Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(1); 2015 > Article
-
Original Article
Transglutaminase 2 Expression and Its Prognostic Significance in Clear Cell Renal Cell Carcinoma - Min Jee Park1, Hae Woon Baek1, Ye-Young Rhee1, Cheol Lee1, Jeong Whan Park1, Hwal Woong Kim2, Kyung Chul Moon,1,3
-
Journal of Pathology and Translational Medicine 2015;49(1):37-43.
DOI: https://doi.org/10.4132/jptm.2014.10.25
Published online: January 15, 2015
1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
2Department of Pathology, Good Moonhwa Hospital, Busan, Korea
3Kidney Research Institute, Medical Research Center, Seoul National University College of Medicine, Seoul, Korea
- Corresponding Author: Kyung Chul Moon, M.D. Department of Pathology, Kidney Research Institute, Medical Research Center, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 110-799, Korea Tel: +82-2-2072-1767 Fax: +82-2-743-5530 E-mail: blue7270@snu.ac.kr
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background:
- A few recent studies have demonstrated a possible role of transglutaminase 2 (TG2) in tumorigenesis or progression of renal cell carcinoma (RCC). The aim of this study was to examine TG2 expression and its clinicopathologic significance in a large number of human clear cell RCCs (CCRCCs).
-
Methods:
- We analyzed 638 CCRCC patients who underwent partial or radical nephrectomy between 1995 and 2005. The expression of TG2 was determined by immunohistochemistry and categorized into four groups, according to staining intensity: negative (0), mild (1+), moderate (2+), and strong (3+).
-
Results:
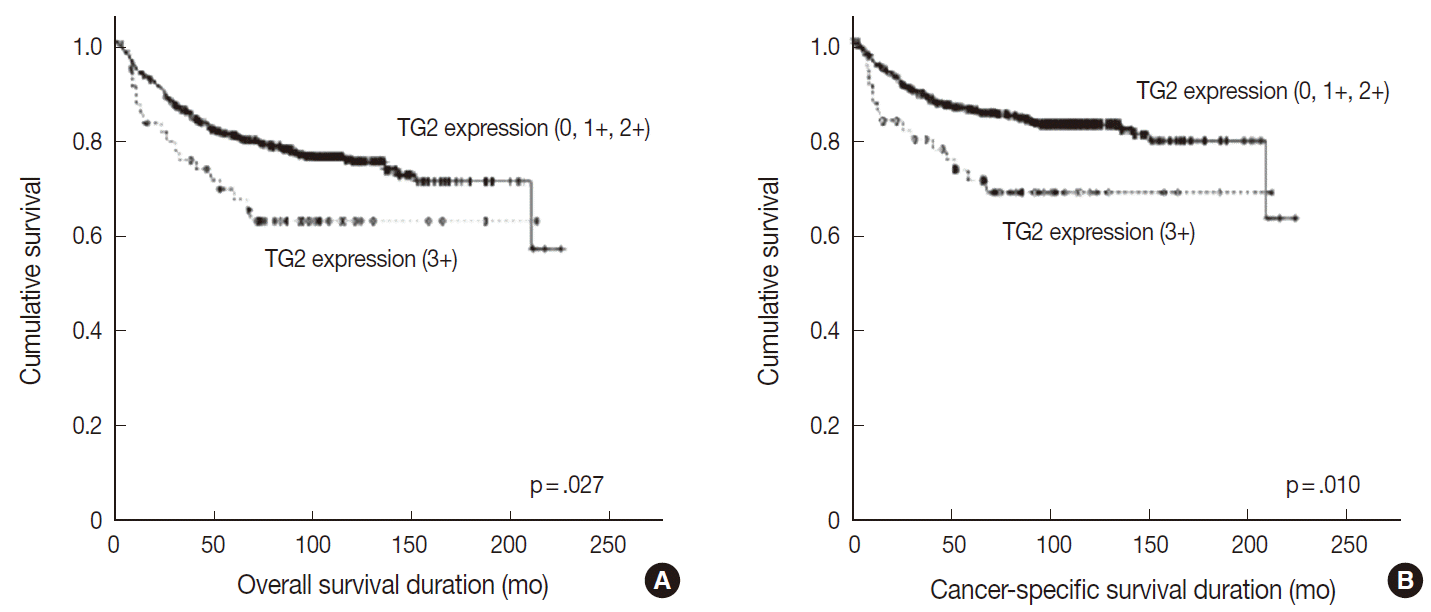
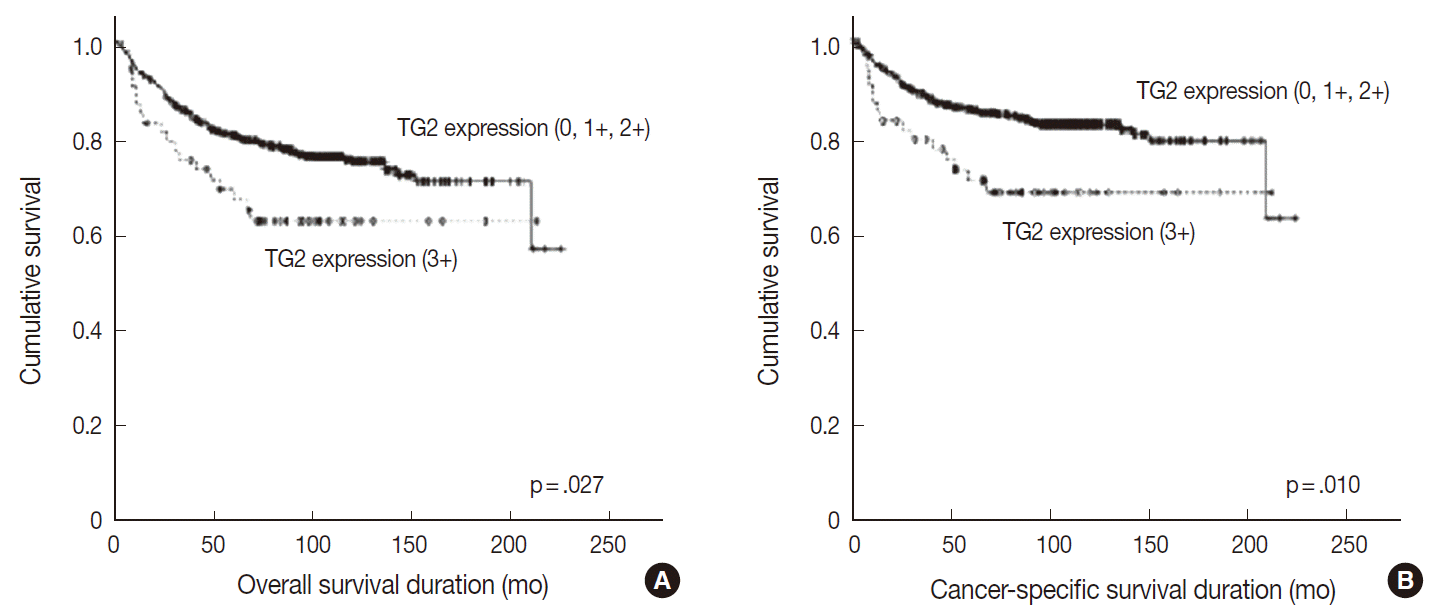
- TG2 staining intensity was negative in 8.5% of CCRCC (n=54), 1+ in 32.6% (n=208), 2+ in 50.5% (n=322), and 3+ in 8.5% (n=54). Strong TG2 expression was correlated with high Fuhrman nuclear grade (p=.011), high T category (p=.049), metastasis (p=.043) and male sex (p<.001) but not with N category.The survival analysis showed a significant association between strong TG2 expression and worse overall and cancer-specific survival (p=.027 and p=.010, respectively). On multivariate analysis, strong TG2 expression was a marginally significant prognostic indicator for Fuhrman nuclear grade and TNM staging (p=.054).
-
Conclusions:
- Our study is the first to demonstrate the clinicopathologic significance of TG2 expression in a large number of human CCRCC samples. Strong TG2 expression was associated with high nuclear grade and poor prognosis.
- Patients and tumor specimens
- We analyzed samples from 638 CCRCC patients who had undergone partial or radical nephrectomy at Seoul National University Hospital in Seoul, Korea between 1995 and 2005. The Seoul National University Hospital Institutional Review Board approved this study. All the clinicopathologic data were obtained from medical records, pathology reports and the review of hematoxylin and eosin (H&E)–stained slides. H&E slides were reviewed for tumor staging and nuclear grade. The tumor stage was determined according to the pTNM staging guidline published by the 2010 American Joint Committee on Cancer (AJCC) [25], and nuclear grading was performed based on the Fuhrman nuclear grading scale [26]. The recurrence or metastasis of CCRCC was determined by the clinical, radiological and pathological parameters. Confirmation of patients’ deaths was obtained from medical records or death certificates.
- Tissue microarray and immunohistochemistry
- Following a review of the tumor slides, a representative area from each tumor block was selected and utilized to construct tissue microarrays (TMA) using a trephine apparatus (Superbiochips Laboratories, Seoul, Korea).
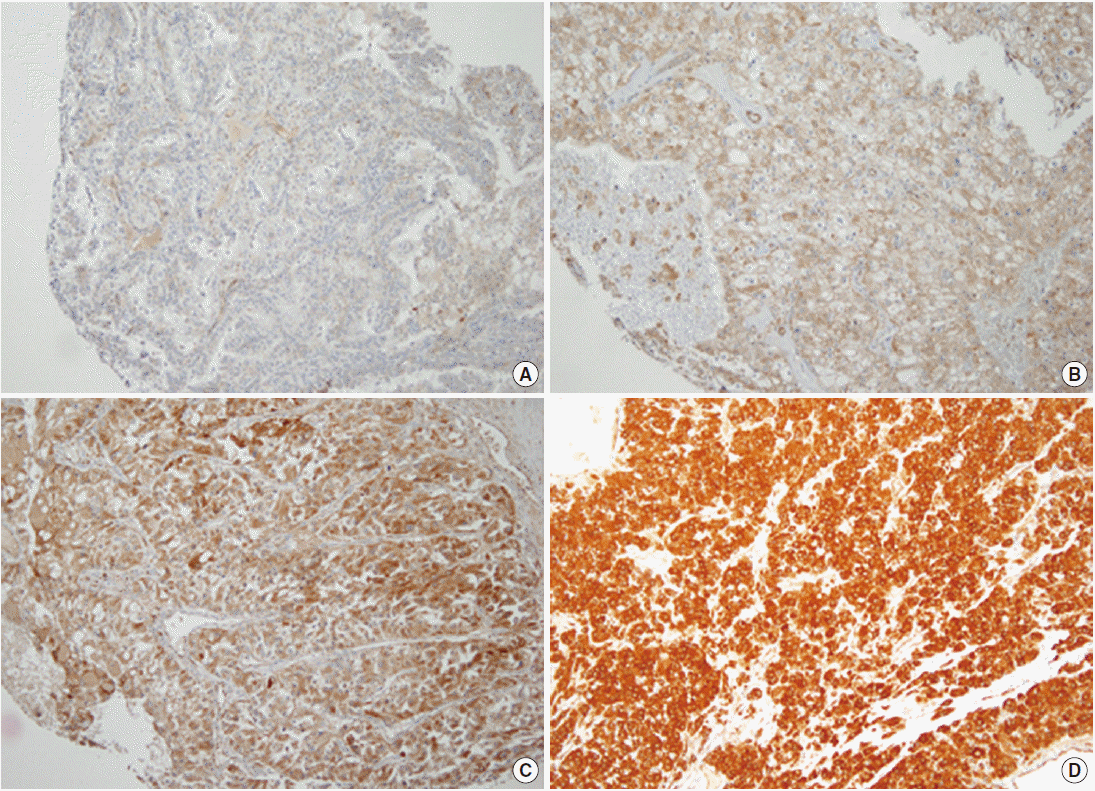
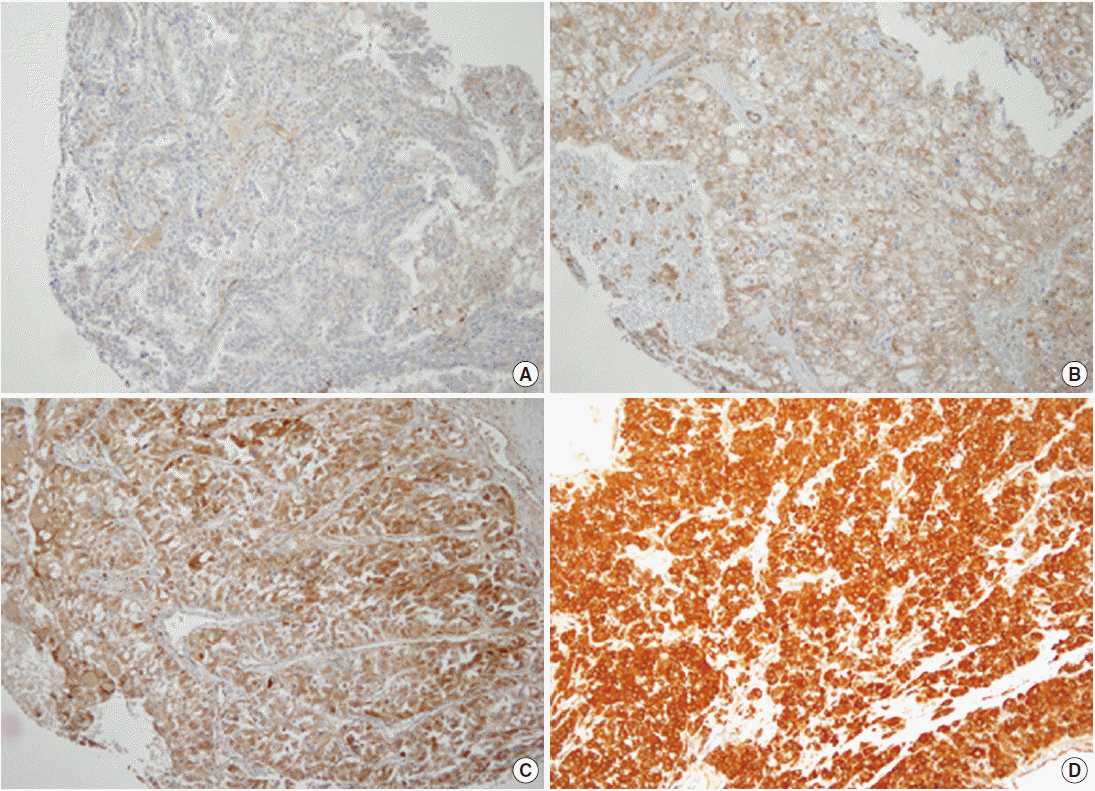
- Immunohistochemical staining was performed on 4-μm-thick sections taken from the TMA slides using the Bond-Max Autostainer (Leica Microsystems, Bannockburn, IL, USA). Polyclonal rabbit anti-TG2 antibody (Neomarkers, Fremont, CA, USA) was diluted 1:100. After heat-induced antigen retrieval, primary antibodies were incubated with the samples for 15 minutes. The binding of the primary antibody was detected using the Bond Polymer Refine Detection kit (Leica Microsystems) according to the manufacturer’s instructions. TG2 immunoreactivity was classified into four categories according to staining intensity: negative (0), weak (1+), moderate (2+), and strong (3+) expression. Constant TG2 staining on endothelial cells was regarded as an internal positive control.
- Statistical analysis
- SPSS ver. 21 (IBM Co., Armonk, NY, USA) was used for statistical analysis. The association between clinicopathologic findings and TG expression was analyzed using the linear by linear association. Overall survival and cancer-specific survival were analyzed using the Kaplan-Meier method supported by the logrank test. The overall survival was defined as the time interval from primary radical or partial nephrectomy to last follow-up or patients’ death. The cancer-specific survival was defined as the time interval from primary radical or partial nephrectomy to last follow-up or cancer-related death and was recorded at final follow-up visit as alive or dead from an unrelated cause. The Cox regression model was used for multivariate analysis. All statistical analyses were 2-tailed, and a p<.05 was regarded as statistically significant.
MATERIALS AND METHODS
- Clinicopathologic characteristics
- The study population included 473 men and 165 women. The ages of the patient population ranged from 24 to 84 years with a mean age of 56.0 years (mean±SD, 56.02±11.53 years). The measured tumors ranged in size from 0.8 to 22 cm with a mean size of 5.6 cm (mean±SD, 5.63±3.40 cm). Patient follow-up times ranged from 2 to 223 months with an average follow-up period of 85.7 months (mean±SD, 85.71±48.46 months). At the time of surgery, lymph node metastases were found in 18 cases (2.8%), and distant metastases were found in 53 cases (8.3%). Four hundred and eight cases were classified as pTNM stage I (63.6%), 82 as stage II (12.8%), 91 as stage III (14.2%), and 57 as stage IV (8.9%). According to Fuhrman nuclear grade, 49 cases were classified as grade 1 (7.7%), 291 as grade 2 (45.6%), 235 as grade 3 (36.8%), and 63 as grade 4 (9.9%).
- TG2 expression in CCRCC
- In the non-neoplastic renal parenchyme, TG2 was faintly expressed, only focally. In CCRCC, TG2 was diffusely expressed in the cytoplasm of tumor cells in most cases (positive in 584 CCRCCs, 91.5%), but the staining intensity was variable. TG2 expression showed a cytoplasmic or membranous pattern. The evaluation of the staining intensity revealed that 54 (8.5%) were negative, 208 showed weak TG2 expression (32.6%), 322 showed moderate TG2 expression (50.5%), and 54 (8.5%) showed strong TG2 expression (Fig. 1).
- The relationship between TG2 expression and pathologic parameters is shown in Table 1. Strong TG2 expression was correlated with high Fuhrman nuclear grade (p=.011), high T category (p=.049), metastasis (p=.043) and male sex (p<.001) but not with N category.
- TG2 expression and survival analysis
- Overall and cancer-specific survival times were not significantly different between groups showing TG2 staining intensities of 0, 1+, and 2+ (p=.872 and p=.816, respectively). In contrast, the strong (3+) TG2 expression grouphad significantly shorter overall and cancer specific survival periods compared with groups showing staining intensities 0, 1+, or 2+ (p=.027 and p=.010, respectively) (Table 2, Fig. 2). Upon multivariate analysis, strong TG2 expression was shown to be a marginal independent prognostic indicator of cancer-specific survival for Fuhrman nuclear grade and TNM staging (p=.054). However, these analyses were not statistically significant for overall survival (p=.154) (Table 3).
RESULTS
- In this study, we investigated the expression level and clinicopathologic significance of TG2 in CCRCC. TG2 was diffusely expressed in most CCRCCs (584/638, 91.5%). Strong TG2 expression in CCRCC was associated with high Fuhrman nuclear grade, but was not associated with TNM stage. In addition, strong expression of TG2 was associated with significantly worse cancer-specific survival in univariate analysis. A multivariate analysis also showed a marginally significant decrease in cancer-specific survival. To our knowledge, this is the first study to elucidate the prognostic significance of TG2 expression in CCRCC.
- Previous studies using cancer cell lines or nude mice have shown the role of TG2 in cancer cell growth, survival and metastasis [13,16,18]. In addition, there have been a few studies showing the clinicopathologic significance of TG2 expression in human cancer tissues. Overexpression of TG2 was associated with poor prognosis in ovarian cancer [14], and high TG2 expression was associated with nodal metastasis and lymphovascular invasion in pancreatic ductal adenocarcinoma [15]. It has also been suggested that TG2 contributes to cancer progression and metastasis by regulating cancer cell migration into the ECM, adhesion to endothelium, invasion, and angiogenesis [20].
- A few previous studies have described the relationship between TG2 and the VHL gene, a well-known gene altered in most CCRCCs [27]. Wykoff et al. [23] showed that TG2 is a novel target gene of VHL in RCC cell lines. Another study demonstrated that TG2 is a critical regulator of VHL [24]. These studies suggest a close relationship between TG2 and CCRCC. In addition to VHL, p53 was also depleted by TG2 in many RCC cell lines [28] A recent study examining microRNA expression in RCC demonstrated that miR-1285, one of the microRNAs that are reduced in RCC specimens and cell lines, inhibited cancer cell proliferation, invasion and migration, as well as directly regulating TG2 expression [21]. Thus, previous studies indicate that TG2 may play a role in pathogenesis or progression of RCC. However, only few studies examined TG2 expression in human RCC samples. Erdem et al. [22] investigated TG2 mRNA expression in 95 primary RCC samples and found that TG2 expression alone was not associated with RCC subtype, nuclear grade, T classification or tumor size. Another study examined TG2 expression by immunohistochemistry in 70 RCC samples, finding an increased TG2 expression in RCC tissue compared to normal kidney tissue and a significant correlation between increased TG2 expression and high T classification [21]. Although these two previous studies showed the significance of TG2 expression in human RCC, in part, they also had some limitations such as relatively small sample sizes and no evaluation of the prognostic significance of TG2 expression.
- We showed the clinicopathologic significance and prognostic implications of TG2 expression in a large number of CCRCCs. The majority of CCRCCs showed diffuse TG2 staining, but the staining intensity was variable. Only a small subset of CCRCCs showed strong TG2 expression (54/638, 6.5%) that correlated with significantly worse prognosis. The remaining cases showing negative to moderate TG2 expression revealed no significant association of TG2 staining intensity with prognosis.
- Although the precise role of TG2 in the progression of CCRCC is not clear, previous studies have suggested some possible mechanisms. First, TG2 can regulate cell adhesion. Overexpression of TG2 in breast cancer cell lines has been reported to contribute to cancer cell invasion and metastasis through the interaction of TG2 with ECM components such as β integrins and fibronectin [13,29].
- In primary RCC samples, increased expression of TG2, along with β1 integrin and syndecan-4, was reported to be associated with metastasis [22]. Second, TG2 has been suggested to regulate apoptosis. Inhibition of TG2 was found to stabilize p53 expression, increasing apoptosis in RCC cell lines [28]. These results suggest that TG2 might enhance metastasis and tumor cell survival.
- Our study also demonstrated that high TG2 expression is associated with tumor aggressiveness such as T category and metastasis. Our results are similar to those of previous studies showing an association between TG2 expression and metastasis or tumor stage of RCC [21,22]. However, there were some differences between these previous studies and our study. First, the study sample was smaller than that of our study (70 and 95 in previous studies versus 638 in our study). Second, they did not separate the RCC subtypes, but we included CCRCC only.
- In addition our study showed that strong TG2 expression was related to high nuclear grade of CCRCC. This result suggests that TG2 expression might be related to the differentiation of CCRCC.
- In conclusion, our study is the first to demonstrate the clinicopathologic significance of TG2 expression in a large number of human CCRCC samples. Strong TG2 expression was related to tumor aggressiveness, high nuclear grade and poor prognosis.
DISCUSSION
Acknowledgments
- 1. Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol 2011; 60: 615-21. ArticlePubMed
- 2. Ljungberg B, Hanbury DC, Kuczyk MA, et al. Renal cell carcinoma guideline. Eur Urol 2007; 51: 1502-10. ArticlePubMed
- 3. Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr Urol Rep 2005; 6: 7-18. ArticlePubMedPDF
- 4. Ljungberg B. Prognostic markers in renal cell carcinoma. Curr Opin Urol 2007; 17: 303-8. ArticlePubMed
- 5. Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 2003; 4: 140-56. ArticlePubMedPDF
- 6. Greenberg CS, Birckbichler PJ, Rice RH. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J 1991; 5: 3071-7. ArticlePubMedPDF
- 7. Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids 2009; 36: 659-70. ArticlePubMedPDF
- 8. Gaudry CA, Verderio E, Aeschlimann D, Cox A, Smith C, Griffin M. Cell surface localization of tissue transglutaminase is dependent on a fibronectin-binding site in its N-terminal beta-sandwich domain. J Biol Chem 1999; 274: 30707-14. PubMed
- 9. Gaudry CA, Verderio E, Jones RA, Smith C, Griffin M. Tissue transglutaminase is an important player at the surface of human endothelial cells: evidence for its externalization and its colocalization with the beta(1) integrin. Exp Cell Res 1999; 252: 104-13. PubMed
- 10. Belkin AM. Extracellular TG2: emerging functions and regulation. Febs J 2011; 278: 4704-16. ArticlePubMedPMC
- 11. Wang Z, Griffin M. TG2, a novel extracellular protein with multiple functions. Amino Acids 2012; 42: 939-49. ArticlePubMedPDF
- 12. Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res 2004; 10: 8068-76. ArticlePubMedPDF
- 13. Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene 2007; 26: 2459-70. ArticlePubMedPDF
- 14. Hwang JY, Mangala LS, Fok JY, et al. Clinical and biological significance of tissue transglutaminase in ovarian carcinoma. Cancer Res 2008; 68: 5849-58. ArticlePubMedPMCPDF
- 15. Verma A, Wang H, Manavathi B, et al. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res 2006; 66: 10525-33. ArticlePubMedPDF
- 16. Verma A, Guha S, Diagaradjane P, et al. Therapeutic significance of elevated tissue transglutaminase expression in pancreatic cancer. Clin Cancer Res 2008; 14: 2476-83. ArticlePubMedPDF
- 17. Park KS, Kim HK, Lee JH, et al. Transglutaminase 2 as a cisplatin resistance marker in non-small cell lung cancer. J Cancer Res Clin Oncol 2010; 136: 493-502. ArticlePubMedPDF
- 18. Fok JY, Ekmekcioglu S, Mehta K, et al. Implications of tissue transglutaminase expression in malignant melanoma. Mol Cancer Ther 2006; 5: 1493-503. ArticlePubMedPDF
- 19. Kumar S, Mehta K. Tissue transglutaminase, inflammation, and cancer: how intimate is the relationship? Amino Acids 2013; 44: 81-8. ArticlePubMedPDF
- 20. Lentini A, Abbruzzese A, Provenzano B, Tabolacci C, Beninati S. Transglutaminases: key regulators of cancer metastasis. Amino Acids 2013; 44: 25-32. ArticlePubMedPDF
- 21. Hidaka H, Seki N, Yoshino H, et al. Tumor suppressive microRNA-1285 regulates novel molecular targets: aberrant expression and functional significance in renal cell carcinoma. Oncotarget 2012; 3: 44-57. ArticlePubMedPMC
- 22. Erdem M, Erdem S, Sanli O, et al. Up-regulation of TGM2 with ITGB1 and SDC4 is important in the development and metastasis of renal cell carcinoma. Urol Oncol 2014; 32: 25.e13-20. ArticlePubMed
- 23. Wykoff CC, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Identification of novel hypoxia dependent and independent target genes of the von Hippel-Lindau (VHL) tumour suppressor by mRNA differential expression profiling. Oncogene 2000; 19: 6297-305. ArticlePubMedPDF
- 24. Kim DS, Choi YB, Han BG, et al. Cancer cells promote survival through depletion of the von Hippel-Lindau tumor suppressor by protein crosslinking. Oncogene 2011; 30: 4780-90. ArticlePubMedPDF
- 25. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer, 2010.
- 26. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982; 6: 655-63. ArticlePubMed
- 27. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press, 2004.
- 28. Ku BM, Kim DS, Kim KH, et al. Transglutaminase 2 inhibition found to induce p53 mediated apoptosis in renal cell carcinoma. FASEB J 2013; 27: 3487-95. ArticlePubMedPDF
- 29. Herman JF, Mangala LS, Mehta K. Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene 2006; 25: 3049-58. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Sex-mediated effects of transglutaminase 2 inhibition on endothelial function in human resistance arteries from diabetic and non-diabetic patients
Khatera Saii, Judit Prat-Duran, Ulf Simonsen, Anders Riegels Knudsen, Jonas Amstrup Funder, Niels Henrik Buus, Estéfano Pinilla
Clinical Science.2025; 139(1): 1. CrossRef - Discovery of novel 1H-benzo[d]imidazole-4,7-dione based transglutaminase 2 inhibitors as p53 stabilizing anticancer agents in renal cell carcinoma
Ga-Ram Kim, Joon Hee Kang, Hyeon Joo Kim, Eunji Im, Jinsu Bae, Woo Sun Kwon, Sun Young Rha, Hyun Cheol Chung, Eun Yi Cho, Soo-Youl Kim, Yong-Chul Kim
Bioorganic Chemistry.2024; 143: 107061. CrossRef - Transglutaminase 2 is associated with adverse colorectal cancer survival and represents a therapeutic target
Patrizia Malkomes, Ilaria Lunger, Elsie Oppermann, Johannes Lorenz, Sara Fatima Faqar-Uz-Zaman, Jiaoyan Han, Sabrina Bothur, Paul Ziegler, Katrin Bankov, Peter Wild, Wolf Otto Bechstein, Michael A. Rieger
Cancer Gene Therapy.2023; 30(10): 1346. CrossRef - Transglutaminase Type 2-MITF axis regulates phenotype switching in skin cutaneous melanoma
Silvia Muccioli, Valentina Brillo, Tatiana Varanita, Federica Rossin, Elisabetta Zaltron, Angelo Velle, Giorgia Alessio, Beatrice Angi, Filippo Severin, Anna Tosi, Manuela D’Eletto, Luca Occhigrossi, Laura Falasca, Vanessa Checchetto, Roberto Ciaccio, Ame
Cell Death & Disease.2023;[Epub] CrossRef - The role of transglutaminase 2 in regulation of the balance between autophagy and apoptosis in tumor cells
Yu. A. Gnennaya, O. M. Semenov, N. A. Barlev
Advances in Molecular Oncology.2023; 10(4): 31. CrossRef - Application of a Fluorescence Anisotropy-Based Assay to Quantify Transglutaminase 2 Activity in Cell Lysates
Sandra Hauser, Paul Sommerfeld, Johanna Wodtke, Christoph Hauser, Paul Schlitterlau, Jens Pietzsch, Reik Löser, Markus Pietsch, Robert Wodtke
International Journal of Molecular Sciences.2022; 23(9): 4475. CrossRef - The Biological and Biomechanical Role of Transglutaminase-2 in the Tumour Microenvironment
Robert Tempest, Sonia Guarnerio, Rawan Maani, Jamie Cooper, Nicholas Peake
Cancers.2021; 13(11): 2788. CrossRef - A Precision Strategy to Cure Renal Cell Carcinoma by Targeting Transglutaminase 2
Soo-Youl Kim, Jeffrey W. Keillor
International Journal of Molecular Sciences.2020; 21(7): 2493. CrossRef - Evaluation of nuclear NF-κB, transglutaminase2, and ERCC1 as predictors of platinum resistance in testicular tumors
Alan A. Azambuja, Paula Engroff, Bruna T. Silva, Roberta C. S. Zorzetti, Fernanda B. Morrone
International braz j urol.2020; 46(3): 353. CrossRef - Transglutaminase 2-Mediated p53 Depletion Promotes Angiogenesis by Increasing HIF-1α-p300 Binding in Renal Cell Carcinoma
Seon-Hyeong Lee, Joon Hee Kang, Ji Sun Ha, Jae-Seon Lee, Su-Jin Oh, Hyun-Jung Choi, Jaewhan Song, Soo-Youl Kim
International Journal of Molecular Sciences.2020; 21(14): 5042. CrossRef - Role of Tissue Transglutaminase Catalytic and Guanosine Triphosphate-Binding Domains in Renal Cell Carcinoma Progression
Burge Ulukan, Ajna Bihorac, Tarik Sipahioglu, Robert Kiraly, Laszlo Fesus, Dilek Telci
ACS Omega.2020; 5(43): 28273. CrossRef - Transglutaminase 2: The Maestro of the Oncogenic Mediators in Renal Cell Carcinoma
Ayca Ece Nezir, Burge Ulukan, Dilek Telci
Medical Sciences.2019; 7(2): 24. CrossRef - Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target
Richard L. Eckert
Molecular Carcinogenesis.2019; 58(6): 837. CrossRef - Allosteric inhibition site of transglutaminase 2 is unveiled in the N terminus
Nayeon Kim, Joon Hee Kang, Won-Kyu Lee, Seul-Gi Kim, Jae-Seon Lee, Seon-Hyeong Lee, Jong Bae Park, Kyung-Hee Kim, Young-Dae Gong, Kwang Yeon Hwang, Soo-Youl Kim
Amino Acids.2018; 50(11): 1583. CrossRef - Renal Cell Carcinoma Is Abrogated by p53 Stabilization through Transglutaminase 2 Inhibition
Seon-Hyeong Lee, Won-Kyu Lee, Nayeon Kim, Joon Hee Kang, Kyung-Hee Kim, Seul-Gi Kim, Jae-Seon Lee, Soohyun Lee, Jongkook Lee, Jungnam Joo, Woo Sun Kwon, Sun Young Rha, Soo-Youl Kim
Cancers.2018; 10(11): 455. CrossRef - Tissue transglutaminase expression is necessary for adhesion, metastatic potential and cancer stemness of renal cell carcinoma
Yesim Bagatur, Ayca Zeynep Ilter Akulke, Ajna Bihorac, Merve Erdem, Dilek Telci
Cell Adhesion & Migration.2017; : 1. CrossRef - Characterization of clear cell renal cell carcinoma by gene expression profiling
Bryan J. Thibodeau, Matthew Fulton, Laura E. Fortier, Timothy J. Geddes, Barbara L. Pruetz, Samreen Ahmed, Amy Banes-Berceli, Ping L. Zhang, George D. Wilson, Jason Hafron
Urologic Oncology: Seminars and Original Investigations.2016; 34(4): 168.e1. CrossRef - Prognostic role of tissue transglutaminase 2 in colon carcinoma
María Jesús Fernández-Aceñero, Sofía Torres, Irene Garcia-Palmero, Cristina Díaz del Arco, J. Ignacio Casal
Virchows Archiv.2016; 469(6): 611. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| Characteristic | Cases (n=638) | TG2 expression |
p-value | |||
|---|---|---|---|---|---|---|
| 0 (n=54, 8.5%) | 1+ (n=208, 32.6%) | 2+ (n=322, 50.5%) | 3+ (n=54, 8.5%) | |||
| Sex | ||||||
| Male | 473 | 33 | 137 | 258 | 45 | <.001 |
| Female | 165 | 21 | 71 | 64 | 9 | |
| Age (yr) | ||||||
| ≤55 | 238 | 17 | 76 | 134 | 11 | .779 |
| >55 | 276 | 28 | 92 | 128 | 28 | |
| Furman grade | ||||||
| 1, 2 | 340 | 32 | 118 | 172 | 18 | .011 |
| 3, 4 | 298 | 22 | 90 | 150 | 36 | |
| T category | ||||||
| 1, 2 | 511 | 47 | 172 | 251 | 41 | .049 |
| 3, 4 | 127 | 7 | 36 | 71 | 13 | |
| N category | ||||||
| 0 or x | 620 | 53 | 201 | 316 | 50 | .453 |
| 1 | 18 | 1 | 7 | 6 | 4 | |
| M category | ||||||
| 0 | 585 | 53 | 194 | 289 | 49 | .043 |
| 1 | 53 | 1 | 14 | 33 | 5 | |
| Stage | ||||||
| I, II | 148 | 45 | 163 | 242 | 40 | .147 |
| III, IV | 9 | 45 | 80 | 14 | ||
| Lung metastasis | ||||||
| Absent | 518 | 47 | 172 | 262 | 37 | .031 |
| Present | 120 | 7 | 36 | 60 | 17 | |
| Bone metastasis | ||||||
| Absent | 588 | 51 | 190 | 298 | 49 | .767 |
| present | 50 | 3 | 18 | 24 | 5 | |
| Prognostic factor | Overall survival |
Cancer-specifi survival |
||
|---|---|---|---|---|
| Survival time (mean ± SE, mo) | p-value | Survival time (mean ± SE, mo) | p-value | |
| TG2 expression | ||||
| 0, 1, 2 | 171.4 ± 4.2 | .027 | 184.7 ± 4.0 | .010 |
| 3 | 143.1 ± 12.5 | 153.3 ± 12.1 | ||
| Nuclear grade | ||||
| 1, 2 | 193.7 ± 4.1 | <.001 | 209.0 ± 3.2 | <.001 |
| 3, 4 | 139.2 ± 5.2 | 149.2 ± 5.0 | ||
| TNM stage | ||||
| I, II | 186.5 ± 3.8 | <.001 | 201.2 ± 3.3 | <.001 |
| III, IV | 105.1 ± 8.7 | 111.6 ± 8.9 | ||
| Prognostic factor | Overall survival |
Cancer specific survival |
|||
|---|---|---|---|---|---|
| HR (95% Cl) | p-value | HR (95% Cl) | p-value | ||
| TG2 expression | 3 vs 0,1,2 | 0.665 (0.409–1.081) | .100 | 0.598 (0.350–1.020) | .059 |
| Histologic grade | 3,4 vs 1,2 | 0.472 (0.327–0.680) | <.001 | 0.295 (0.177–0.490) | <.001 |
| TNM stage | III, IV vs I, II | 0.190 (0.136–0.265) | <.001 | 0.107 (0.069–1.165) | <.001 |
CCRCC, clear cell renal cell carcinoma; TG2, transglutaminase 2.
CCRCC, clear cell renal cell carcinoma; SE: standard error, TG2, transglutaminase 2.
CCRCC, clear cell renal cell carcinoma; HR, hazard ratio; Cl, confidence interval; TG2, transglutaminase 2.

 E-submission
E-submission