (p=.217). The Ct values were not significantly different between the FFPE and MFPE samples (p=.129, data not shown).
- Effects of the duration of formalin fixation
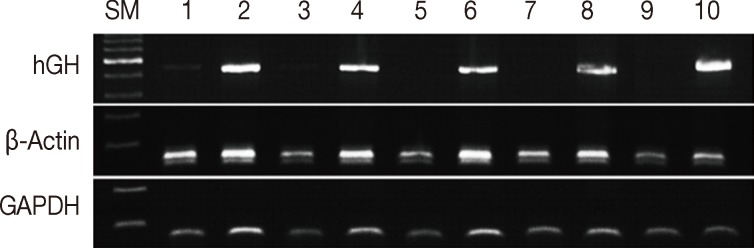
- Formalin fixation is the standard method used for routine tissue preservation. However, a standard duration of fixation has not been established, and duration of fixation may be prolonged to more than 48 hours because of holidays, more gross examination, or other circumstances. In order to evaluate the effects of the duration of formalin fixation, three human cancer samples were fixed in formalin for 3, 7, 30, 90, and 180 days at room temperature. As a control, tissue from the same samples was fixed in formalin for one day and then stored in 70% ethanol at 4℃ for 2, 6, 29, 89, and 179 days. The DNA integrity in the time-course treatment was assessed (Fig. 2), and there were considerable differences in the PCR results. Except for a weak band in the 3-day sample, amplification of hGH was unsuccessful in the samples with prolonged exposure to formalin. However, all samples kept in ethanol after 1-day formalin fixation had amplified hGH bands. Although the differences were not significant, the amount of GAPDH and β-actin PCR products decreased with increasing exposure to formalin. The DNA integrity was well preserved in the tissues kept in ethanol after 1-day formalin fixation.
- The yield of RNA was greater for the samples fixed in formalin for 90 and 180 days than for the other samples (540.03±232.90 ng/µL vs 286.39±78.69 ng/µL, p=.026). However, prolonged exposure to formalin had no significant effect on the purity of RNA and the real-time RT-PCR products of β-actin (data not shown).
- Effects of decalcification
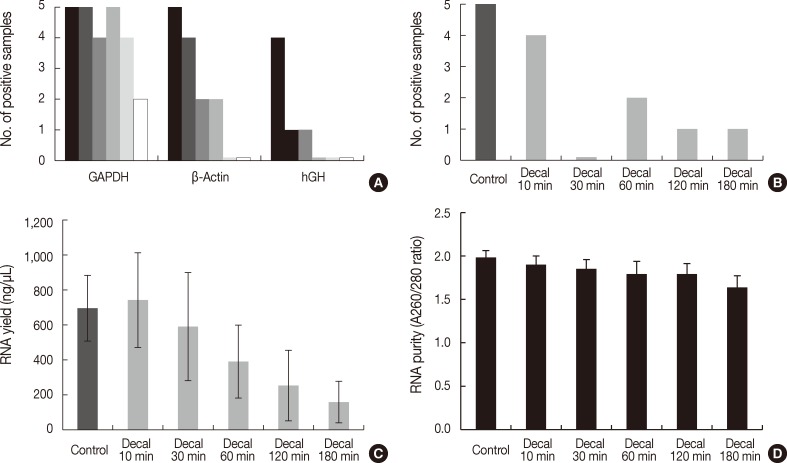
- To investigate the effects of decalcification, we decalcified five human cancer tissue samples for 0, 10, 30, 60, 120, and 180 minutes. The PCR results were significantly affected by the decalcification time. GAPDH was amplified in all five samples treated with decalcification solution for 0 minute, 10 minutes, and 60 minutes, but it was amplified in four samples decalcified for 30 minutes and 120 minutes and two samples for 180 minutes (p=.017) (Fig. 3A). β-Actin was amplified in all five control samples, four samples decalcified for 10 minutes, two samples for 30 minutes and 60 minutes, and none for 120 and 180 minutes (p<.001) (Fig. 3A). In contrast, hGH was amplified in four control samples, one sample for 10 minutes and 30 minutes, and none for 60, 120, and 180 minutes (p<.001) (Fig. 3A)
- Real-time RT-PCR of β-Actin was successful in four of five samples decalcified for 10 minutes, none for 30 minutes, two for 60 minutes, and one for 120 minutes and 180 minutes (p=.004) (Fig. 3B). The yield of RNA from decalcified tissues decreased gradually with increasing decalcification time (p=.004) (Fig. 3C). In addition, the purity (260/280 nm ratios) also decreased with increasing decalcification time (p=.009) (Fig. 3D).
- Effects of the storage period
- FFPE tissue stored at room temperature for 3 to 10 years has been used as an archival resource in many molecular studies. Thirty samples of human cancer tissue were randomly selected to investigate the effects of storage time on DNA preservation. All selected samples were from cases with enough cancer tissue for further experiments. These consisted of five samples stored for less than one month, five stored for six months, five stored for one year, five stored for three years, five stored for five years, and five stored for eight years. The PCR results were as follows; 13 out of 15 samples stored for 3 to 8 years were positive for GAPDH, 14 were positive for β-actin, and four were positive for hGH. Out of the 15 samples stored for ≤1 year, all 15 samples were positive for GAPDH and β-actin, and seven were positive for hGH. The PCR results were not significantly different between 3-8 year and ≤1 year storage groups (p>.05). The quantity and quality of the DNA did not significantly differ with the duration of storage (p>.05, data not shown).
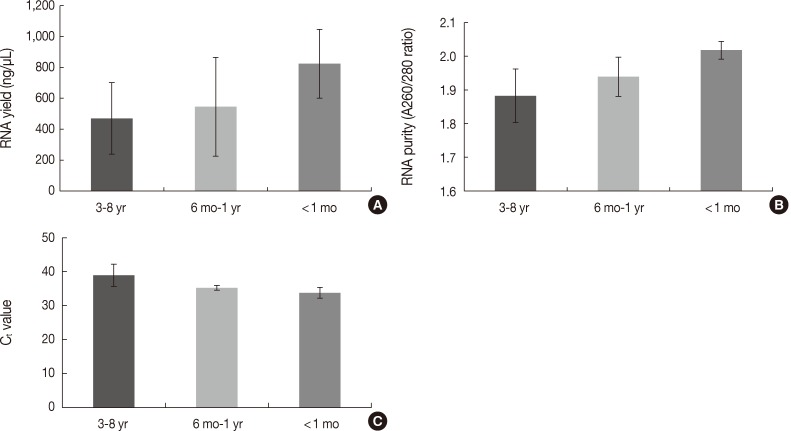
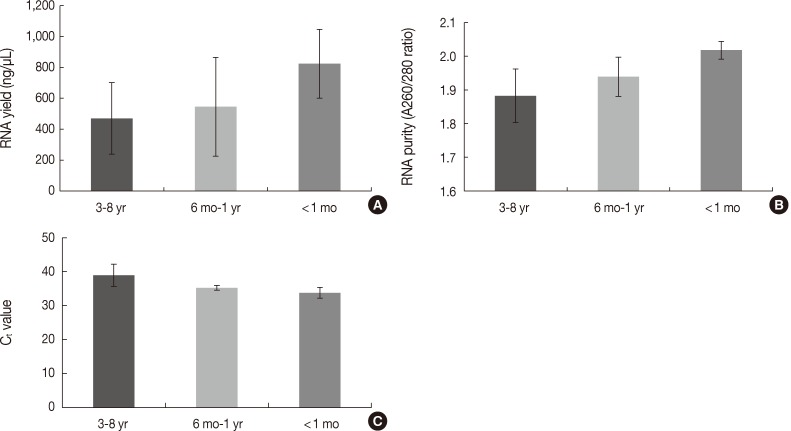
- The quantity and quality of the RNA differed with the duration of storage. Tissues stored for longer periods had a smaller amount of RNA detected using the NanoDrop, although the difference was not statistically significant between 3-8 years and ≤1 year (469.86±231.87 ng/µL vs 637.87±312.17 ng/µL, p=.101) (Fig. 4A). Furthermore, there was a significant difference in RNA yield between >1 month and <1 month (499.90±264.97 ng/µL vs 823.66±223.06 ng/µL, p=.037). The purity also decreased with increasing storage periods (1.88±0.08 ng/µL for 3-8 years vs 1.97±0.06 ng/µL for ≤1 year, p=.008) (Fig. 4B). According to the real-time RT-PCR results, β-actin was amplified in seven of 12 samples (58.3%) with a 3-8 year storage time, five of eight samples (62.5%) with a one month to one year storage time, and all four samples (100%) with less than a one month storage time. Increasing the storage period increased the Ct value, which indicates fewer initial templates (37.83±1.18 for 3-8 years, 35.19±0.70 for one month to one year vs 33.73±1.60 for <1 month, p=.003) (Fig. 4C).
RESULTS
- High-quality nucleic acid is important in molecular pathologic diagnosis, especially for cancer specimens. Accurate prediction of the prognosis and therapeutic response of cancer patients mainly depends on the sensitivity and specificity of the molecular diagnosis. However, nucleic acids from fixed tissues are reported to be easily damaged during pretreatment, which may lead to test failure or considerably different diagnostic results. Despite the previous studies showing nucleic acid damage during fixation and storage, the exact quality of extracted nucleic acids is still uncertain in daily practice. The fixation method and tissue storage should therefore be standardized to improve the quality of molecular pathologic diagnosis. In order to investigate the effects of fixatives, fixation method, and storage period, we tested the yield, purity, and integrity of nucleic acids from samples of gastrointestinal cancer tissue.
- The fixatives affected the quality of extracted nucleic acids from human tissue samples. The formalin fixation method is currently the most common. However, the toxicity of formalin causes many problems.7 We compared formalin to methacarn with respect to nucleic acid integrity. The yield and purity of DNA from the FFPE samples did not differ from those of the MFPE samples (data not shown), and the yield and purity of RNA from the FFPE samples were higher than from the MFPE samples. hGH PCR was possible in all MFPE samples, but it was possible in only seven of the 15 corresponding FFPE samples. Furthermore, real-time RT-PCR for β-actin (97 bp) was possible in all MFPE samples but only in nine of the 12 FFPE samples. Therefore, the integrity of nucleic acids is likely to be greater in the MFPE samples than in the FFPE samples. Many pathologic laboratories employ a formalin-based fixation method. Formalin is a relatively good fixative for molecular diagnosis and FFPE samples are sufficiently high quality for PCR or RT-PCR of small-sized nucleic acids. However, considering the higher integrity of nucleic acids in the MFPE samples than in the FFPE samples, methacarn-based fixation may therefore be recommended to improve the integrity of nucleic acids.
- Prolonged formalin fixation adversely affects the quality of tissue DNA, although it has only a minor effect on histopathology.11 The average size of DNA extracted from tissues fixed in buffered formalin decreases with increasing duration of fixation. Tissues fixed in buffered formalin for 3 to 6 hours yield greater amounts of high molecular-weight DNA.15 Srinivasan et al.7 recommended fixing tissue with buffered formalin for 3 to 6 hours to preserve nucleic acids. However, in practice tissues are routinely fixed for 24 to 48 hours, and sometimes the duration of fixation may be longer than 48 hours because of holidays or more sectioning. In this study, PCR of β-actin and GAPDH was successful in samples subjected to prolonged formalin fixation. However, PCR for larger DNA fragments (i.e., hGH, 434 bp) was not possible following prolonged formalin fixation. In addition, there were more β-actin and GAPDH PCR products in the samples subjected to 1-day formalin fixation that were stored in ethanol at 4℃ than in the samples subjected to prolonged formalin fixation. While PCR of small-sized DNA fragments is possible following prolonged formalin fixation, formalin fixation for one day and storage in ethanol is recommended to improve the quality of molecular diagnosis.
- In various cancer specimens including papillary thyroid carcinoma, mucinous adenocarcinoma, as well as some bone and soft tissue tumor dystrophic calcification, is observed. Bone is also a common site of metastasis in various cancers (e.g., breast, prostate, thyroid, lung, and kidneys).16 The calcified tissue or bone specimens should be decalcified in order to obtain 4 to 8 µm thick FFPE tissue sections. Molecular pathology is also necessary in calcified tumor tissues for diagnosis and for determining a prognosis or predicting therapeutic responses. However, the degree of nucleic acid damage caused by decalcification is considered to be significant.12,17 To demonstrate the effects of a decalcification solution on tissue nucleic acids, we compared the yield, purity, and integrity of nucleic acids following various decalcification times. With a prolonged duration of decalcification, even small-sized DNA such as β-actin could not be amplified. Molecular diagnosis of calcified tissue should therefore be performed with caution, and nondecalcified paraffin blocks are recommended for accurate molecular diagnosis.
- Most paraffin blocks are stored at room temperature. The rate of late recurrence in cancer patients has increased, and paraffin blocks that have been stored for more than five years are being used for molecular diagnosis.18 These archival paraffin blocks have also been a major source of many retrospective molecular studies. Archival specimens have shown great potential for use in PCR techniques.13,19 The present study demonstrated that small-sized DNA or RNA fragments were successfully amplified in most FFPE samples even if they were kept for several years at room temperature. However, recent specimens gave better PCR results than specimens that had been stored for several years. In particular, the yield, purity, and integrity of RNA by RT-PCR were better in the recent specimens. Real-time RT-PCR showed that β-actin was amplified in all samples (100%) with less than one month of storage time, but in 62.5% of samples stored for one month to one year, and 58.3% of samples stored for 3 to 8 years. Increasing the storage period also increased the Ct value, which was 37.83±1.18 for samples stored for 3 to 8 years, 35.19±0.70 for one month to one year, and 33.73±1.60 for <1 month. Therefore, the integrity of RNA is more affected by storage within the first year than for periods longer than one year. Some older samples could be used for real-time RT-PCR with optimal PCR product size, but the results of quantitative RT-PCR should be cautiously interpreted in the context of longer durations of storage. Although these results demonstrated a significant difference in the yield, purity, and integrity of RNA according to the storage period, this study is limited by a small sample size, and further large scale studies are needed.
- In summary, we investigated the effects of fixatives, duration of formalin fixation, decalcification, and storage periods of paraffin blocks on the yield, purity, and integrity of nucleic acids from human cancer samples. Methacarn was better than formalin for nucleic acid preservation, especially high molecular-weight DNA. Prolonged formalin fixation negatively affected the integrity of DNA, but DNA quality could be preserved by storing the samples in ethanol at 4℃ following1-day formalin fixation. With decalcification, most nucleic acids were degraded, and PCR was unsuccessful. The yield, purity, and integrity of mRNA progressively decreased with prolonged storage of the paraffin blocks. Therefore, optimal fixatives and standardized procedures are essential for improving the quality of molecular pathologic diagnosis.
DISCUSSION
- 1. Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359: 1757-1765. ArticlePubMed
- 2. Marisa L, de Reyniès A, Duval A, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med 2013; 10: e1001453.ArticlePubMedPMC
- 3. Oh JR, Kim DW, Lee HS, et al. Microsatellite instability testing in Korean patients with colorectal cancer. Fam Cancer 2012; 11: 459-466. ArticlePubMed
- 4. Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010; 138: 2073-2087.e3. ArticlePubMedPMC
- 5. Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol 2010; 28: 1254-1261. ArticlePubMed
- 6. Botti G, Franco R, Carbone A. Sample conservation: freezing, fixation and quality control. Pathologica 2008; 100: 76-85. ArticlePubMed
- 7. Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol 2002; 161: 1961-1971. ArticlePubMedPMC
- 8. Delfour C, Roger P, Bret C, et al. RCL2, a new fixative, preserves morphology and nucleic acid integrity in paraffin-embedded breast carcinoma and microdissected breast tumor cells. J Mol Diagn 2006; 8: 157-169. ArticlePubMedPMC
- 9. Shibutani M, Uneyama C, Miyazaki K, Toyoda K, Hirose M. Methacarn fixation: a novel tool for analysis of gene expressions in paraffin-embedded tissue specimens. Lab Invest 2000; 80: 199-208. ArticlePubMed
- 10. Dotti I, Bonin S, Basili G, et al. Effects of formalin, methacarn, and fineFIX fixatives on RNA preservation. Diagn Mol Pathol 2010; 19: 112-122. ArticlePubMed
- 11. Foss RD, Guha-Thakurta N, Conran RM, Gutman P. Effects of fixative and fixation time on the extraction and polymerase chain reaction amplification of RNA from paraffin-embedded tissue. Comparison of two housekeeping gene mRNA controls. Diagn Mol Pathol 1994; 3: 148-155. ArticlePubMed
- 12. Brown RS, Edwards J, Bartlett JW, Jones C, Dogan A. Routine acid decalcification of bone marrow samples can preserve DNA for FISH and CGH studies in metastatic prostate cancer. J Histochem Cytochem 2002; 50: 113-115. ArticlePubMed
- 13. Guerrero RB, Batts KP, Brandhagen DJ, Germer JJ, Perez RG, Persing DH. Effects of formalin fixation and prolonged block storage on detection of hepatitis C virus RNA in liver tissue. Diagn Mol Pathol 1997; 6: 277-281. ArticlePubMed
- 14. Lee HS, Park KU, Park JO, Chang HE, Song J, Choe G. Rapid, sensitive, and specific detection of Mycobacterium tuberculosis complex by real-time PCR on paraffin-embedded human tissues. J Mol Diagn 2011; 13: 390-394. ArticlePubMedPMC
- 15. Douglas MP, Rogers SO. DNA damage caused by common cytological fixatives. Mutat Res 1998; 401: 77-88. ArticlePubMed
- 16. Coleman RE. Skeletal complications of malignancy. Cancer 1997; 80(8 Suppl):1588-1594. ArticlePubMed
- 17. Shao YY, Wang L, Hicks DG, Ballock RT. Analysis of gene expression in mineralized skeletal tissues by laser capture microdissection and RT-PCR. Lab Invest 2006; 86: 1089-1095. ArticlePubMed
- 18. Jatoi I, Anderson WF, Jeong JH, Redmond CK. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol 2011; 29: 2301-2304. ArticlePubMedPMC
- 19. Talaulikar D, Shadbolt B, McNiven M, Dahlstrom JE. DNA amplification from formalin-fixed decalcified paraffin-embedded bone marrow trephine specimens: does the duration of storage matter? Pathology 2008; 40: 702-706. ArticlePubMed
REFERENCES
Fig. 1Comparison between formalin and methacarn fixatives by polymerase chain reaction of DNA of various sizes. Lanes 1 and 3, formalin fixation; lanes 2 and 4, methacarn fixation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 60 bp), β-actin (148 bp), and human growth hormone (hGH; 434 bp). SM, size marker.
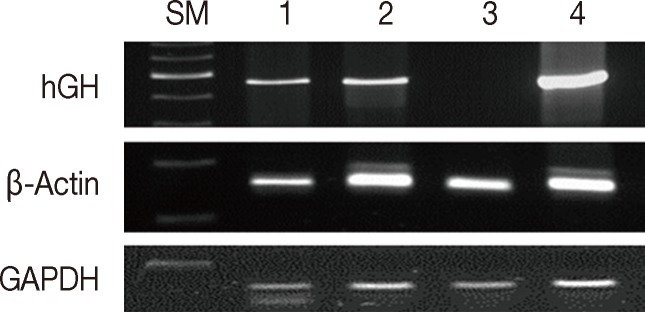

Fig. 2Assessment of DNA integrity according to the duration of formalin fixation. Lanes 1/3/5/7/9, fixed in formalin for 3/7/30/90/180 days at room temperature; lanes 2/4/6/8/10, fixed in formalin for one day and stored in ethanol at 4℃ for 2/6/29/79/179 days. SM, size marker; hGH, human growth hormone; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
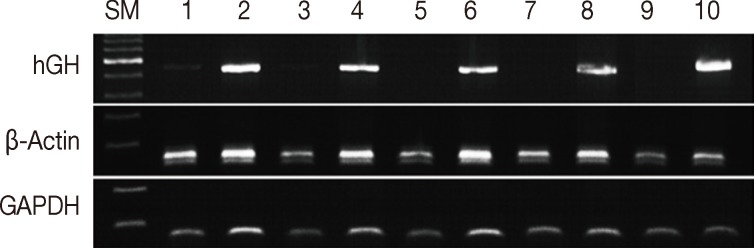

Fig. 3Bar graph representing polymerase chain reaction (PCR) results, reverse transcription (RT)-PCR results, the quantity of RNA, and the quality of RNA extracted from formalin-fixed paraffin-embedded tissues treated with decalcification solution. (A) PCR results of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), β-actin, and human growth hormone (hGH) according to duration of decalcification (0/10/30/60/120/180 minutes). (B) RT-PCR results of β-actin (97 bp). (C) RNA yield (ng/µL). (D) RNA purity (A260/280 ratios). Decal, decalcification.
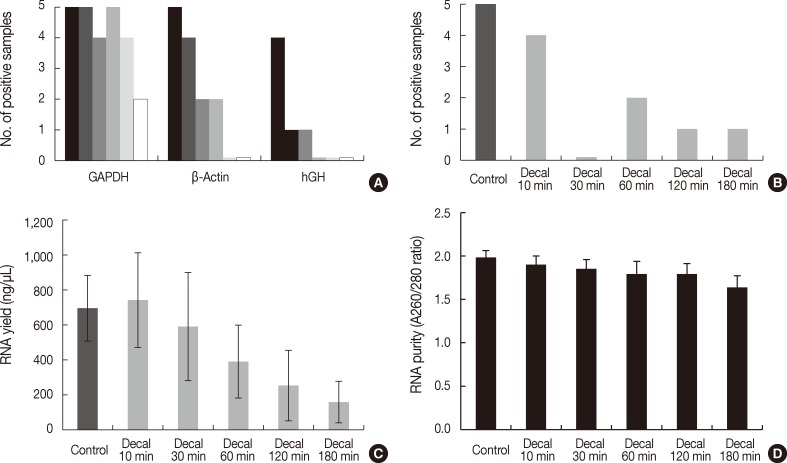

Figure & Data
References
Citations
Citations to this article as recorded by 

- InDEL instability in two different tumoral tissues and its forensic significance
İpek Gürel, Faruk Aşıcıoğlu, Gökhan Ersoy, Özlem Bülbül, Tülin Öztürk, Gönül Filoğlu
Forensic Science, Medicine and Pathology.2024;[Epub] CrossRef - Utility of bronchoscopically obtained frozen cytology pellets for next-generation sequencing
Chihiro Mimura, Rei Takamiya, Shodai Fujimoto, Takafumi Fukui, Atsuhiko Yatani, Jun Yamada, Mizuki Takayasu, Naoya Takata, Hiroki Sato, Kiyoko Fukuda, Koichi Furukawa, Daisuke Hazama, Naoko Katsurada, Masatsugu Yamamoto, Shingo Matsumoto, Koichi Goto, Mot
BMC Cancer.2024;[Epub] CrossRef - Predictive molecular pathology after prolonged fixation: A study on tissue from anatomical body donors
Anja Böckers, Leon Schurr, Michael Schön, Tatjana Scholl, Tobias M. Böckers, Konrad Steinestel, Annette Arndt
Experimental and Molecular Pathology.2024; 137: 104899. CrossRef - Tiempos y condiciones de almacenamiento de las muestras en anatomía patológica. Recomendaciones de la Sociedad Española de Anatomía Patológica parte 1: muestras destinadas al diagnóstico
Francesc Tresserra Casas, Esther Rosello Sastre, María Jesús Fernández Aceñero, Lara Zaragoza Macián, Javier Azúa Romeo, Clara Alfaro-Cervelló, Samuel Navarro Fos, Eugenia García Fernández, Jordi Temprana-Salvador, Mar Iglesias Coma, Francesc Olivares Veg
Revista Española de Patología.2024; 57(4): 235. CrossRef - RNA-Seq Analysis in Non-Small Cell Lung Cancer: What Is the Best Sample from Clinical Practice?
Lorenzo Nibid, Giovanna Sabarese, Luca Andreotti, Benedetta Canalis, Daniela Righi, Filippo Longo, Margherita Grazi, Pierfilippo Crucitti, Giuseppe Perrone
Journal of Personalized Medicine.2024; 14(8): 851. CrossRef - A Study on the Stainability and DNA Conservation of Tissue Slides according to Fixation Time and Temperature
Da-som JEONG
Korean Journal of Clinical Laboratory Science.2024; 56(3): 217. CrossRef - Selection and Evaluation of mRNA and miRNA Reference Genes for Expression Studies (qPCR) in Archived Formalin-Fixed and Paraffin-Embedded (FFPE) Colon Samples of DSS-Induced Colitis Mouse Model
Ana Unkovič, Emanuela Boštjančič, Aleš Belič, Martina Perše
Biology.2023; 12(2): 190. CrossRef - The quality of DNA isolated from autopsy formalin-fixed and formalin-fixed paraffin-embedded tissues: study of 1662 samples
Katarina Vitošević, Danijela Todorović, Živana Slović, Tatjana Varljen, Ivana Radaković, Dušan Radojević, Vanja Čanović, Miloš Todorović
Molecular Biology Reports.2023; 50(8): 6323. CrossRef - Morphometric and Molecular Analysis of Five-Spine Epidinium Morphotypes Taken from the Rumen of European Bison, Bison bonasus
Silvia Ivorová, Anna Kopčaková, Peter Pristaš, Svetlana Kišidayová
Life.2023; 13(12): 2350. CrossRef - Retrieving high-quality genomic DNA from formalin-fixed paraffin-embedded tissues for multiple molecular analyses
Ha Thi Nguyen, Vinay Bharadwaj Tatipamula, Duy Ngoc Do, Thien Chi Huynh, Mai Kim Dang
Preparative Biochemistry & Biotechnology.2022; 52(1): 48. CrossRef - Evaluation of DNA Isolation and Amplification from Various Organs Preserved through Frozen, Formalin-Fixed and Paraffin-Embedded Tissue Sample method
Mifta Rizqina Amalia, Anna Roosdiana, Yudit Oktanella, Andreas Bandang Hardian, Dini Agusti Paramanandi, Kharisma Kurnia Utami, Andi Tri Rakhmat Akbar, Made Venika Nareswari, Fajar Shodiq Permata
Journal of Experimental Biology and Agricultural Sciences.2022; 10(3): 643. CrossRef - DNA isolated from formalin-fixed paraffin-embedded healthy tissue after 30 years of storage can be used for forensic studies
Katarina Vitošević, Miloš Todorović, Živana Slović, Tatjana Varljen, Stevan Matić, Danijela Todorović
Forensic Science, Medicine and Pathology.2021; 17(1): 47. CrossRef - Extraction of DNA and RNA from Formalin-fixed Paraffin-embedded Tissue Specimens
NIdhi Shukla, Narmadhaa Siva, Madhu Sivakumar, Rabia Parveen, Ashwani Mishra, Avadh Shah, Krishna Medicherla, Prashanth Suravajhala
BIO-PROTOCOL.2021;[Epub] CrossRef - Cyclin dependent kinase inhibitor 2A and miR-671-5p expression profile in Iranian glioblastoma multiforme
Tayyebali Salmani, Sayyed Mohammad Hossein Ghaderian, Mohammadreza Hajiesmaeili, Omidvar Rezaei Mirghaed, Azadeh Rakhshan, Mohammad Javad Nasiri, Mahan Mohammadi
Gene Reports.2020; 19: 100620. CrossRef - Comparison between Fluorescence in-situ Hybridization (FISH), Reverse Transcriptase PCR (RT-PCR) and fragment analysis, for detection of t (X; 18) (p11; q11) translocation in synovial sarcomas
Omshree Shetty, Trupti Pai, Mamta Gurav, Bharat Rekhi
Indian Journal of Pathology and Microbiology.2020; 63(1): 64. CrossRef - Comparison of HPV detection rate in formalin‐fixed paraffin‐embedded tissues of head and neck carcinoma using two DNA extraction kits and three amplification methods
Ljiljana Božić, Tanja Jovanović, Aleksandra Šmitran, Marko Janković, Aleksandra Knežević
European Journal of Oral Sciences.2020; 128(6): 501. CrossRef - Formalin Fixation of Human Healthy Autopsied Tissues: The Influence of Type of Tissue, Temperature and Incubation Time on the Quality of Isolated DNA
Danijela Todorovic, Katarina Vitosevic, Milos Todorovic, Zivana Slovic
Serbian Journal of Experimental and Clinical Research .2020; 21(4): 307. CrossRef - Detection of Disease-specific Fusion Genes of Soft Tissue Tumors Using Formalin-fixed Paraffin-embedded Tissues; Its Diagnostic Usefulness and Factors Affecting the Detection Rates
Takahiro Matsushige, Satoshi Kuwamoto, Michiko Matsushita, Lusi Oka Wardhani, Yasushi Horie, Kazuhiko Hayashi, Yukisato Kitamura
Yonago Acta Medica.2019; 62(1): 115. CrossRef - The Biospecimen Preanalytical Variables Program: A Multiassay Comparison of Effects of Delay to Fixation and Fixation Duration on Nucleic Acid Quality
Latarsha J. Carithers, Rachana Agarwal, Ping Guan, Hana Odeh, Michael C. Sachs, Kelly B. Engel, Sarah R. Greytak, Mary Barcus, Conrado Soria, Chih-Jian (Jason) Lih, P. Mickey Williams, Philip A. Branton, Leslie Sobin, Benjamin Fombonne, Therese Bocklage,
Archives of Pathology & Laboratory Medicine.2019; 143(9): 1106. CrossRef - Pathobiology and innate immune responses of gallinaceous poultry to clade 2.3.4.4A H5Nx highly pathogenic avian influenza virus infection
Kateri Bertran, Mary J. Pantin-Jackwood, Miria F. Criado, Dong-Hun Lee, Charles L. Balzli, Erica Spackman, David L. Suarez, David E. Swayne
Veterinary Research.2019;[Epub] CrossRef - Optimized Storage Methods of RNA Extraction from Formalin Fixed Paraffin Embedded Tissue
Mehdi Barati, Mahdieh Shokrollahi Barough, Fatemeh Pak, Vahid Semnani, Mehrnoosh Pashaei, Parviz Kokhaei
Middle East Journal of Rehabilitation and Health.2018;[Epub] CrossRef - Effect of formalin fixation on pcr amplification of DNA isolated from healthy autopsy tissues
Katarina Vitošević, Miloš Todorović, Tatjana Varljen, Živana Slović, Stevan Matić, Danijela Todorović
Acta Histochemica.2018; 120(8): 780. CrossRef - Evaluation of the optimal provision of formalin-fixed, paraffin-embedded material for reverse transcription-PCR in soft-tissue tumour diagnosis
Khin Thway, Dorte Wren, Jasmin Lee, Lisa Thompson, Cyril Fisher, David Gonzalez
Journal of Clinical Pathology.2017; 70(1): 20. CrossRef - Molecular Testing for Gastrointestinal Cancer
Hye Seung Lee, Woo Ho Kim, Yoonjin Kwak, Jiwon Koh, Jeong Mo Bae, Kyoung-Mee Kim, Mee Soo Chang, Hye Seung Han, Joon Mee Kim, Hwal Woong Kim, Hee Kyung Chang, Young Hee Choi, Ji Y. Park, Mi Jin Gu, Min Jin Lhee, Jung Yeon Kim, Hee Sung Kim, Mee-Yon Cho
Journal of Pathology and Translational Medicine.2017; 51(2): 103. CrossRef - Reference genes for studies in infectious parasitic diseases in five types of human tissues
Cristina Silva Meira-Strejevitch, Vera Lucia Pereira-Chioccola, Marta Marques Maia, Daise Damaris Carnietto de Hippolito, Hui-Tzu Lin Wang, Gabriela Motoie, Aparecida Helena de Souza Gomes, Cristina Takami Kanamura, Roosecelis Brasil Martines, Cinara Cáss
Gene Reports.2017; 7: 98. CrossRef - Establishment of a primary culture of polymorphous low grade adenocarcinoma cells
Lucas Novaes Teixeira, Victor Angelo Martins Montalli, Silvia Borges Pimentel de Oliveira, Thais Fernanda Santos Toledo, Elizabeth Ferreira Martinez, Vera Cavalcanti de Araújo
Archives of Oral Biology.2017; 82: 188. CrossRef - DNA degrades during storage in formalin-fixed and paraffin-embedded tissue blocks
Alice Guyard, Alice Boyez, Anaïs Pujals, Cyrielle Robe, Jeanne Tran Van Nhieu, Yves Allory, Julien Moroch, Odette Georges, Jean-Christophe Fournet, Elie-Serge Zafrani, Karen Leroy
Virchows Archiv.2017; 471(4): 491. CrossRef - Prevalence of exon 11 internal tandem duplications in the C‐KIT proto‐oncogene in Australian canine mast cell tumours
VS Tamlin, AE Kessell, RJ Mccoy, EC Dobson, TS Smith, M Hebart, L Brown, D Mitrovic, AE Peaston
Australian Veterinary Journal.2017; 95(10): 386. CrossRef - Robust transcriptional tumor signatures applicable to both formalin-fixed paraffin-embedded and fresh-frozen samples
Rou Chen, Qingzhou Guan, Jun Cheng, Jun He, Huaping Liu, Hao Cai, Guini Hong, Jiahui Zhang, Na Li, Lu Ao, Zheng Guo
Oncotarget.2017; 8(4): 6652. CrossRef - Clinical impact of targeted amplicon sequencing for meningioma as a practical clinical-sequencing system
Sayaka Yuzawa, Hiroshi Nishihara, Shigeru Yamaguchi, Hiromi Mohri, Lei Wang, Taichi Kimura, Masumi Tsuda, Mishie Tanino, Hiroyuki Kobayashi, Shunsuke Terasaka, Kiyohiro Houkin, Norihiro Sato, Shinya Tanaka
Modern Pathology.2016; 29(7): 708. CrossRef - Comparison of the Diagnostic Value Between Real-Time Reverse Transcription-Polymerase Chain Reaction Assay and Histopathologic Examination in Sentinel Lymph Nodes for Patients With Gastric Carcinoma
Yoonjin Kwak, Soo Kyung Nam, Eun Shin, Sang-Hoon Ahn, Hee Eun Lee, Do Joong Park, Woo Ho Kim, Hyung-Ho Kim, Hye Seung Lee
American Journal of Clinical Pathology.2016; 145(5): 651. CrossRef - WITHDRAWN: Selection of reference genes in five types of human tissues for normalization of gene expression studies in infectious diseases
Cristina Silva Meira-Strejevitch, Vera Lucia Pereira-Chioccola, Marta Marques Maia, Daise Damaris Carnietto de Hipólito, Hui-Tzu Lin Wang, Gabriela Motoie, Aparecida Helena de Souza Gomes, Cristina Takami Kanamura, Roosecelis Brasil Martines, Cinara Cássi
Gene.2016;[Epub] CrossRef - A Comparison of Fresh Frozen vs. Formalin-Fixed, Paraffin-Embedded Specimens of Canine Mammary Tumors via Branched-DNA Assay
Florenza Lüder Ripoli, Annika Mohr, Susanne Conradine Hammer, Saskia Willenbrock, Marion Hewicker-Trautwein, Silvia Hennecke, Hugo Murua Escobar, Ingo Nolte
International Journal of Molecular Sciences.2016; 17(5): 724. CrossRef - Expression of caspase-3 predicts prognosis in advanced noncardia gastric cancer
Sousana Amptoulach, Andreas C. Lazaris, Ioanna Giannopoulou, Nikolaos Kavantzas, Efstratios Patsouris, Nikolaos Tsavaris
Medical Oncology.2015;[Epub] CrossRef - The Importance of Reference Gene Analysis of Formalin-Fixed, Paraffin-Embedded Samples from Sarcoma Patients — An Often Underestimated Problem
Ninna Aggerholm-Pedersen, Akmal Safwat, Steen Bærentzen, Marianne Nordsmark, Ole Steen Nielsen, Jan Alsner, Brita S. Sørensen
Translational Oncology.2014; 7(6): 687. CrossRef - Comparison of histomorphology and DNA preservation produced by fixatives in the veterinary diagnostic laboratory setting
William F. Craft, Julia A. Conway, Michael J. Dark
PeerJ.2014; 2: e377. CrossRef - Histotechnical solutions for quality improvement of nucleic acid specimens extracted from paraffin blocks
A. N Vaganova
Genes & Cells.2014; 9(2): 96. CrossRef
Effects of Fixation and Storage of Human Tissue Samples on Nucleic Acid Preservation




Fig. 1 Comparison between formalin and methacarn fixatives by polymerase chain reaction of DNA of various sizes. Lanes 1 and 3, formalin fixation; lanes 2 and 4, methacarn fixation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 60 bp), β-actin (148 bp), and human growth hormone (hGH; 434 bp). SM, size marker.
Fig. 2 Assessment of DNA integrity according to the duration of formalin fixation. Lanes 1/3/5/7/9, fixed in formalin for 3/7/30/90/180 days at room temperature; lanes 2/4/6/8/10, fixed in formalin for one day and stored in ethanol at 4℃ for 2/6/29/79/179 days. SM, size marker; hGH, human growth hormone; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Fig. 3 Bar graph representing polymerase chain reaction (PCR) results, reverse transcription (RT)-PCR results, the quantity of RNA, and the quality of RNA extracted from formalin-fixed paraffin-embedded tissues treated with decalcification solution. (A) PCR results of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), β-actin, and human growth hormone (hGH) according to duration of decalcification (0/10/30/60/120/180 minutes). (B) RT-PCR results of β-actin (97 bp). (C) RNA yield (ng/µL). (D) RNA purity (A260/280 ratios). Decal, decalcification.
Fig. 4 Differences in the quantity and quality of RNA according to the storage period of paraffin blocks. (A) RNA yield (ng/µL). (B) RNA purity (A260/280 ratios). (C) Ct values of real-time reverse transcription-polymerase chain reaction.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Effects of Fixation and Storage of Human Tissue Samples on Nucleic Acid Preservation

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article