Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(6); 2014 > Article
-
Brief Case Report
Hybrid Granular Cell Tumor/Perineurioma - Sung Sun Kim, Yoo Duk Choi,, Jae Hyuk Lee, Chan Choi, Chang Soo Park
-
Korean Journal of Pathology 2014;48(6):409-412.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.6.409
Published online: December 31, 2014
Department of Pathology, Chonnam National University Medical School, Gwangju, Korea
- Corresponding Author: Yoo Duk Choi, M.D. Department of Pathology, Chonnam National University Hospital, Department of Pathology, Chonnam National University Medical School, 42 Jebong-ro, Dong-gu, Gwangju 501-757, Korea Tel: +82-62-220-5688, Fax: +82-62-225-0480, E-mail: drydchoi@chonnam.ac.kr
• Received: December 1, 2013 • Revised: March 4, 2014 • Accepted: March 24, 2014
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
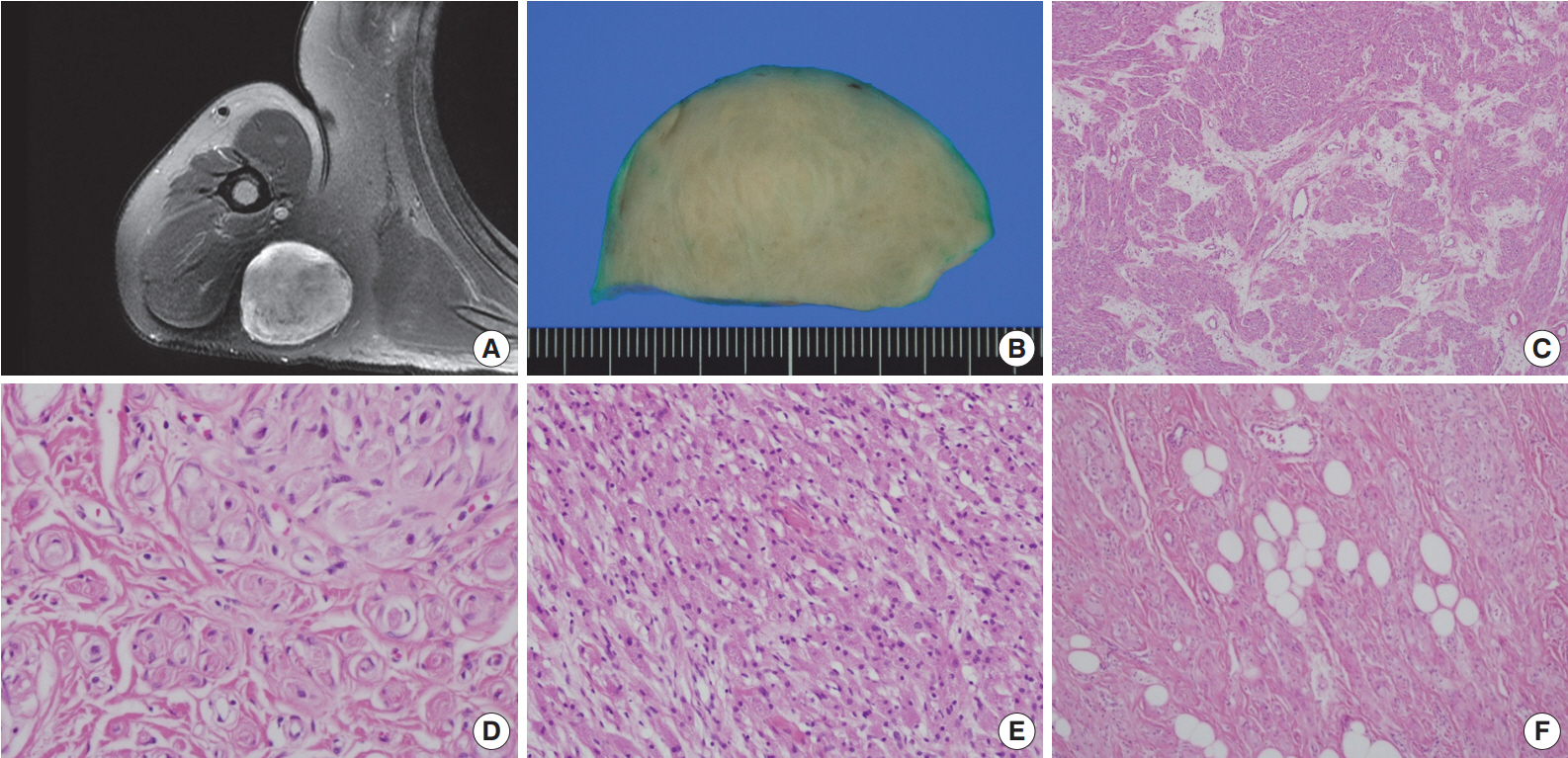
- A 54-year-old man presented with a 2-year history of a well-circumscribed movable mass in the posterior aspect of the right axilla. He did not complain of tenderness or neuropathic pain. He had no specific familial or medical history of neurogenic tumors, including neurofibromatosis. Laboratory test results, including electrolyte levels and blood cell counts, were all within the normal ranges. The magnetic resonance imaging revealed a well-circumscribed mass in the subcutaneous fat layer of the right posterior axilla (Fig. 1A). The patient underwent an excisional biopsy of the mass.
- Macroscopically, the lesion was a well-circumscribed nodular mass measuring 5.5×5 cm in the greatest dimension. It was tan to gray in color and rubbery to firm in consistency (Fig. 1B). No hemorrhage or necrosis was evident. There were no macroscopic associations with a nerve.
- Microscopically, the neoplasm was a well-circumscribed but nonencapsulated deep soft tissue mass. At first glance, there were nests or rounded aggregations of granular cells and prominent encircling spindle cells. Because the spindle cells showed a whorled and storiform growth pattern, the overall appearance of the lesion looked like whorls or eddies (Fig. 1C, D). Although the proportion of granular cells and spindle cells was variable depending on the location of the tumor, the overall proportion was similar (Fig. 1C-E). Two components of the lesion had no transition area and were intimately admixed throughout the lesion. Occasionally, mature adipose tissue was admixed with the lesion, which may have constituted a third component of the lesion (Fig. 1F). Although it occupied a small area, it was distributed widely and not restricted to the periphery. Cytologically, granular cells had a round to polygonal shape and conspicuous eosinophilic granular cytoplasm. The nuclei were small, centrally located, and mildly pleomorphic. Spindle cells were fusiform to spindle-shaped with indistinct cell borders. They had inconspicuous pale eosinophilic cytoplasm and bipolar cytoplasmic processes. No mitotic figures were seen.
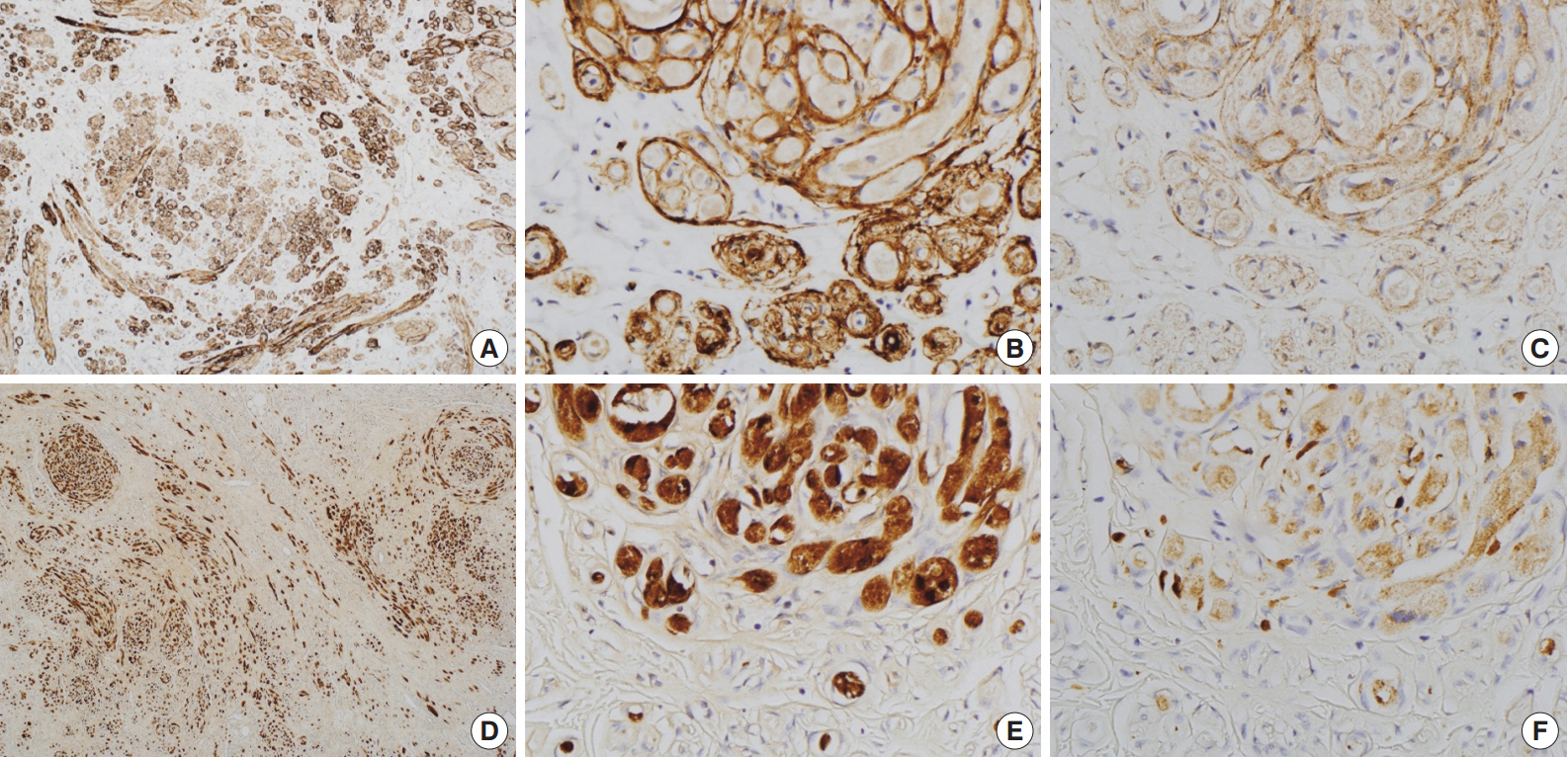
- Immunohistochemically, granular cells were positive for S-100 (1:1,000, polyclonal, Dako, Glostrup, Denmark) and CD68 (1:3,000, KP1, NeoMarkers, Fremont, CA, USA) but not for epithelial membrane antigen (EMA; 1:100, E29, Dako), glucose transporter 1 (GLUT-1; 1:100, polyclonal, Millipore, CA, USA), or claudin-1 (1:200, polyclonal, Biocare Medical, Concord, CA, USA). However, the spindle cells were immunoreactive for EMA, GLUT-1, and claudin-1 and did not stain for S-100 (Fig. 2). In particular, EMA and GLUT-1 highlighted the cytoplasmic processes of the spindle cells. The tumor cells were not immunoreactive for CD34 (1:300, QBEnd10, Dako), cytokeratin (1:200, AE1/AE3, Dako), desmin (1:100, D33, Dako), neurofilament protein (1:2,000, 2F11, Dako), or smooth muscle actin (1:200, 1A4, Dako).
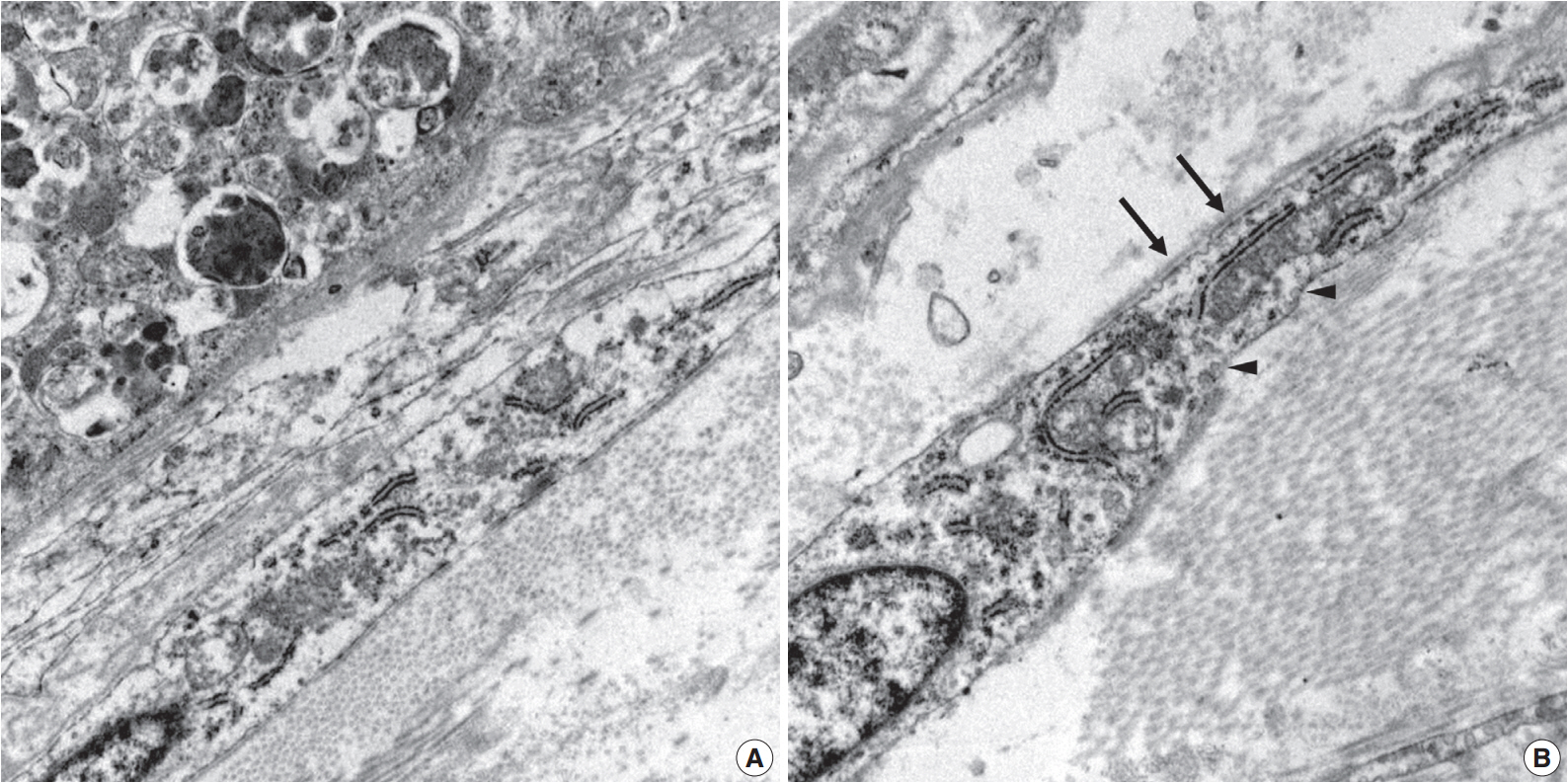
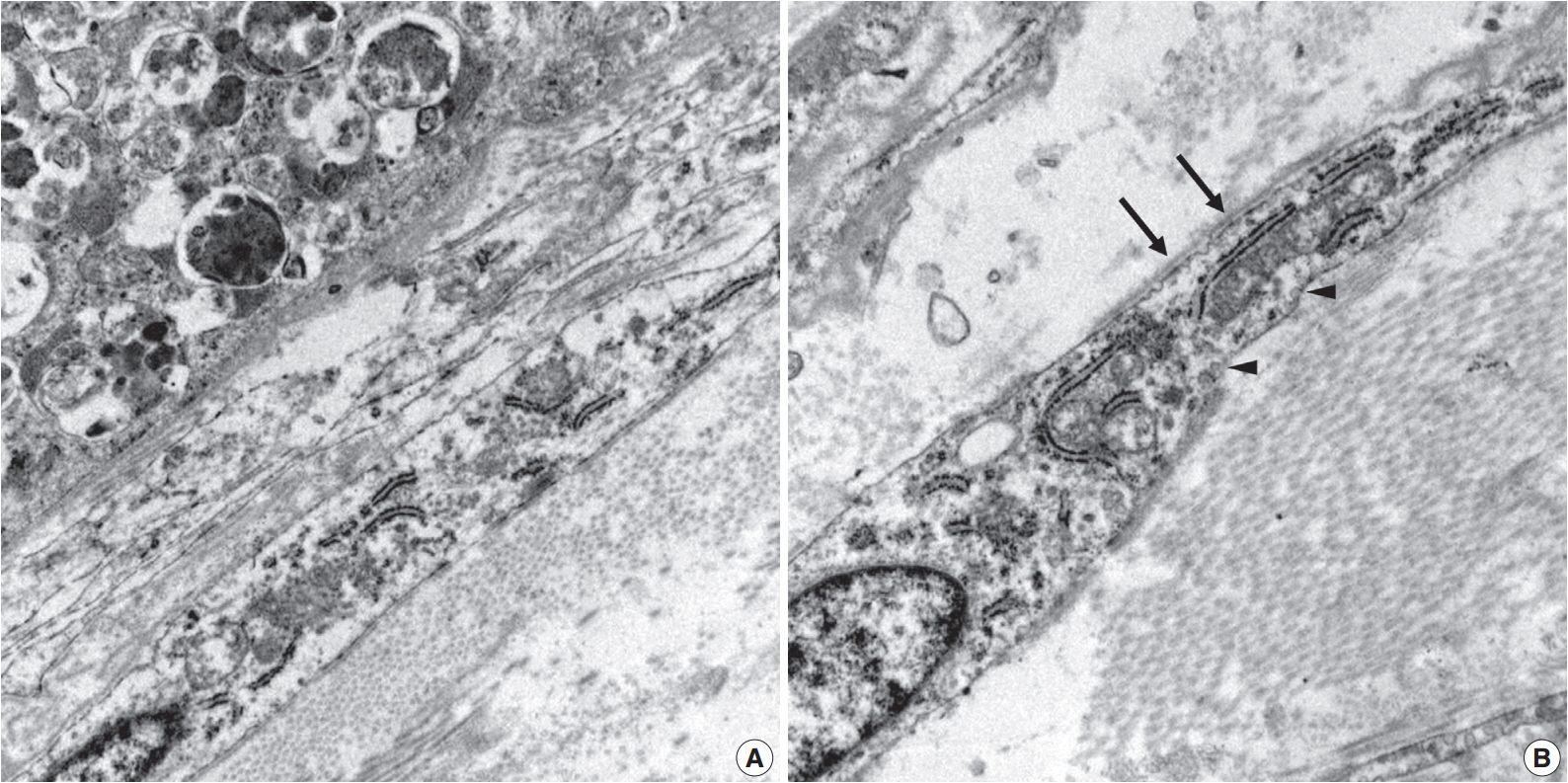
- Ultrastructurally, two populations of the lesion were easily identified. Granular cells exhibited abundant cytoplasm filled with phagolysosomes with electron-dense debris. Spindle cells revealed discontinuous and irregular basal lamina, rudimentary intercellular junction, thin cytoplasmic processes, and pinocytic vesicles (Fig. 3).
CASE REPORT
- Benign PNSTs include schwannomas, neurofibromas, and perineuriomas, which have distinct histomorphological and immunohistochemical profiles. They originate from the connective tissue sheath of peripheral nerves, such as Schwann cells, perineurial cells, and fibroblasts. PNSTs comprising more than one distinct histologic cell type, called “hybrid PNSTs,” have recently been described. After Feany et al. [5] first described a hybrid PNST in 1998, these tumors have been increasingly identified, likely due to the aid of ancillary tests, such as immunohistochemical staining. Among these tumors, hybrid schwannoma/perineurioma and hybrid neurofibroma/perineurioma are the most common combinations [6]. Hybrid granular cell tumor/perineurioma is a somewhat rare combination, and there have been only a few reports in the English literature [1-4].
- Perineuriomas are uncommon benign peripheral nerve sheath neoplasms composed exclusively of well-differentiated perineurial cells. Perineurial cells are neurosustentacular cells that surround nerve fascicles and constitute a blood-nerve barrier that acts to preserve the nerve tract by creating tight junctions with endothelial cells [1]. These cells are also involved in other tumors of the peripheral nerve sheath, such as neurofibromas, schwannomas, and malignant PNSTs. Perineurial cells are immunoreactive for EMA, CD34, GLUT-1, and claudin-1, which are used as diagnostic markers [7].
- When granular cells and perineurial cells are seen as predominant tumor components, granular cell changes of perineurioma and reactive hyperplastic changes of perineurial cells in a granular cell tumor must be excluded before a final diagnosis of hybrid granular cell tumor/perineurioma can be made [8,9]. Since perineurial cells can be observed as an additional minor component in PNSTs, such as neurofibromas and granular cell tumors, it is important to determine if they are hyperplastic or neoplastic. Reactive hyperplastic or remnant perineurial cells in neo-plasms of neurosustentacular cells are usually located in the periphery or perivascular area. They are usually related to nerve fascicles and arranged in a concentric pattern [8]. In our case, perineurial and granular cells are major tumor components that are intimately admixed with each other in most areas of the lesion and are not associated with nerves. On the basis of these findings, a hybrid granular cell tumor/perineurioma was diagnosed rather than a single PNST.
- Granular cell changes can be seen in various non-neoplasitc or neoplastic lesions, including granular cell traumatic neuroma, dermatofibrosarcoma protuberans, leiomyoma, leiomyosarcoma, basal cell carcinoma, and perineurioma [9,10]. These changes may be nonspecific morphologic changes due to lysosomal accumulation as a result of metabolic alterations. Recently, there was a reported case of granular perineurioma based on granular cells negative for S-100 and perineurial cells positive for EMA, claudin-1, and GLUT-1 [7]. Immunoreactivity for S-100, which represents the neurogenic origin of granular cell components, was a main differention point between granular perineurioma and hybrid granular cell tumor/perineurioma. That study stated that granular cell changes were nonspecific findings resulting from lysosomal defects, and the granular components were not neoplastic.
- The origin of hybrid PNSTs is unclear; however, there are a few suggestions. It has been proposed that, because both components of hybrid PNST are of schwannian origin, any combination of individual PNSTs is possible by a hypothetically divergent differentiation [1], which is supported by reports of various combinations of PNSTs. Others have indicated that cellular dimorphism is a result of metaplastic processes based on transition cells with immunohistochemical dual-lineage expression [4]. In the present case, there were multiple foci of intralesional adipose tissue proliferation that were thought to be minor tumor components rather than entrapped tissue. Although one case cannot represent all tumors, this feature suggests that this composite tumor is a type of hamartomatous lesion or originates from pluripotent mesenchymal cells.
- With improvement in ancillary studies, such as immunohistochemistry, hybrid PNSTs will be more readily recognized and diagnosed. In the present case, granular cell and perineurial components were easily detected due to distinct histomorphologies. Granular perineurioma and granular cell tumor with reactive perineurial cell hyperplasia should be excluded during diagnosis. A careful histomorphologic examination of perineurial cell location within the tumor and immunohistochemical stains for S-100, EMA, GLUT-1, and claudin-1 are needed to make a definitive diagnosis. We reported herein a rare case of hybrid granular cell tumor/perineurioma.
DISCUSSION
Fig. 1.Right shoulder magnetic resonance imaging reveals a well-circumscribed mass in the right posterior axilla (A). Grossly, it is tan to gray in color (B). At low power, the tumor reveals a whorled and storiform growth pattern (C) and rounded aggregations of granular cells with encircling spindle cells (D). (E) Predominant aggregation of granular cells is also observed. (F) Mature adipose tissue is occasionally admixed with main components of the tumor.
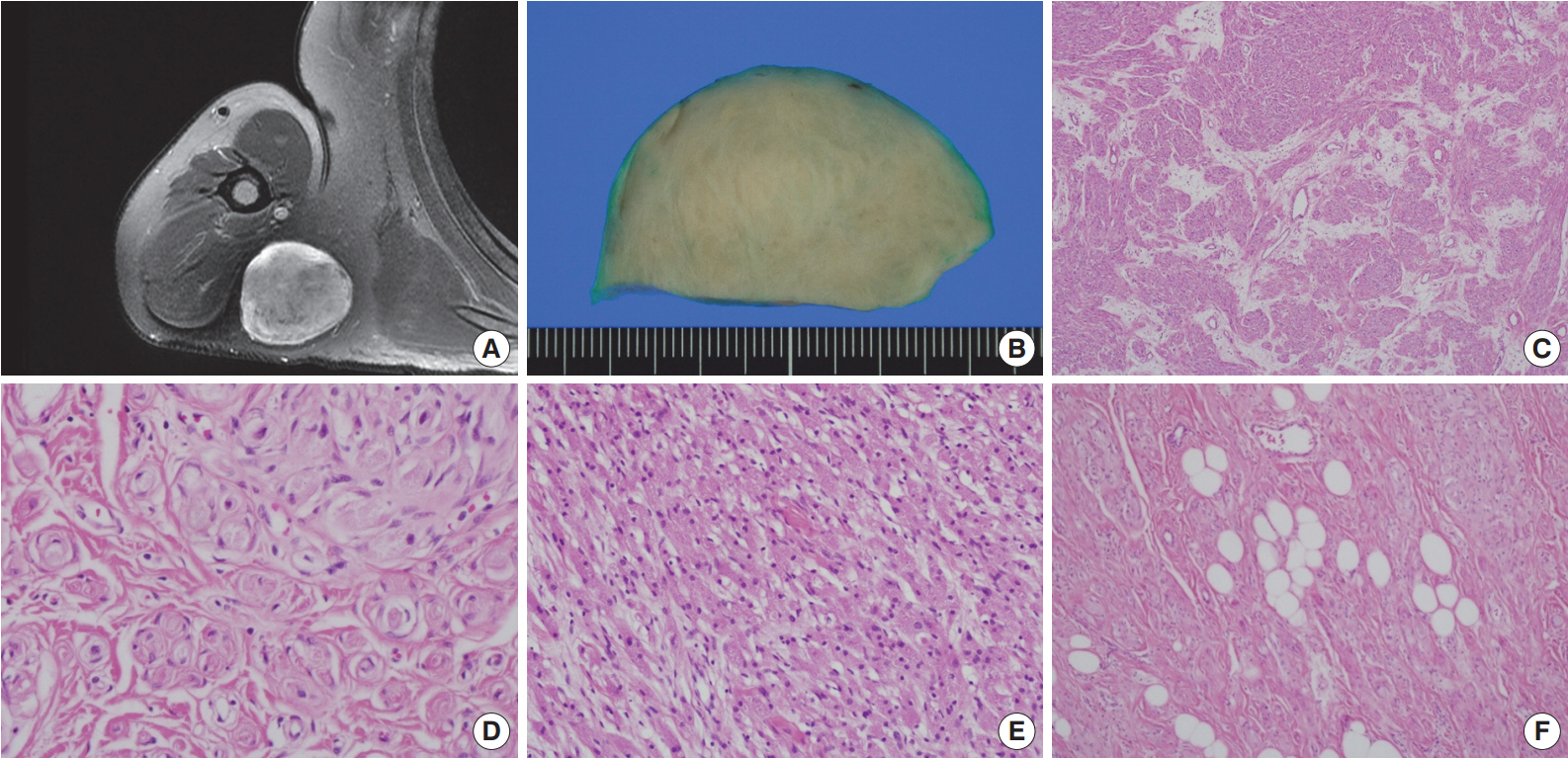

Fig. 2.Immunohistochemical findings of the area in Fig. 1D. Spindle cells are immunopositive for glucose transporter 1 (A, B) and epithelial membrane antigen (C). Granular cells are immunoreactive for S-100 (D, E) and CD68 (F).


Fig. 3.Electron microscopy of the granular cells and perineurial cells derived from formalin-fixed tissue. Granular cells reveal abundant cytoplasm filled with phagolysosomes (A, ×8,000), and spindle cells show irregular basal lamina (arrows) and pinocytic vesicles (arrowheads) (B, ×15,000).

- 1. Zarineh A, Costa ME, Rabkin MS. Multiple hybrid granular cell tumor-perineuriomas. Am J Surg Pathol 2008; 32: 1572-7. ArticlePubMed
- 2. Izquierdo FM, Suárez-Vilela D, Honrado E. Intraneural hybrid granular cell tumor-perineurioma. APMIS 2013; 121: 678-80. ArticlePubMed
- 3. Diaz-Flores L, Alvarez-Arguelles H, Madrid JF, Varela H, Gonzalez MP, Gutierrez R. Perineurial cell tumor (perineurioma) with granular cells. J Cutan Pathol 1997; 24: 575-9. ArticlePubMed
- 4. Matter A, Hewer E, Kappeler A, Fleischmann A, Vajtai I. Plexiform hybrid granular cell tumor/perineurioma: a novel variant of benign peripheral nerve sheath tumor with divergent differentiation. Pathol Res Pract 2012; 208: 310-4. ArticlePubMed
- 5. Feany MB, Anthony DC, Fletcher CD. Nerve sheath tumours with hybrid features of neurofibroma and schwannoma: a conceptual challenge. Histopathology 1998; 32: 405-10. ArticlePubMed
- 6. Park JY, Park NJ, Kim SP, Kwon KY, Lee SS. A soft tissue perineurioma and a hybrid tumor of perineurioma and schwannoma. Korean J Pathol 2012; 46: 75-8. ArticlePubMedPMC
- 7. Hornick JL, Fletcher CD. Soft tissue perineurioma: clinicopathologic analysis of 81 cases including those with atypical histologic features. Am J Surg Pathol 2005; 29: 845-58. ArticlePubMed
- 8. Izquierdo F, Suárez-Vilela D, Honrado E. Perineurial cells in granular cell tumors and neoplasms with perineural invasion: an immunohistochemical study. Am J Dermatopathol 2012; 34: 800-9. ArticlePubMed
- 9. Al-Daraji WI. Granular perineurioma: the first report of a rare distinctive subtype of perineurioma. Am J Dermatopathol 2008; 30: 163-8. ArticlePubMed
- 10. Mentzel T, Wadden C, Fletcher CD. Granular cell change in smooth muscle tumours of skin and soft tissue. Histopathology 1994; 24: 223-31. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Rare Hybrid Perineurioma and Granular Cell Tumor: A Pediatric Case
Kennedy H. Sun, Sonia P. Goyal, Evelyn M. Kim, Esperanza Mantilla-Rivas, Gary F. Rogers, Sam P. Gulino
Pediatric and Developmental Pathology.2024;[Epub] CrossRef - What’s new in nerve sheath tumors
Anders Meyer, Steven D. Billings
Virchows Archiv.2020; 476(1): 65. CrossRef - A Rare Perineurioma/Granular Cell Tumor Hybrid Peripheral Nerve Sheath Tumor
Koorosh Haghayeghi, Gladys Telang, Sonja Chen, Jack Bevivino, Shamlal Mangray, Yiang Hui, Leslie Robinson-Bostom
The American Journal of Dermatopathology.2020; 42(10): 762. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Hybrid Granular Cell Tumor/Perineurioma



Fig. 1. Right shoulder magnetic resonance imaging reveals a well-circumscribed mass in the right posterior axilla (A). Grossly, it is tan to gray in color (B). At low power, the tumor reveals a whorled and storiform growth pattern (C) and rounded aggregations of granular cells with encircling spindle cells (D). (E) Predominant aggregation of granular cells is also observed. (F) Mature adipose tissue is occasionally admixed with main components of the tumor.
Fig. 2. Immunohistochemical findings of the area in Fig. 1D. Spindle cells are immunopositive for glucose transporter 1 (A, B) and epithelial membrane antigen (C). Granular cells are immunoreactive for S-100 (D, E) and CD68 (F).
Fig. 3. Electron microscopy of the granular cells and perineurial cells derived from formalin-fixed tissue. Granular cells reveal abundant cytoplasm filled with phagolysosomes (A, ×8,000), and spindle cells show irregular basal lamina (arrows) and pinocytic vesicles (arrowheads) (B, ×15,000).
Fig. 1.
Fig. 2.
Fig. 3.
Hybrid Granular Cell Tumor/Perineurioma

 E-submission
E-submission