Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(6); 2014 > Article
-
Brief Case Report
A Case of Type II Enteropathy-Associated T-Cell Lymphoma with Epstein-Barr Virus Positivity - Min Jeong Song, Chan Sik Park, Hee Sang Hwang, Cheol Won Suh1, Dok Hyun Yoon1, Jooryung Huh,
-
Korean Journal of Pathology 2014;48(6):426-429.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.6.426
Published online: December 31, 2014
Departments of Pathology, Asan Medical Center, University of Ulsan Collage of Medicine, Seoul, Korea
1Departments of Oncology, Asan Medical Center, University of Ulsan Collage of Medicine, Seoul, Korea
- Corresponding Author: Jooryung Huh, M.D. Department of Pathology, Asan Medical Center, University of Ulsan Collage of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 138-736, Korea Tel: +82-2-3010-4553, Fax: +82-2-472-7898, E-mail: jrhuh@amc.seoul.kr
• Received: March 13, 2014 • Revised: May 16, 2014 • Accepted: May 21, 2014
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
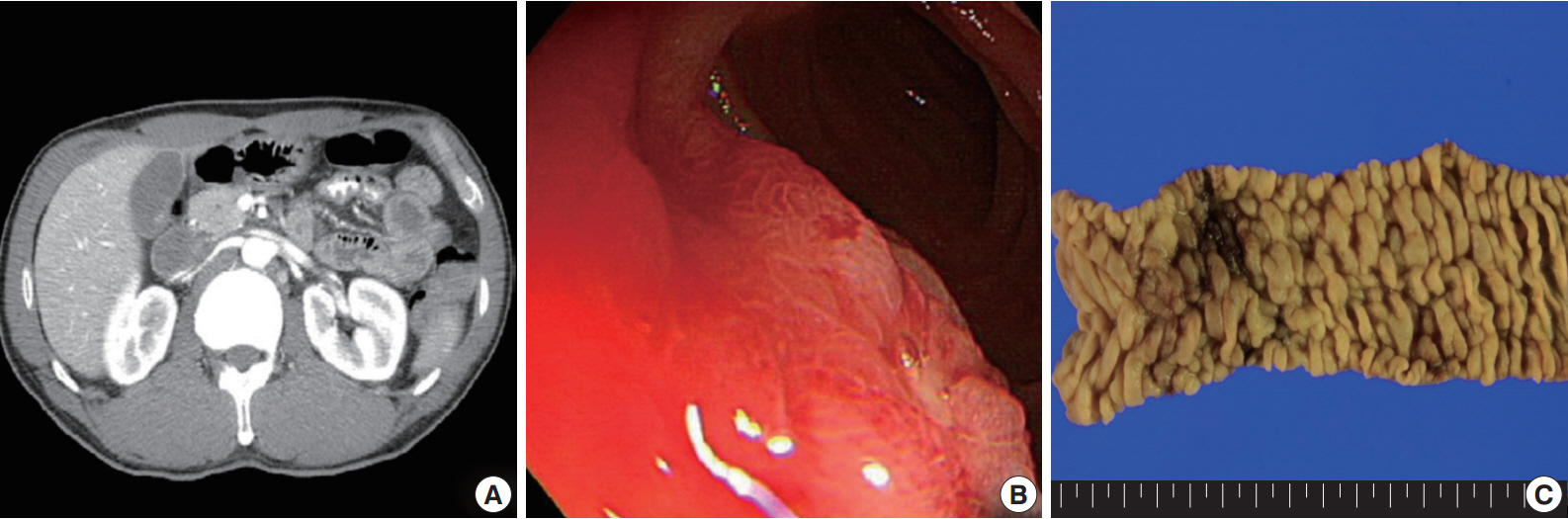
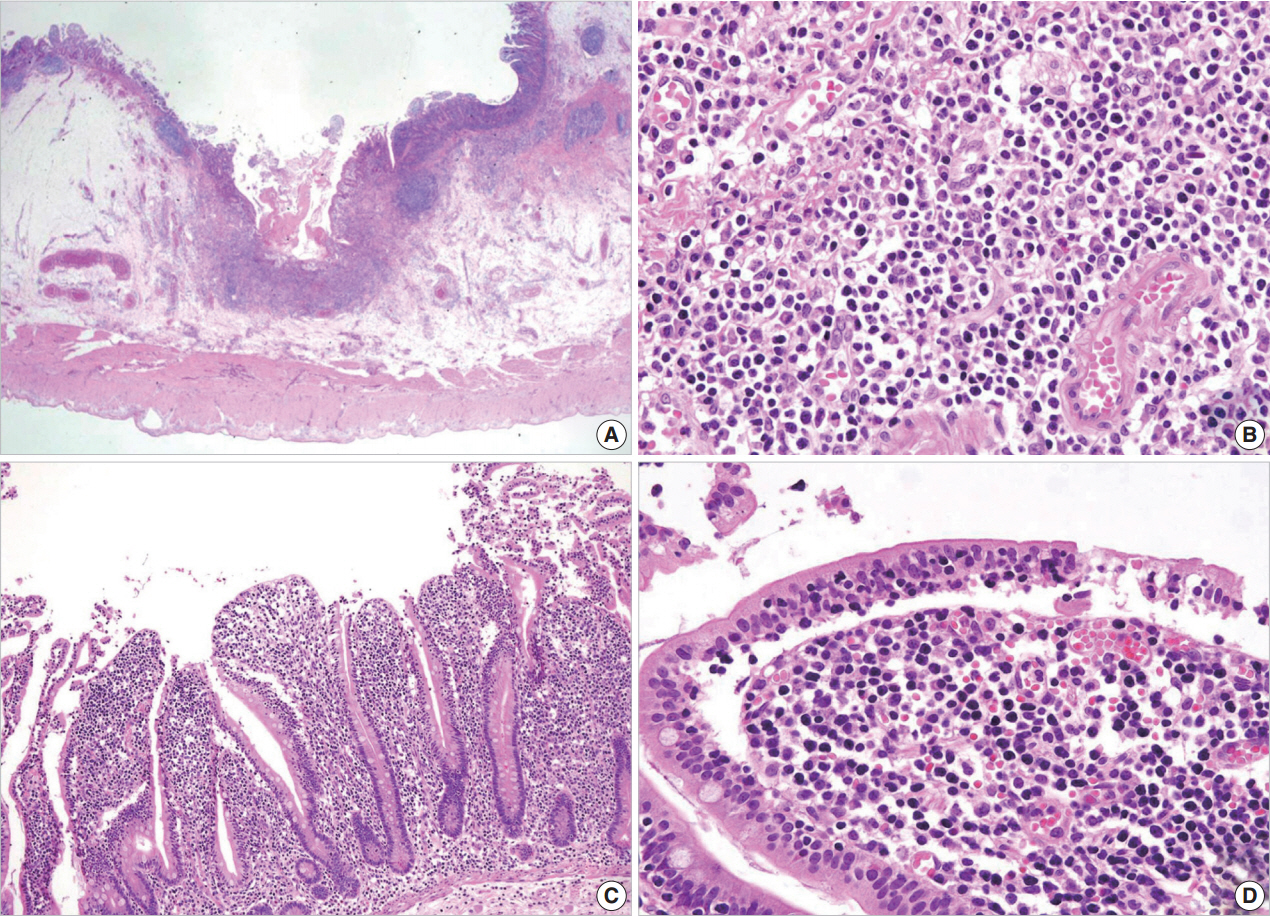
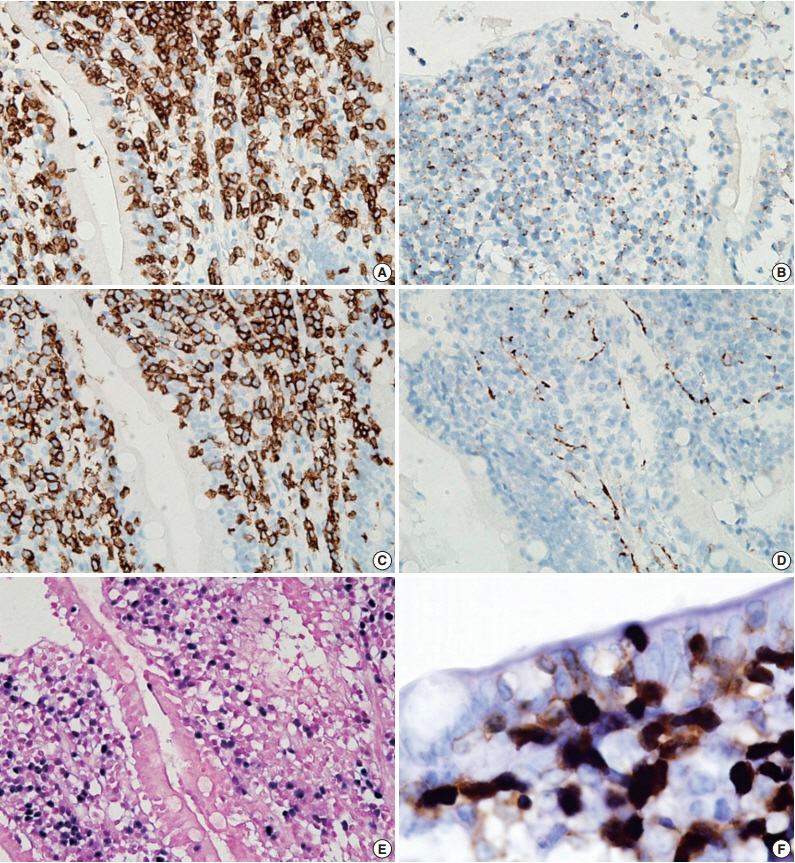
- A 54-year-old Korean man was referred to our institute for evaluation of recurrent hematochezia. In the previous three years, the patient had experienced five episodes of hematochezia, the last occurring three days prior to presentation, and all occasions required embolization of the superior mesenteric artery. He appeared chronically ill but was mentally alert. Initial vital signs were within normal limits: blood pressure, 112/75 mm Hg; heart rate, 82/min; respiration, 16/min; and body temperature, 36.4°C. Complete blood counts were as follows: white blood cell count, 6,100/µL; hemoglobin, 10.4 g/dL; and platelet count, 187×103/µL with normocytic normochromic peripheral blood morphology. Abdominopelvic computed tomography showed diffuse thickening of the proximal jejunal wall without obstruction and multiple enlarged mesenteric lymph nodes (Fig. 1A). Endoscopy showed multiple discrete geographic ulcers oozing fresh blood in the proximal jejunum (Fig. 1B). The patient underwent a segmental resection of the small intestine. On macroscopic examination, multiple ill-demarcated geographic ulcers covered with hemorrhagic exudate were identified in the jejunum. Obstruction or perforation was not observed. The intestinal mucosa was diffusely edematous (Fig. 1C). Microscopically, the central zone of the ulcer showed dense lymphocytic infiltration in the lamina propria and submucosa. The infiltrate was composed of monotonous, small- to medium-sized lymphocytes with scanty cytoplasm and round, hyperchromatic nuclei (Fig. 2A, B). Angiocentricity, angioinvasion, or coagulative necrosis was not observed. On immunophenotyping, the tumor cells were CD3+CD5+CD8+TIA-1+TCR-βF1+CD20–CD56– and CD4– (Fig. 3). In situ hybridization for EBER showed strong signals in the majority of tumor cells, which were CD3+ T-cells. The adjacent jejunal mucosa showed extensive intraepithelial lymphocytosis in the epithelium, mild villous atrophy, and crypt hyperplasia (Fig. 2C, D). These lymphocytes revealed cytological and immunologic features identical to those of the central zone. Polymerase chain reaction analysis using Biomed primers showed clonally rearranged TCR γ genes. Based on these findings, the lesion was diagnosed as type II EATL with EBV positivity. Chemotherapy with cyclophosphamide, doxorubicin, and vincristine was started, and the patient was alive with no evidence of recurrence at 23 months after the surgery.
CASE REPORT
- Type II EATL is a newly recognized entity that requires further characterization and exhibits distinctive clinicopathological features. This lymphoma is the predominant type of EATL in Asian populations and is not associated with celiac disease. Histologically, type II EATL reveals monotonous small- to medium-sized tumor cells and lack of EBV infection of the tumor cells. The immunophenotype of this tumor is typically CD3+CD8+CD56+CD4– and frequently γδ TCR+ [2].
- On histological and immunohistochemical analyses, the present case is compatible with type II EATL as shown by enteropathic features (villous atrophy, crypt hyperplasia, and diffuse intraepithelial lymphocytosis) and a CD3+CD8+TIA-1+TCR-βF1+CD4– immunophenotype. Although type II EATL typically expresses CD56, approximately 9% of cases are CD56- as in the present case [3]. However, EBV positivity is not a typical feature of type II EATL [1,2,4,5], and most authors report a complete absence of EBV in type II EATL [2,4,5]. Several authors have suggested that diffuse positivity of EBER is an indication that the tumor in question should be diagnosed as extranodal NK/T-cell lymphoma [4]. However, Tse et al. [6] reported three cases with extensive EBER expression but without histological features of NK/T-cell lymphoma in a study of 34 type II EATLs in an Asian population. Furthermore, two of the EBER-positive cases in that study had clonal TCR gene rearrangement, implying T-cell lineage. In addition, other authors have also reported EBV-positive EATL (Table 1). Although the CD56 status was not specified in that study, except for one case positive for CD56, our case resembles those of Tse et al. [6], suggesting that a small minority of type II EATL cases may be EBV-positive. These observations suggest that type II EATL is a heterogeneous disease with distinct phenotypic and genotypic characteristics.
DISCUSSION
Fig. 1.Abdominopelvic computed tomography reveals diffuse thickening of the proximal jejunum with no discrete mass lesions or obstruction and multiple enlarged mesenteric nodes (A). (B) On endoscopy, multiple discrete ulcers oozing fresh blood are observed. (C) The jejunum shows multiple geographic ulcers covered with hemorrhagic exudates without obstruction or perforation. The adjacent mucosa is diffusely edematous.
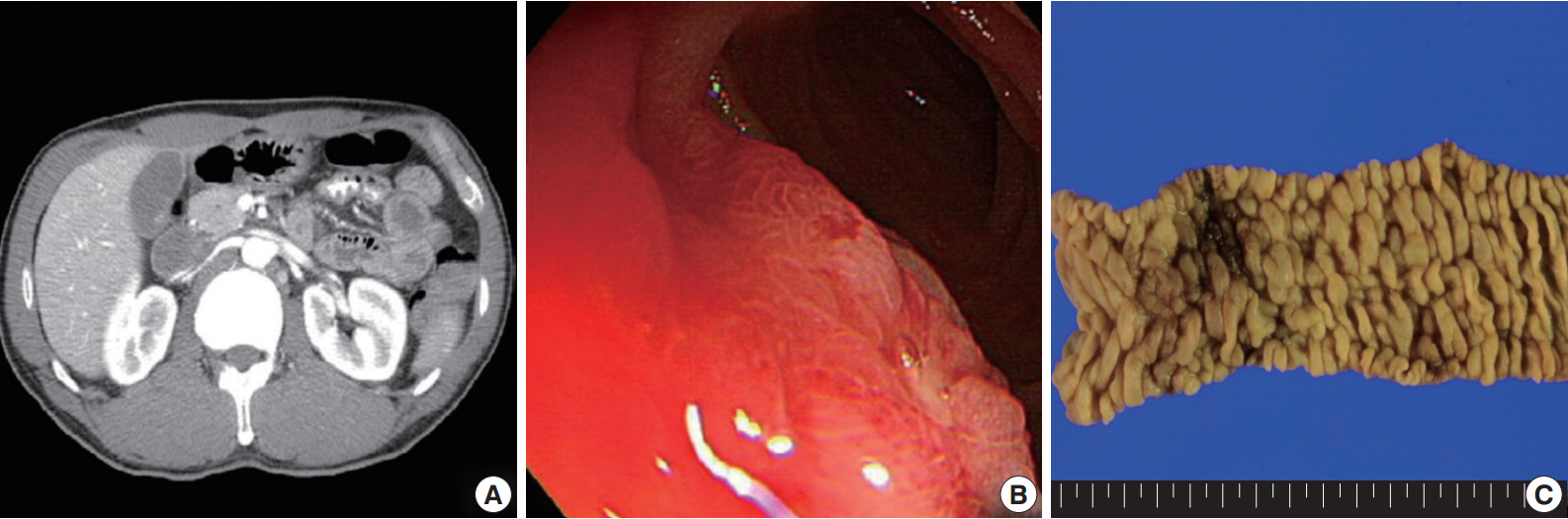

Fig. 2.Microscopic examination. (A, B) The central zone of the tumor is ulcerated with monotonous small- to medium-sized lymphocytic infiltration in the mucosa and submucosa. The adjacent mucosa shows mild villous atrophy and crypt hyperplasia (C) and extensive intraepithelial lymphocytosis (D).
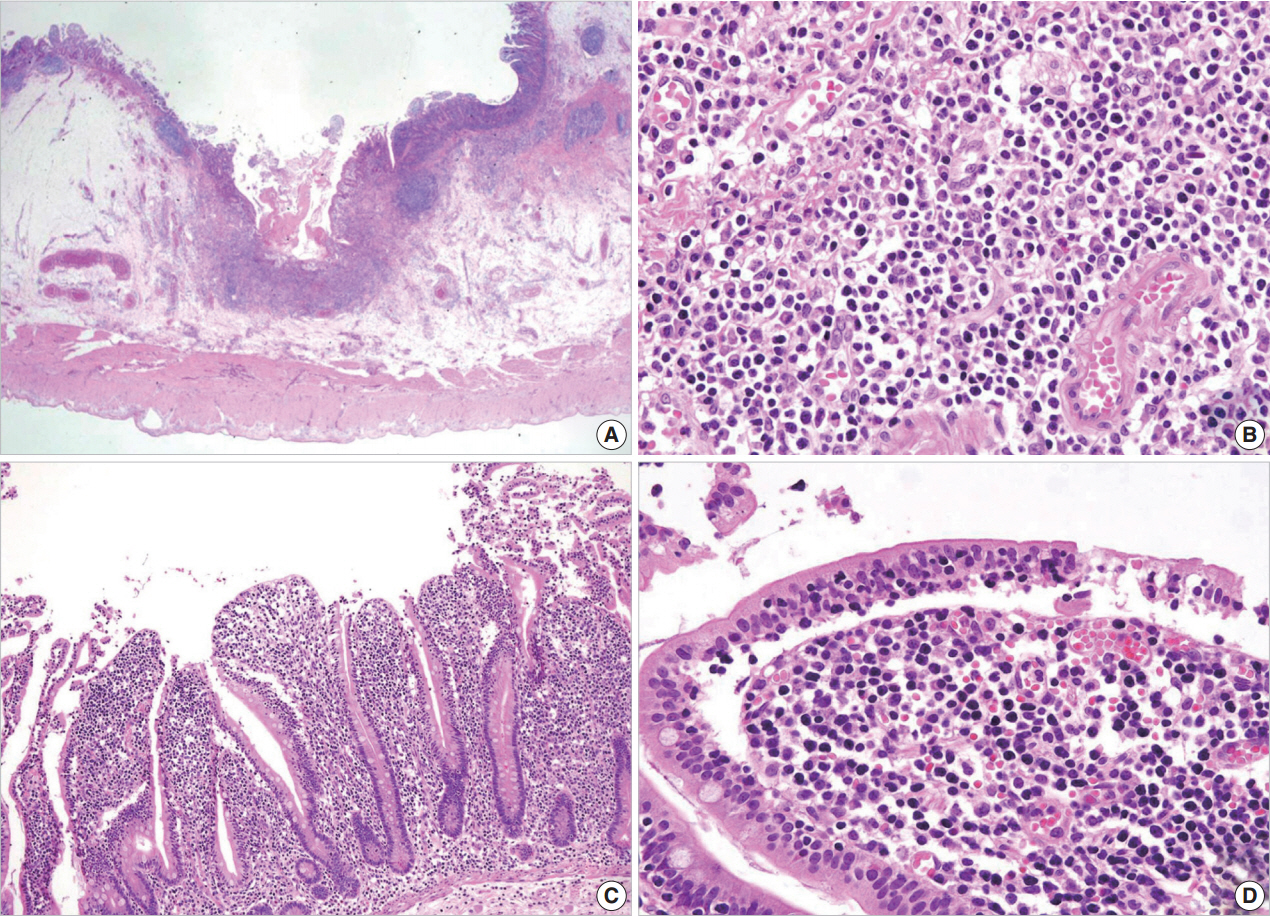

Fig. 3.Immunohistochemical stains. Tumor cells are positive for CD3 (A), TIA-1 (B), and CD8 (C), but negative for CD56 (D). (E) In situ hybridization for Epstein-Barr virus (EBV)–encoded RNA (EBER) shows diffuse nuclear positivity in tumor cells. (F) Dual staining for EBER (black) and CD3 (brown) reveals that the EBV-positive cells are CD3-positive T-cells.
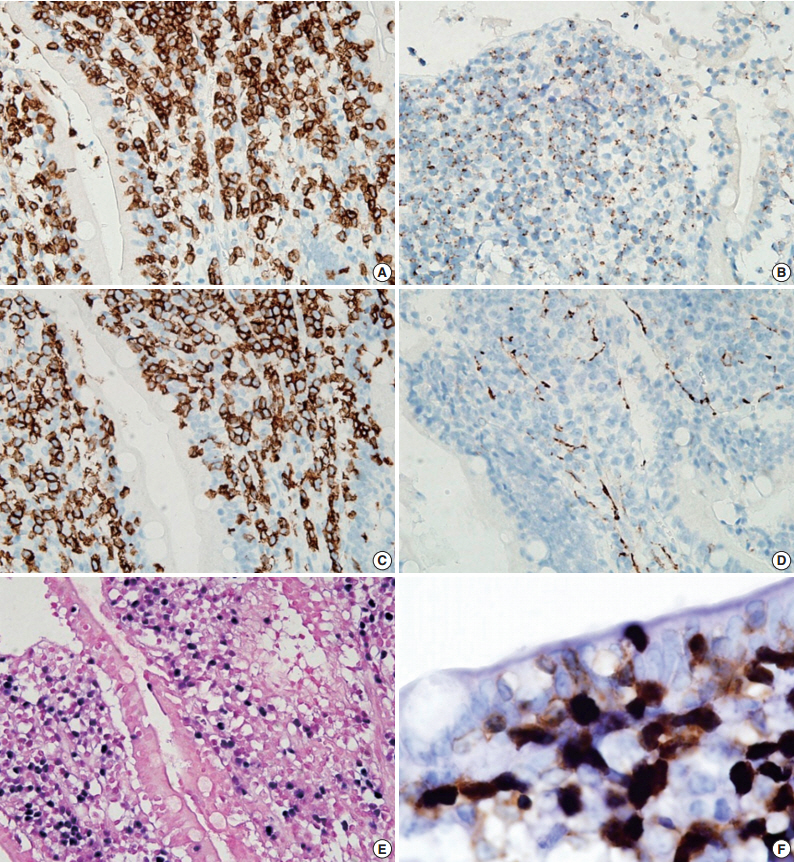

Table 1.Summary of previously published reports including a patient with EBV-positive EATL
| Reference | Country | No. of cases | No. of EBV-posi- tive cases | EBV-positive cell population |
|---|---|---|---|---|
| Tse et al. (2012) [6] | Multinational (Asia) | 34 | 3 | Cytotoxic T cells |
| Ng (2012) [7] | Singapore | 1 | 1 | CD3+, CD4–, CD8–, CD56– tumor cells |
| Delabie et al. (2011) [1] | Multinational (North America, Europe, and Asia) | 58 | 4 | Subpopulation of likely reactive cells |
| Takeshita et al. (2011) [8] | Japan | 24 | 2 | CD56–, CD8– T cells |
| 1 | CD56+, CD8+ T cells | |||
| Quintanilla-Martinez et al. (1997) [9] | Multinational (Mexico, Austria, and Germany) | 6 | 1 | Pleomorphic medium and large cells |
| Pan et al. (1993) [10] | UK | 11 | 3 | Large CD3+ tumor cells |
- 1. Delabie J, Holte H, Vose JM, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood 2011; 118: 148-55. ArticlePubMedPDF
- 2. Chan JK, Chan AC, Cheuk W, et al. Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent γδ T-cell receptor expression. Am J Surg Pathol 2011; 35: 1557-69. PubMed
- 3. Tan SY, Chuang SS, Tang T, et al. Type II EATL (epitheliotropic intestinal T-cell lymphoma): a neoplasm of intra-epithelial T-cells with predominant CD8αα phenotype. Leukemia 2013; 27: 1688-96. ArticlePubMedPDF
- 4. Tan SY, Nakamura S, Tan HC, Liu YH, Chuang SS. Diagnosis of type II enteropathy-associated T-cell lymphoma should be limited to EBER-cases. Am J Hematol 2012; 87: E129-30. Article
- 5. Arps DP, Smith LB. Classic versus type II enteropathy-associated T-cell lymphoma: diagnostic considerations. Arch Pathol Lab Med 2013; 137: 1227-31. ArticlePubMedPDF
- 6. Tse E, Gill H, Loong F, et al. Type II enteropathy-associated T-cell lymphoma: a multicenter analysis from the Asia Lymphoma Study Group. Am J Hematol 2012; 87: 663-8. ArticlePubMed
- 7. Ng SB. Lymphoma Workshop No. 00011. EBV+ Intestinal T-cell Lymphoma. XVI Meeting of the European Association for Haematopathology; Lisbon, Portugal. 2012 Oct 20-25.
- 8. Takeshita M, Nakamura S, Kikuma K, et al. Pathological and immunohistological findings and genetic aberrations of intestinal enteropathy-associated T cell lymphoma in Japan. Histopathology 2011; 58: 395-407. ArticlePubMed
- 9. Quintanilla-Martinez L, Lome-Maldonado C, Ott G, et al. Primary non-Hodgkin’s lymphoma of the intestine: high prevalence of Epstein-Barr virus in Mexican lymphomas as compared with European cases. Blood 1997; 89: 644-51. ArticlePubMedPDF
- 10. Pan L, Diss TC, Peng H, et al. Epstein-Barr virus (EBV) in enteropathy-associated T-cell lymphoma (EATL). J Pathol 1993; 170: 137-43. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Various Endoscopic Features in Monomorphic Epitheliotropic Intestinal T-Cell Lymphoma
Yasuhiro Aoki, Tomohisa Sujino, Kaoru Takabayashi, Makoto Mutakuchi, Katsura Emoto, Naoki Hosoe, Haruhiko Ogata, Takanori Kanai
Case Reports in Gastroenterology.2021; 15(1): 312. CrossRef - A viral map of gastrointestinal cancers
Natália R. Costa, Rui M. Gil da Costa, Rui Medeiros
Life Sciences.2018; 199: 188. CrossRef - Type II Enteropathy-Associated T-cell Lymphoma: A Rare Report from Iran
Neda Nozari
Middle East Journal of Digestive Diseases.2017; 9(1): 55. CrossRef - Unusual Cause of Dysphagia
Shahram Agah, Ramak Ghavam, Ahmad Darvishi Zeidabadi, Arash Sarveazad
Middle East Journal of Digestive Diseases.2017; 9(1): 58. CrossRef - Multiple lesions of gastrointestinal tract invasion by monomorphic epitheliotropic intestinal T-cell lymphoma, accompanied by duodenal and intestinal enteropathy-like lesions and microscopic lymphocytic proctocolitis: a case series
Hideki Ishibashi, Satoshi Nimura, Yoshiyuki Kayashima, Yasushi Takamatsu, Kunihiko Aoyagi, Naohiko Harada, Masanori Kadowaki, Takihiko Kamio, Shotaro Sakisaka, Morishige Takeshita
Diagnostic Pathology.2016;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
A Case of Type II Enteropathy-Associated T-Cell Lymphoma with Epstein-Barr Virus Positivity



Fig. 1. Abdominopelvic computed tomography reveals diffuse thickening of the proximal jejunum with no discrete mass lesions or obstruction and multiple enlarged mesenteric nodes (A). (B) On endoscopy, multiple discrete ulcers oozing fresh blood are observed. (C) The jejunum shows multiple geographic ulcers covered with hemorrhagic exudates without obstruction or perforation. The adjacent mucosa is diffusely edematous.
Fig. 2. Microscopic examination. (A, B) The central zone of the tumor is ulcerated with monotonous small- to medium-sized lymphocytic infiltration in the mucosa and submucosa. The adjacent mucosa shows mild villous atrophy and crypt hyperplasia (C) and extensive intraepithelial lymphocytosis (D).
Fig. 3. Immunohistochemical stains. Tumor cells are positive for CD3 (A), TIA-1 (B), and CD8 (C), but negative for CD56 (D). (E) In situ hybridization for Epstein-Barr virus (EBV)–encoded RNA (EBER) shows diffuse nuclear positivity in tumor cells. (F) Dual staining for EBER (black) and CD3 (brown) reveals that the EBV-positive cells are CD3-positive T-cells.
Fig. 1.
Fig. 2.
Fig. 3.
A Case of Type II Enteropathy-Associated T-Cell Lymphoma with Epstein-Barr Virus Positivity
| Reference | Country | No. of cases | No. of EBV-posi- tive cases | EBV-positive cell population |
|---|---|---|---|---|
| Tse et al. (2012) [6] | Multinational (Asia) | 34 | 3 | Cytotoxic T cells |
| Ng (2012) [7] | Singapore | 1 | 1 | CD3+, CD4–, CD8–, CD56– tumor cells |
| Delabie et al. (2011) [1] | Multinational (North America, Europe, and Asia) | 58 | 4 | Subpopulation of likely reactive cells |
| Takeshita et al. (2011) [8] | Japan | 24 | 2 | CD56–, CD8– T cells |
| 1 | CD56+, CD8+ T cells | |||
| Quintanilla-Martinez et al. (1997) [9] | Multinational (Mexico, Austria, and Germany) | 6 | 1 | Pleomorphic medium and large cells |
| Pan et al. (1993) [10] | UK | 11 | 3 | Large CD3+ tumor cells |
Table 1. Summary of previously published reports including a patient with EBV-positive EATL
EBV, Epstein-Barr virus; EATL, enteropathy-associated T-cell lymphoma.

 E-submission
E-submission