Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(6); 2014 > Article
-
Brief Case Report
Digital Papillary Carcinoma - Sharon Lim, Inju Cho, Mi Ja Lee,
-
Korean Journal of Pathology 2014;48(6):438-441.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.6.438
Published online: December 31, 2014
Department of Pathology, Chosun University School of Medicine, Gwangju, Korea
- Corresponding Author: Mi Ja Lee, M.D. Department of Pathology, Chosun University School of Medicine, 309 Pilmun-daero, Dong-gu, Gwangju 501-759, Korea Tel: +82-62-230-6345, Fax: +82-62-234-4584, E-mail: mjblee@chosun.ac.kr
• Received: June 17, 2014 • Revised: July 15, 2014 • Accepted: July 16, 2014
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
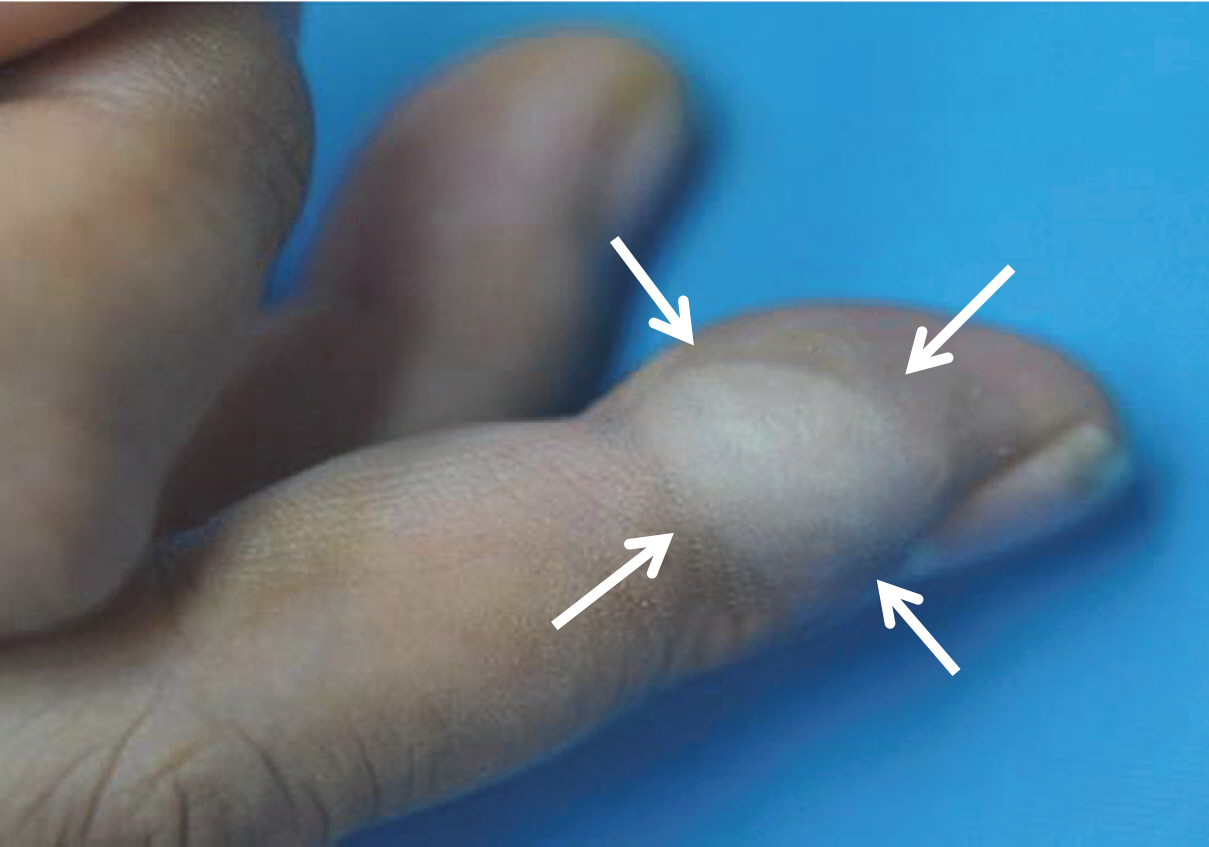
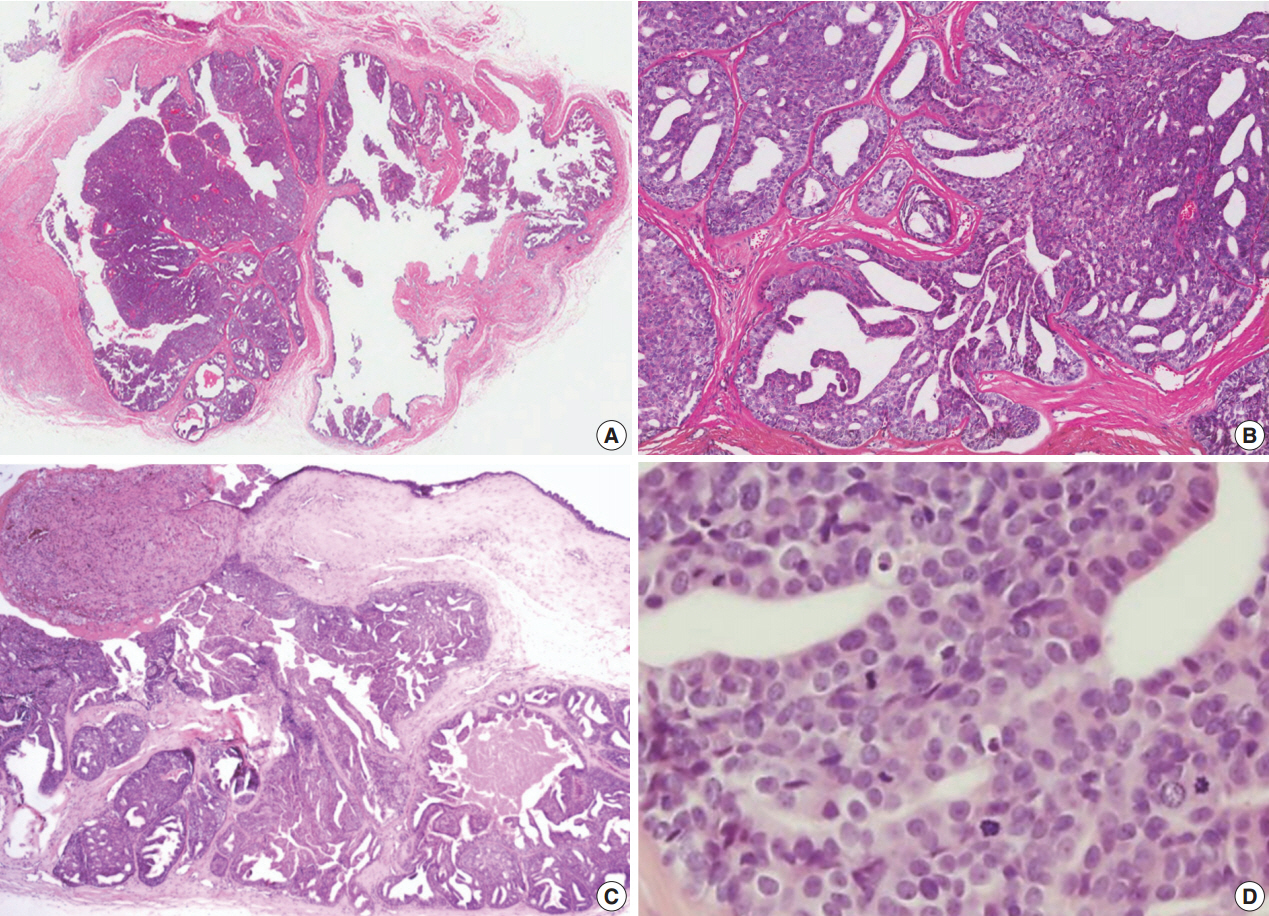
- A 39-year-old male had a small, nontender, painless nodule in the right third finger which had lasted for 20 years, but had increased in size (1.8×1.5 cm) and became extremely painful in the last 7 months (Fig. 1). Complete surgical resection of the mass was performed and there was no evidence of recurrence or metastasis over a period of 2.5 years. Grossly, the cystic nodule had a white-to-yellow colored surface and a well-defined margin. Microscopic findings (Fig. 2) of the tumor revealed a solid portion and cystic area with papillary projection into the cystic lumen with eosinophilic secretory materials. The tumor cells were polygonal and showed gland-like structure with mild atypia. The mitotic rate was high (2–7/high-power field [HPF]) and there was no necrosis. The nucleus showed dispersed chromatin and vesicular nuclei with small but prominent nucleoli. Immunohistochemistry (Fig. 3) revealed two distinct cell layers with a pronounced basal layer and luminal cells. p63 and smooth muscle actin were expressed in the basal layer, and S-100 protein and carcinoembryonic antigen were stained in luminal cells. The p53 activity expression and Ki-67 index were about 30%, but epithelial membrane antigen did not react with both basal and luminal cells.
CASE REPORT
- DPC is an uncommon malignant adnexal neoplasm that was first classified by the WHO as a skin tumor in 2006 [4]. The condition was documented and established from 1979 [1] until 1987 [2], however, no large-scale study has been conducted due to the rarity of this condition. In the Republic of Korea, only one case report was published in a domestic journal [5], and to our knowledge this was the first Korean DPC case reported in the English literature.
- Clinically, DPC presents as solitary non-tender masses that arise from fingers and toes and/or adjacent skin of the palms and soles. The distal portion of a finger or thumb is frequently documented as a DPC location. Clinical diagnoses included cysts, vascular lesions, and giant cell tumors. Lesions may range in size from 10 to 40 mm (mean, 20 mm), with variable duration [6].
- Histopathological findings include a well-circumscribed or infiltrative growth pattern and tubuloalveolar ductal structures with areas of papillary projection that protrude into the cystic lumen. The papillary projections are associated with fibrovascular cores in some foci. Lesions have solid and cystic components with closely aggregated back-to-back glandular architectures lined by cuboidal or columnar epithelia that are surrounded by a basal myoepithelial cell layer. Cytologic atypia or mitotic counts have been observed but are variable. Cysts contain either necrotic debris or eosinophilic secretory material.
- Historically, this tumor has also been designated as ADPA or ADPAca. However, these designations are inadequate and limit understanding of the malignant potential of ADPAca. In 1987, Kao et al. [2] reviewed 57 cases of ADPA or ADPAca. They defined malignancy criteria: poor glandular differentiation, necrosis, cytologic atypia, high mitotic rate (3–6/HPF), and invasion of soft tissue, bone, and blood vessels. In addition they distinguished ADPAca from ADPA. However, regardless the histologic malignant potential, the tumor recurrence rate was reported to be 50% [2] and tumor metastasis was 14% with or without recurrence [3]. The histological differences between ADPA and ADPAca were not associated with clinical outcomes [3]. The degree of tubule formation, nuclear grade or atypia, mitotic rate, circumscription, and necrosis were not helpful in differentiating tumors that behaved more aggressively [3,6]. Therefore it is suggested that all aggressive digital papillary tumors be designated as ADPAca.
- A series of 31 cases of DPC was published by Suchak et al. [6] based on reports from three different institutions. Thirty-one cases were reviewed by at least two authors that identified several histologic features. However, these histopathologic features were not correlated with malignant potential. Cases with minimal atypia that may have previously been classified as ADPA did develop into metastatic disease. Conversely, some cases with histologic features suggestive of malignancy had a benign course. Similarly, clinical parameters such as age at diagnosis, duration of tumor or tumor size, were not correlated with the aggressive behavior of DPC [3]. Based on the report by Duke et al. [3], we hypothesize that histologic features are not reliable parameters for predicting high-grade malignant behavior.
- In the WHO classification, ADPA and ADPAca were changed to DPC without any benign counterpart [4]. But, DPC does not always exhibit an aggressive malignant behavior. Our case had an extremely long duration of 20 years and the patient did not experience local recurrence or distant metastasis within the follow-up period. Similarly, we reviewed another case in a domestic journal [5]. The case was about a patient with a 15-year history of a painless mass before the size changed over the past several months; additionally, the patient did not experience local recurrence or distant metastasis for over 2 years [5]. We suggest that these cases are DPC with a low-grade malignant potential. Although DPC was formerly classified as adenoma or adenocarcinoma, there were no clinical or histologic characteristics that can differentiate the tumors that are likely to locally recur or distantly metastasize. According to the Armed Forces Institute of Pathology (AFIP) [7], DPC are divided with two low- and highgrade malignant potential groups.
- Although DPC is considered to be a malignant tumor, it is not recommended to perform a vigorous surgical excision-involving amputation for all patients who have had a lesion that has not changed for many years. Hsu et al. [8] found that p53, Ki-67, and p63 could be useful in therapeutic decision-making, and wide excision should be considered rather than amputation for patients who had a long-standing history without evidence of bone invasion and with low intensity Ki-67. Expression of p63 seemed to be a valuable basal marker and Ki-67, a nuclear proliferation marker, has shown to have prognostic value. Parsa et al.[9] explained that double staining in p63 and Ki-67 revealed an active proliferation phase of tumor cells. However, the prognostic value of p53, p63, and Ki-67 in ADPAca was not identified completely. Further evaluation and analysis are required to estimate recurrence or metastatic risk in DPC. Therefore, complete surgical excision with or without lymph node dissection should be performed immediately after pathologic diagnosis [10].
- Our patient had a 20-year history of a painless mass before the size changed over the last 7 months. The overall course of disease was unclear due to the absence of previous biopsy in an earlier disease stage. Over 2.5 years after the complete excision, no recurrence or metastasis was found. However, this lesion had typical histologic features of DPC with high mitotic activity and a high proliferation index. Therefore, meticulous follow-up to evaluate for signs of recurrence and metastasis is necessary for the patient’s health.
DISCUSSION
Acknowledgments
Fig. 1.A 1.8×1.5 cm-sized round protruding mass is presented at the lateral side of the right third finger.


Fig. 2.(A) The lesion shows a multilobular tumor with solid and papillary or micropapillary projections into the cystic area. Solid portion of tumor consists of glandular structure and papillary pattern (B) with eosinophilic secretory materials (C) and shows mild atypia with frequent mitosis (D).


Fig. 3.The basal cells of the tumor react positively for p63 (A), whereas luminal cells react for CEA (B). The tumor cells stain diffusely for p53 (C) and Ki-67 (D).


- 1. Helwig EB. Aggressive digital papillary adenoma. American Academy of Dermatology and Clinical Pathology Conference; 1979 Dec 5; Chicago, IL, USA.
- 2. Kao GF, Helwig EB, Graham JH. Aggressive digital papillary adenoma and adenocarcinoma: a clinicopathological study of 57 patients, with histochemical, immunopathological, and ultrastructural observations. J Cutan Pathol 1987; 14: 129-46. ArticlePubMed
- 3. Duke WH, Sherrod TT, Lupton GP. Aggressive digital papillary adenocarcinoma (aggressive digital papillary adenoma and adenocarcinoma revisited). Am J Surg Pathol 2000; 24: 775-84. PubMed
- 4. LeBoit PE, Burg G, Weedon D, Sarasin A. World Health Organization classification of tumours: pathology and genetics of skin tumours. Lyon: IARC Press, 2006; 133-4.
- 5. Heo EP, Kim CY, Oh CW. A case of aggressive digital papillary adenoma. Korean J Dermatol 2002; 40: 811-5.
- 6. Suchak R, Wang WL, Prieto VG, et al. Cutaneous digital papillary adenocarcinoma: a clinicopathologic study of 31 cases of a rare neoplasm with new observations. Am J Surg Pathol 2012; 36: 1883-91. PubMed
- 7. Patterson JW, Wick MR. Atlas of tumor pathology fourth series: nonmelanocytic tumors of the skin. AFIP atlas of tumor pathology, series 4. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology, 2006; 157-8.
- 8. Hsu HC, Ho CY, Chen CH, Yang CH, Hong HS, Chuang CH. Aggressive digital papillary adenocarcinoma: a review. Clin Exp Dermatol 2010; 35: 113-9. ArticlePubMed
- 9. Parsa R, Yang A, McKeon F, Green H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol 1999; 113: 1099-105. ArticlePubMed
- 10. Horii T, Sekiya H, Takatoku K, Hoshino Y. Aggressive digital papillary adenocarcinoma 5 years after surgical resection of adenoma on index finger. Eur J Orthop Surg Traumatol 2010; 20: 397-9. ArticlePDF
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Digital Papillary Adenocarcinoma: Uncommon Malignancy of Sweat Glands - Two Rare Cases
Neeti Goyal, Pawan Dhaman, Jasvinder Kaur Bhatia, Pragya Sharma, Prabha Shankar Mishra, Vikram Singh, Anvesh Rathore
Journal of Marine Medical Society.2025; 27(1): 103. CrossRef - Digital papillary adenocarcinoma: A case report of a rare malignant tumour with recommendations on management and follow-up
Varanindu Mudduwa, Mohammad Goodarzi, Richard Chalmers, Haitham Khashaba
International Journal of Surgery Case Reports.2025; 127: 110922. CrossRef - Digital papillary adenocarcinoma: A case report
Betty A. Kasimo, Vivian Akello, James J. Yahaya
Clinical Case Reports.2021;[Epub] CrossRef - A rare case of a digital papillary carcinoma of the hand with secondary conservative management
Rabeet Khan, Renu Irri, Effie Katsarma
Journal of Surgical Case Reports.2020;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Digital Papillary Carcinoma



Fig. 1. A 1.8×1.5 cm-sized round protruding mass is presented at the lateral side of the right third finger.
Fig. 2. (A) The lesion shows a multilobular tumor with solid and papillary or micropapillary projections into the cystic area. Solid portion of tumor consists of glandular structure and papillary pattern (B) with eosinophilic secretory materials (C) and shows mild atypia with frequent mitosis (D).
Fig. 3. The basal cells of the tumor react positively for p63 (A), whereas luminal cells react for CEA (B). The tumor cells stain diffusely for p53 (C) and Ki-67 (D).
Fig. 1.
Fig. 2.
Fig. 3.
Digital Papillary Carcinoma

 E-submission
E-submission