Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(4); 2014 > Article
-
Brief Case Report
Chordoid Meningioma in a Pediatric Patient with Tuberous Sclerosis Complex - Jiwon Lee, Hee Joon Yu, Jeehun Lee, Ji Hye Kim1, Hyung Jin Shin2, Yeon-Lim Suh3, Munhyang Lee
-
Korean Journal of Pathology 2014;48(4):302-306.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.4.302
Published online: August 26, 2014
Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
1Department of Radiology and Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
3Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding Author: Munhyang Lee, M.D. Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-3522, Fax: +82-2-3410-0043, mhlee091@skku.edu
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- A 17-year-old female was admitted for recurrent vomiting and headaches that had been occurring for several months. The patient had been born as a term baby, with no perinatal problems. However, the patient had been diagnosed with infantile spasms at 3 months of age, and had been taking antiepileptic drugs since this time. Based on typical skin lesions such as shagreen patches on her back and hypopigmented spots, the patient's history of epilepsy, and her brain MRI findings, the patient was clinically diagnosed with TSC. Even though the patient continued to have seizures which required antiepileptic medications, she developed normally and attended a regular high school. Genetic analysis of TSC1 and TSC2 genes was not performed, as no family member had ever been diagnosed with TSC.
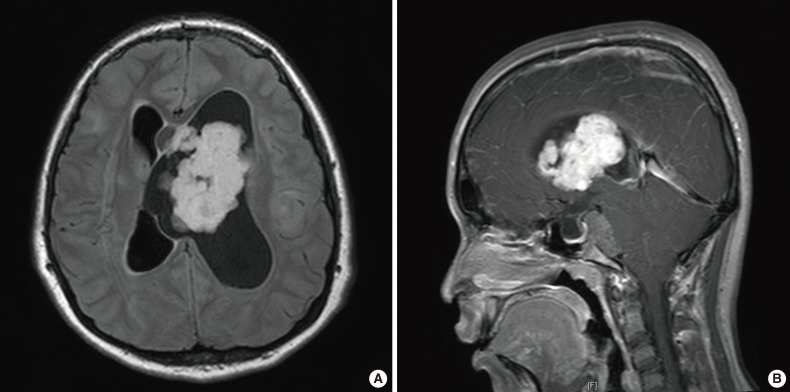
- At her follow-up appointment, the patient complained about intermittent headaches and vomiting, which occurred approximately once every three or four months. These symptoms lasted for two or three days, regardless of seizures, and waxed and waned in severity. On her follow-up visit, the patient showed subtle asymmetric facial expressions and described an increased frequency of headaches and vomiting; therefore, she underwent brain MRI. Imaging scans revealed two tumors located in the left lateral ventricle and prepontine cistern, generating obstructive hydrocephalus and herniation of the cerebellar tonsil and brainstem (Fig. 1). Considering the clinical information, SEGA was the most probable radiological diagnosis; however, the presence of two lesions and the existence of prepontine tumors were considered to be atypical.
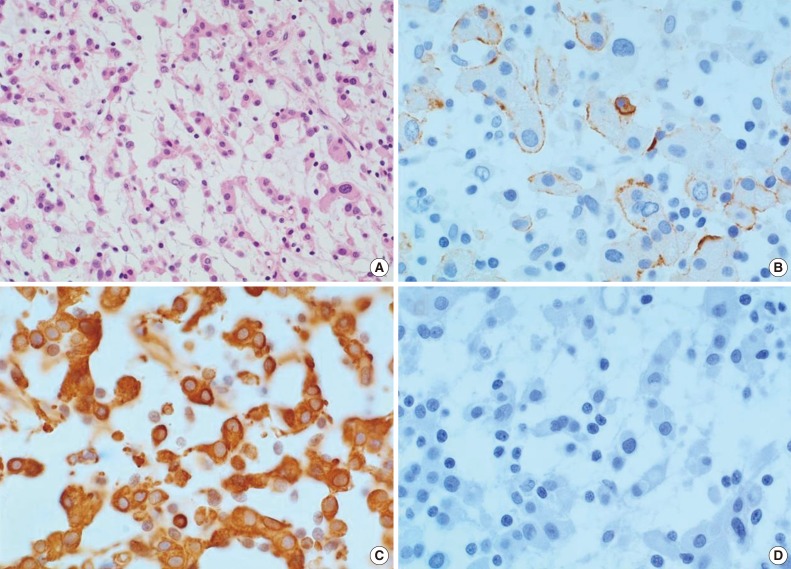
- Upon clinical diagnosis of SEGA, the patient underwent a tumor removal operation for the mass in the left lateral ventricle. During the operation, a hypervascular, pink-colored, and friable mass was discovered in the lateral ventricle which was subjected to gross total resection. Histopathologic examination revealed a predominance of cord-like or trabecularly arranged eosinophilic tumor cells in the mucoid matrix background (Fig. 2). Tumor cells were polygonal and epithelioid, and focal infiltration of lymphoid cells within the tumor was also observed. The tumor did not show any characteristics of meningothelial or transitional meningioma, with no evidence for either necrosis or mitosis found. Immunohistochemical analysis revealed that tumor cells were focally reactive for epithelial membrane antigen (EMA) (Fig. 2B) and strongly positive for vimentin (Fig. 2C). Tumor cells were negative for glial fibrillary acidic protein (GFAP), S-100 protein, synaptophysin, neurofilament, and CD34 (Fig. 2D). The MIB-1 proliferative index was 1.8%. Collectively, these histopathologic findings of the tumor are consistent with CM.
- For the prepontine tumor, we planned a short-term follow-up consisting of brain MRI scans after taking into account the surgical risks and benefits. After the operation, the patient did not receive additional treatments for the mass in the lateral ventricle, such as chemotherapy or radiotherapy. The patient's second brain MRI scan, performed 10 months after the operation, showed no evidence of recurrence of the operated lesion. However, the prepontine mass was increased in size. Therefore, gamma knife surgery was performed with a total dose of 13 Gy for the prepontine tumor (Fig. 1).
- During the patient's follow-up period, consisting of 23 months postinitial operation (6 months from the gamma knife surgery), she did not exhibit any symptoms. Also, a follow-up brain MRI scan revealed no evidence of tumor recurrence in the lateral ventricle and a decrease in the size of the prepontine enhancing mass (Fig. 1).
CASE REPORT
- Initially, the patient was suspected to have SEGA based on her clinical diagnosis of TSC and the findings of the brain MRI scans. One of the tumors was located in the left lateral ventricle and caused hydrocephalus, a finding frequently associated with SEGA.5 However, pathological studies revealed that the tumor was CM. Additionally, another tumor in the prepontine area was observed. It is unusual to have two or more SEGA lesions in TSC patients; furthermore, the tumor's prepontine location is also unusual for a SEGA lesion. This case study is the first to our knowledge describing a TSC patient with a prepontine mass. Radiological findings suggested that the prepontine mass was more likely to be a meningioma, in accordance with the pathologic diagnosis of CM. We did not obtain any tissue from the prepontine lesion because of the accompanying risk of brainstem destruction. As an alternative approach, we treated the prepontine lesion with gamma knife surgery and did not perform a biopsy.
- Meningiomas are common, and constitute about one-third of all intracranial tumors in adults. However, meningiomas are rare in children, with their incidence reported to be less than 6%.8 Furthermore, CM is also very rare, comprising only 0.5% to 1.0% of all meningiomas. Moreover, only 27 cases of CM have been reported in children since November 2012.2,3,7 Under the WHO classification guidelines, CM is a grade II tumor; CM is also considered to be a high-grade brain tumor due to its recurrence potential and its biologic behavior.7 CM is mainly located in the supra-tentorial area, especially in fronto-parietal convexities.2 In brain MRI scans, CM appears to be hypo-intense on the T1 weighted image (T1WI) and iso- or hyper-intense on the T2 weighted image (T2WI). Furthermore, CM shows homogenous enhancement on contrast images. Regarding the pathology of CM tumors, lobular arrangements with myxoid stroma are generally seen.2 In addition, CM tumors are composed of cords and lobules of the tumor cells with eosinophilic cytoplasm; CM tumors are usually elongated or round in shape, a feature similar to SEGA lesions.7 However, differential diagnosis is possible by comparing the two tumors according to their immunohistochemical features. In the present case of CM, tumor cells stained positive for EMA and vimentin, two classic immunohistochemical markers of meningioma. Furthermore, tumor cells stained negative for GFAP and S-100, two proteins commonly expressed in SEGA lesions.8 SEGA lesions may show staining for the neurofilament proteins synaptophysin and neuron-specific enolase. However, the tumor cells obtained from the patient described in this report did not show staining with these antibodies. Chordoid glioma is a rare, low-grade neoplasm with a unique chordoid appearance. The most distinctive immunohistochemical feature of chordoid glioma cells is their strong, diffuse reactivity for GFAP and CD34. However, chordoid glioma was ruled out for this patient based on the absence of GFAP and CD34 immunoreactivity in the present tumor. Therefore, the histopathological and immunohistochemical findings of our case are compatible with CM.
- TSC is an autosomal dominant genetic disorder resulting from mutation of either the TSC1 or TSC2 gene. TSC is manifested as a multisystem, neuro-cutaneous syndrome associated with hamartoma formation in multiple organs.3 Cortical tubers, SENs and SEGAs are the characteristic intracranial lesions of TSC.6 SEGA lesions have been reported in 5% to 14% of patients with TSC.5 SEGA lesions develop mainly around the foramen of Monro, and appear hypointense on the T1WI and T2WI, with marked contrast enhancement on brain MRI scans.5 SEGA lesions are benign tumors characterized by a mixture of well-circumscribed, large astrocytic-like cells and multinucleated spindle cells, often with calcification.5 Surgical removal is the treatment of choice for SEGA lesions. If SEGA lesions are not removed in a timely fashion, tumor growth can eventually result in hydrocephalus and neurological deficits.6 On one hand, CM appears benign from its histopathologic features; on the other hand, CM is classified as a WHO grade II tumor due to its recurrence potential and aggressive growth. Several indicators are suggestive of aggressive meningioma, including a mitotic index of 4 or more per 10 high power field, a mucin-rich chordoid component of the tumor, and a high MIB-1 labeling index. These indicators are related to tumor proliferation and have been shown to accurately predict patient prognosis. The more mitosis or mucin-producing activity of CM, the more aggressive biological behavior is shown by the tumor. Similarly, a high MIB-1 labeling index is correlated with tumor proliferation.9 In this case study, the MIB-1 labeling index was low compared with the tumor grade I value defined by WHO (MIB-1 index value, 1.0% to 1.35%), and mitosis was not seen.9 These findings are in accordance with the patient's lack of tumor recurrance. A previous clinicopathologic study focusing on 12 cases of CM reported low MIB-1 labeling indexes, <2% in all cases except two. These two cases showed 6% and 8% MIB-1 labeling indexes, respectively.7
- The patient's CM arose in the lateral ventricle, an unusual site for CM, which is usually located in the cerebral convexities.7 Although SEGA lesions are the most common brain tumors in TSC patients, it is also possible that different kinds of tumors develop in TSC patients. For example, one case of sphenoid transitional meningioma in a TSC patient has been reported.3 Additionally, other tumors, such as hemangioma, astroblastoma, and glioblastoma multiforme have also been reported in TSC patients, although rarely.10 This case is the first CM case, with high similarity to SEGA lesions, reported in a TSC patient.
- Brain imaging studies are only required if the TSC patient has epilepsy, intellectual disabilities, behavioral changes, hydrocephalus, or exhibits signs of increased intracranial pressure, such as vomiting and headaches. Additionally, regular check-ups and early detection for brain tumors in TSC patients are encouraged because SEN is almost asymptomatic and can transform into SEGA.5 Although most intraventricular tumors can be SEGA lesions, other tumor types are also possible, considering all aspects of this case. Accurate diagnosis and well-timed treatments are important influences on a patient's prognosis, since CM can show malignant behavior despite exhibiting benign pathologic features. Here, we report the first case of CM, presenting a similar appearance to SEGA, in a TSC patient.
DISCUSSION
- 1. Santos MV, Furlanetti L, Valera ET, Brassesco MS, Tone LG, de Oliveira RS. Pediatric meningiomas: a single-center experience with 15 consecutive cases and review of the literature. Childs Nerv Syst 2012; 28: 1887-1896. ArticlePubMed
- 2. Couce ME, Aker FV, Scheithauer BW. Chordoid meningioma: a clinicopathologic study of 42 cases. Am J Surg Pathol 2000; 24: 899-905. ArticlePubMed
- 3. Castillon-Benavides NK, Salinas-Lara C, Ponce-Guerrero F, León P, Gelista N, Tena-Suck ML. Tuberous sclerosis complex and sphenoid meningioma. Arq Neuropsiquiatr 2010; 68: 455-458. ArticlePubMed
- 4. Goh S, Butler W, Thiele EA. Subependymal giant cell tumors in tuberous sclerosis complex. Neurology 2004; 63: 1457-1461. ArticlePubMed
- 5. Cuccia V, Zuccaro G, Sosa F, Monges J, Lubienieky F, Taratuto AL. Subependymal giant cell astrocytoma in children with tuberous sclerosis. Childs Nerv Syst 2003; 19: 232-243. ArticlePubMedPDF
- 6. Moavero R, Pinci M, Bombardieri R, Curatolo P. The management of subependymal giant cell tumors in tuberous sclerosis: a clinician's perspective. Childs Nerv Syst 2011; 27: 1203-1210. ArticlePubMed
- 7. Epari S, Sharma MC, Sarkar C, Garg A, Gupta A, Mehta VS. Chordoid meningioma, an uncommon variant of meningioma: a clinicopathologic study of 12 cases. J Neurooncol 2006; 78: 263-269. ArticlePubMed
- 8. Tena-Suck ML, Collado-Ortiz MA, Salinas-Lara C, García-López R, Gelista N, Rembao-Bojorquez D. Chordoid meningioma: a report of ten cases. J Neurooncol 2010; 99: 41-48. ArticlePubMed
- 9. Ko KW, Nam DH, Kong DS, Lee JI, Park K, Kim JH. Relationship between malignant subtypes of meningioma and clinical outcome. J Clin Neurosci 2007; 14: 747-753. ArticlePubMed
- 10. Tsuchida T, Kamata K, Kawamata M, Okada K, Tanaka R, Oyake Y. Brain tumors in tuberous sclerosis. Report of 4 cases. Childs Brain 1981; 8: 271-283. ArticlePubMed
REFERENCES
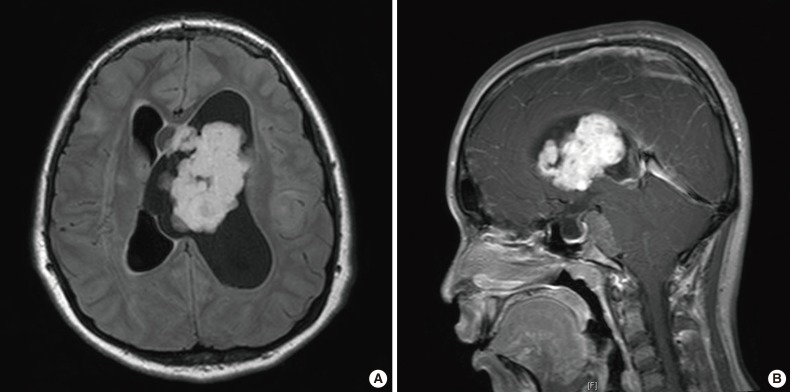
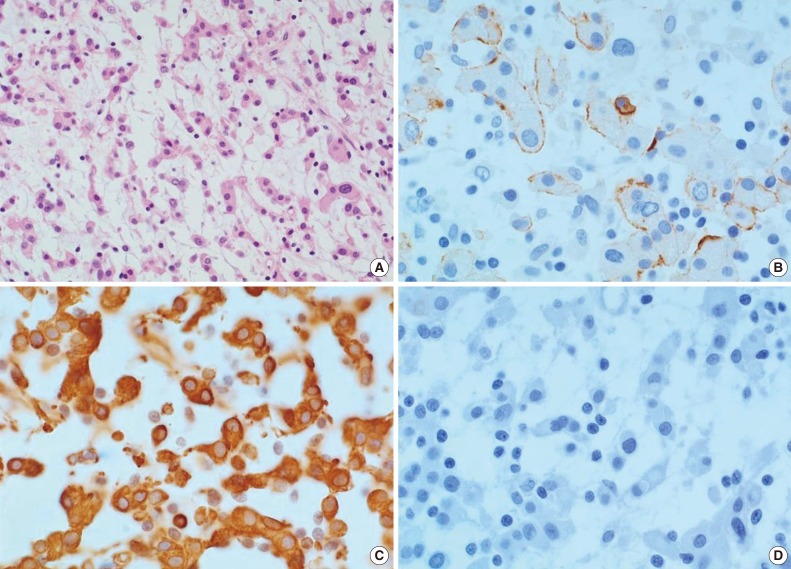
Figure & Data
References
Citations

- Somatic mutation landscape in a cohort of meningiomas that have undergone grade progression
Sarah A Cain, Bernard Pope, Stefano Mangiola, Theo Mantamadiotis, Katharine J Drummond
BMC Cancer.2023;[Epub] CrossRef - Innumerable Meningiomas Arising in a Patient With Tuberous Sclerosis Complex Decades After Radiation Therapy
Ahmed Gilani, Julieann C Lee, BK Kleinschmidt-DeMasters
Pediatric and Developmental Pathology.2021; 24(5): 471. CrossRef - Predictors of recurrence in the management of chordoid meningioma
Winward Choy, Leonel Ampie, Jonathan B. Lamano, Kartik Kesavabhotla, Qinwen Mao, Andrew T. Parsa, Orin Bloch
Journal of Neuro-Oncology.2016; 126(1): 107. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



 E-submission
E-submission