Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(3); 2015 > Article
-
Case Study
Follicular Proliferative Lesion Arising in Struma Ovarii - Min Jee Park, Min A Kim, Mi Kyung Shin1, Hye Sook Min,2
-
Journal of Pathology and Translational Medicine 2015;49(3):262-266.
DOI: https://doi.org/10.4132/jptm.2015.03.26
Published online: May 15, 2015
Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
1Department of Pathology, Hallym University College of Medicine, Seoul, Korea
2Department of Epidemiology and Preventive Medicine, Graduate School of Public Health, Seoul National University, Seoul, Korea
- Corresponding Author Hye Sook Min, M.D., Ph.D. Department of Epidemiology and Preventive Medicine, Graduate School of Public Health, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 151-742, Korea Tel: +82-2-880-2743 Fax: +82-2-762-9105 E-mail: lilloa@snu.ac.kr
• Received: February 25, 2015 • Revised: March 21, 2015 • Accepted: March 26, 2015
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Malignant struma ovarii is extremely rare and difficult to diagnose histologically, particularly in cases of follicular carcinoma. This case study is intended to describe three cases of follicular proliferative lesion arising in struma ovarii that we experienced. The first case was clearly malignant given the clinical picture of multiple recurrences, but there was little histological evidence of malignancy. Our second case featured architectural and cellular atypia and necrosis and was diagnosed as malignant despite the absence of vascular and stromal invasion. Our third case exhibited solid microfollicular proliferation without any definite evidence of malignancy (even the molecular data was negative); however, we could not completely exclude malignant potential after conducting a literature review. In cases such as our third case, it has been previously suggested that a diagnostic term recognizing the low-grade malignant potential, such as “proliferative stromal ovarii” or “follicular proliferative lesion arising in the stromal ovarii” would be appropriate.
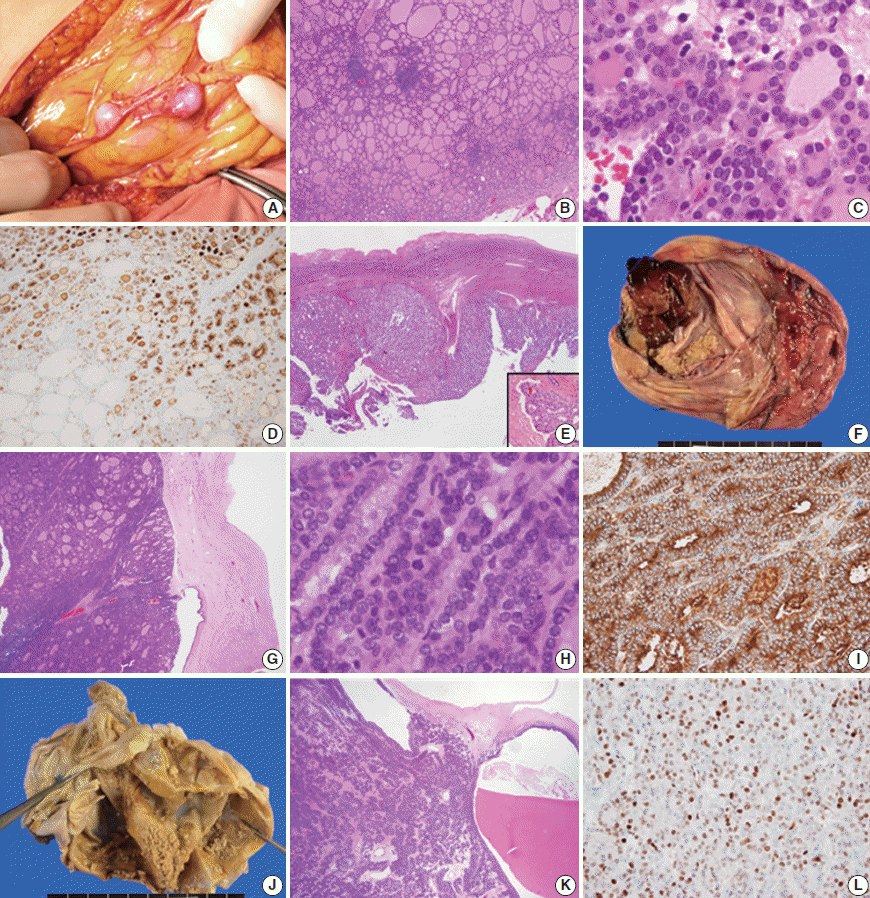
- A 35-year-old female visited the hospital complaining of abdominal discomfort, and pelvic magnetic resonance imaging (MRI) scan revealed multiple nodules in the peritoneum and omentum with a large volume of ascitic fluid. She had a history of surgery to treat struma ovarii in the left ovary 9 years ago. The mass was removed under the suspicion of struma ovarii recurrence. Three months later, multiple enhanced nodules in the adnexa, omentum, perihepatic space, and peritoneum (Fig. 1A) were evident on follow-up imaging, and the patient underwent re-surgery, total thyroidectomy, and radioactive iodine (RAI) therapy. The histological features of nodules from the second and third surgeries were similar. The nodules exhibited mixed micro- and macro-follicular proliferation, with scattered lymphocytic infiltration, and were covered with thin fibrous capsules (Fig. 1B). The tumor cell nuclei were round, uniform, and normochromatic; neither mitosis nor necrosis was evident (Fig. 1C). Immunohistochemically, galectin-3, cyclin D1, and HBME1 were focally positive, and HBME1 expression was limited principally to regions of microfollicular proliferation (Fig. 1D). The Ki-67 positivity level was enhanced by up to 10%, in the microfollicles. The cervical thyroid showed features of chronic lymphocytic thyroiditis but there was no evidence of malignancy. We reviewed all slides of the struma ovarii initially operated on to compare later lesions with the initial ovarian mass. The original mass had both solid and cystic components, and was fibrotically encapsulated (Fig. 1E). Follicles of variable size and papillary structure were observed, and one microscopic focus of vascular invasion was observed after meticulous examination (Fig. 1E, inset). No mutation in any of BRAF (V600E) or RAS (HRAS codon 61, NRAS codon 61, and KRAS codon 12/13), and no PPARy rearrangement (explored using fluorescence in situ hybridization), was evident in the recurring nodules. The lesion was diagnosed as follicular carcinoma arising in the struma ovarii, based on the clinical and pathological findings, and the patient underwent RAI therapy (200 mCi). There has not been local recurrence or distant metastasis in the 25 months of follow-up to date.
- The second case was an 80-year-old female who visited the hospital complaining of acute abdominal discomfort. A 20 cm-diameter mass was observed in the right ovary on pelvic computed tomography and was thought to be a mature cystic teratoma. The tumor was removed with no evidence of peritoneal adhesions, ascites, or seeding nodules apparent intraoperatively. The mass was grossly multicystic and partially solid (~10%) (Fig. 1F). Histologically, the cystic wall was composed of normal thyroid tissue (>50%), skin, and fat tissue. However, the solid portion was surrounded by fibrotic tissue (separating the solid portion from the ovarian stroma), and exhibited proliferation of macro- and micro-follicles (Fig. 1G), crowded nuclei, occasional mitosis (Fig. 1H), and necrosis. However, there was no evidence of vascular invasion. The solid region was galectin-3–negative, but HBME1-positive in the cytoplasm but not the cytoplasmic membrane (Fig. 1I). Cyclin D1 and Ki-67 levels were focally increased in the solid portion of the mass (by ~10%) and thyroglobulin expression was retained. No BRAF or RAS mutation was present and PPARy was not rearranged. The lesion was diagnosed as a follicular carcinoma arising in struma ovarii, which might progress to a poorly differentiated carcinoma requiring RAI therapy and close follow-up. There was no local recurrence or distant metastasis during 20 months of follow-up.
- The last case was a 58-year-old female with a palpable pelvic mass. MRI revealed a 16 cm-diameter multiloculated, solid cystic mass in the left ovary, without adhesions, ascites, or peritoneal seeding. On removal, the tumor was grossly multicystic and filled with thick brownish fluid; small yellowish solid portions were scattered within the inner cystic wall (Fig. 1J). These exhibited a microfollicular proliferation pattern admixed with occasional macrofollicles. No capsule was evident (Fig. 1K). The nuclei were uniformly round and mildly atypical, but there was no evident necrosis or vascular invasion. The mass was HBME1 and p53 negative, but focally positive for galectin-3 and cyclin D1 (Fig. 1L). The Ki-67 positivity rate was approximately 1%–3%. All molecular studies were negative. This lesion was diagnosed as a follicular proliferative lesion arising in the struma ovarii, requiring regular long-term follow-up. There was no recurrence or metastasis noted during 18 months of follow-up.
- Results of clinicopathological studies on the three aforementioned patients are summarized in Table 1.
CASE REPORT
- Some earlier cases reported as malignant struma ovarii are now recognized to have been strumal carcinoids [2]. Malignant struma ovarii currently refers to thyroid-type carcinomas, including papillary and follicular carcinomas. Follicular carcinoma is the second most common type of malignant struma ovarii. The age range for patients with malignant struma ovarii is 22 to 70 years [3]. Typical follicular carcinomas commonly metastasize to distant sites including the lung, liver, bone, and central nervous system [3], as do thyroid gland carcinomas.
- However, histological diagnosis of follicular carcinoma arising in struma ovarii is rather difficult; it is not clear whether the malignant criteria for the thyroid gland are wholly transferable to the struma ovarii [4]. Capsular invasion is the major criterion for thyroid malignancy, but the normal ovary usually lacks a capsule. Furthermore, many struma ovarii associated with distant metastases lacked tumor capsules [5]. Thus, invasion of the surrounding ovarian stroma and/or serosa and vascular invasion have been regarded as histological evidence for follicular carcinoma of the ovary [3,6]. However, it is difficult to define the term “invasion into the stroma and serosa”; some authors consider invasion to be when infiltrating tumor cells are surrounded by thin fibrotic tissue, not by thickened ovarian cortical tissue [4]. The concept of vascular invasion is also somewhat controversial; some authors consider this to be present only when more than three invasive foci are found [7] or, indeed, only when many well-separated vessels are involved [8]. Also, less-differentiated forms of follicular carcinoma can exhibit architectural abnormalities of the trabecular pattern, nuclear atypia, and increased mitotic activity [5].
- A new subtype of follicular carcinoma of the ovary, described by Roth and Karseladze [9], presents an even greater diagnostic challenge. Highly differentiated follicular carcinoma of ovarian origin (HDFCO) was previously considered to reflect extra-ovarian dissemination of normal thyroid tissue, and was termed “peritoneal strumosis.” However, based on both clinical experience and a literature review [3,9], the authors suggested that the term peritoneal strumosis should be replaced by HDFCO, as these lesions behaved as did other thyroid-type carcinomas except that HDFCO was of a much low grade. In line with this suggestion, Robboy et al. [4] reported on 15 cases of biological malignancy (thus characterized by recurrence and metastasis) that exhibited histologically normal thyroid patterns, and/or normal micro- or macro-follicular proliferative patterns, without any definite histological evidence of malignancy. The cited authors and others [7,8] argued that the histological patterns of follicular lesions were not predictive of clinical behavior, and proposed that the diagnostic term “proliferative struma ovarii” should be used to describe a proliferative follicular lesion of the ovary without evident malignant features; the term aptly recognized the latent malignant potential of these masses.
- Since microscopic features do not predict the clinical outcomes of malignant struma ovarii, pathologists cannot be certain that a follicular proliferative lesion in the struma ovarii is benign. Instead, we should consider clinical behavior suggestive of malignancy, such as extraovarian spread, adhesion to adjacent organs, significant volume of ascites (1 L or more), a stromal diameter >5 cm, and a lesion comprised of >50% proliferative thyroid tissue [8].
- Our first case was obviously malignant, given the mutiple recurrences. However, initially, it was difficult to diagnose a malignant struma ovarii because the lesion exhibited no histological evidence of stromal/serosal invasion or vascular invasion. Although no ascites or peritoneal adhesion was evident during the first surgery, the tumor diameter was presumed to be over 5 cm and follicular proliferations (of various patterns) constituted more than half of the lesion. Thus, the lesion was a “proliferative strumal ovarii” according to the terminology defined by Robboy et al. [4], although this terminology is not universally employed. The second case exhibited necrosis and features of a less-differentiated follicular carcinoma including architectural and nuclear atypia, and there was no difficulty with a diagnosis of malignant stromal ovarii. The third case exhibited rather innocuous histological features. Microfollicular proliferation was observed in most regions of the solid lesion; however, the tumor was largely cystic and the solid portions were tiny and scattered, so it was unclear whether proliferative thyroid tissue constituted >50% of the lesion. Both ascites and adhesion were absent, and the tumor diameter was far greater than 5 cm due to its cystic nature. We could not completely exclude malignant potential, and we diagnosed the lesion as a “follicular proliferative lesion, requiring long-term follow-up.”
- Although BRAF and RAS mutations and RET/PTC rearrangement have been reported in malignant struma ovarii exhibiting histological features of papillary carcinoma [10], we did not find any molecular studies that examined follicular proliferative lesions of struma ovarii in the literature. Previous results on papillary carcinoma suggested that the pathogenesis of malignant struma ovarii might be similar to that of carcinoma of the thyroid gland. Thus, we analyzed tissue samples for BRAF and RAS mutations and PPARy rearrangements, but the results were negative in each of the three cases.
- In summary, a follicular proliferative lesion in struma ovarii, including typical follicular carcinoma, HDFCO, and follicular proliferation without evident malignant features, is extremely rare. Diagnoses are not straightforward, and pathologists should be cautious in diagnosing such lesions as benign because a malignant potential may co-exist with innocuous histological features. In particular, even if a follicular proliferative lesion lacks evident malignant features, a diagnostic term recognizing low-grade malignant potential should be used. The literature review suggests that “proliferative sturmal ovarii” or “follicular proliferative lesion arising in the stromal ovarii” would be appropriate.
DISCUSSION
Acknowledgments
Fig. 1.Peritoneal nodules found intraoperatively (A) and the microscopic findings (B, C). HBME1 positivity of microfollicles of the first case (D) is observed and the initial ovarian lesion of the first case (E) shows vascular invasion (E, inset). The gross features of the second case (F) and the histological findings (G, H) are suggestive of malignancy, and HBME1 status is positive only in the cytoplasm (I). In the third case, the solid regions are tiny and scattered (J). Microscopically, microfollicles are predominant (K), and cyclin D1 expression is increased (L).
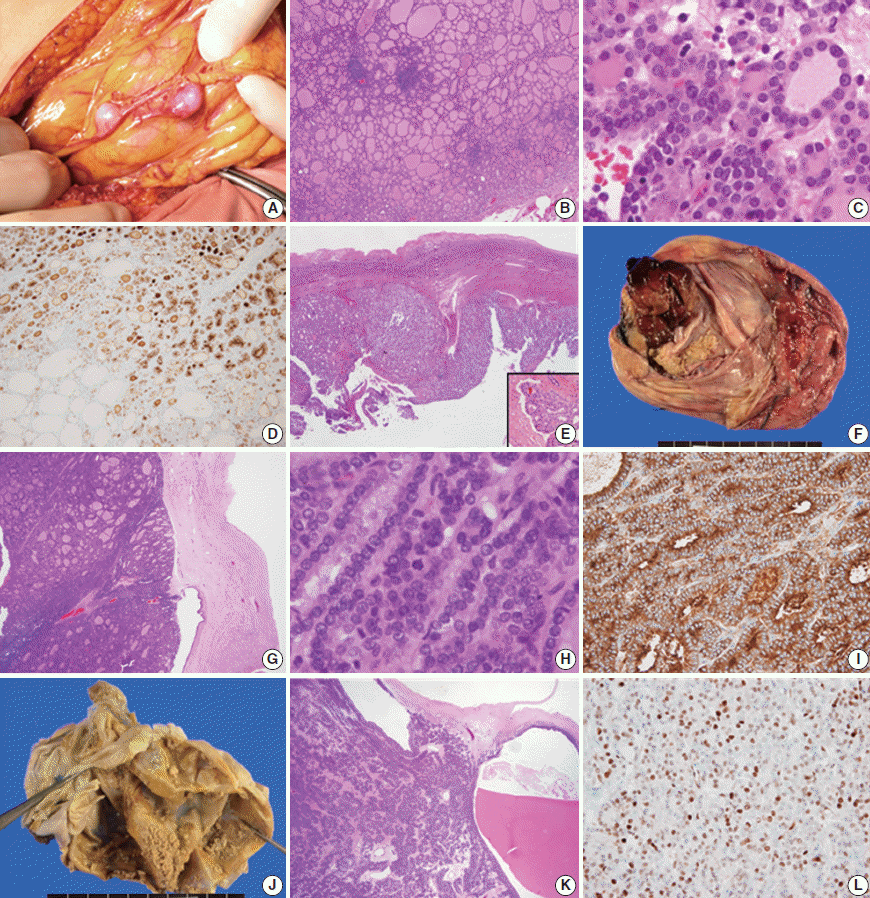

Table 1.Summary of patient clinicopathological features
- 1. Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: IARC Press, 2014.Article
- 2. Kurman RJ, Ellenson LH, Ronnett BM. Blaustein’s pathology of the female genital tract. 6th ed. New York: Springer, 2010.Article
- 3. Roth LM, Miller AW 3rd, Talerman A. Typical thyroid-type carcinoma arising in struma ovarii: a report of 4 cases and review of the literature. Int J Gynecol Pathol 2008; 27: 496-506. ArticlePubMed
- 4. Robboy SJ, Shaco-Levy R, Peng RY, et al. Malignant struma ovarii: an analysis of 88 cases, including 27 with extraovarian spread. Int J Gynecol Pathol 2009; 28: 405-22. ArticlePubMed
- 5. Zhang X, Axiotis C. Thyroid-type carcinoma of struma ovarii. Arch Pathol Lab Med 2010; 134: 786-91. ArticlePubMed
- 6. Roth LM, Talerman A. The enigma of struma ovarii. Pathology 2007; 39: 139-46. ArticlePubMed
- 7. Shaco-Levy R, Bean SM, Bentley RC, Robboy SJ. Natural history of biologically malignant struma ovarii: analysis of 27 cases with extraovarian spread. Int J Gynecol Pathol 2010; 29: 212-27. ArticlePubMed
- 8. Shaco-Levy R, Peng RY, Snyder MJ, et al. Malignant struma ovarii: a blinded study of 86 cases assessing which histologic features correlate with aggressive clinical behavior. Arch Pathol Lab Med 2012; 136: 172-8. ArticlePubMed
- 9. Roth LM, Karseladze AI. Highly differentiated follicular carcinoma arising from struma ovarii: a report of 3 cases, a review of the literature, and a reassessment of so-called peritoneal strumosis. Int J Gynecol Pathol 2008; 27: 213-22. ArticlePubMed
- 10. Boutross-Tadross O, Saleh R, Asa SL. Follicular variant papillary thyroid carcinoma arising in struma ovarii. Endocr Pathol 2007; 18: 182-6. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Role of gene sequencing in classifying struma ovarii: BRAF p.G469A mutation and TERT promoter alterations favour malignant struma ovarii
Sophie Neyrand, Alexis Trecourt, Jonathan Lopez, Pierre Alexandre Just, Françoise Descotes, Françoise Borson‐Chazot, Isabelle Ray‐Coquard, Myriam Decaussin‐Petrucci, Mojgan Devouassoux‐Shisheboran
Histopathology.2024; 84(2): 291. CrossRef - Malignant struma ovarii: next-generation sequencing of six cases revealed Nras, Braf, and Jak3 mutations
Roberta Poli, Maria Scatolini, Enrico Grosso, Francesca Maletta, Marco Gallo, Daniele Liscia, Anna Nelva, Flora Cesario, Giuseppe Forte, Jasna Metovic, Marco Volante, Emanuela Arvat, Mauro Papotti
Endocrine.2021; 71(1): 216. CrossRef - Proliferative struma ovarii: A rare case report
Shankhanila Mazumdar, GaganKumar Rangari, Neeraj Dhameja, NishaRani Agrawal
International Journal of Clinicopathological Correlation.2021; 5(2): 85. CrossRef - Malignant struma ovarii presenting with follicular carcinoma: A case report with molecular analysis
Takafumi Tsukada, Hiroshi Yoshida, Mitsuya Ishikawa, Yuka Asami, Kouya Shiraishi, Tomoyasu Kato
Gynecologic Oncology Reports.2019; 30: 100498. CrossRef - A Rare Case: Struma Ovarii in a 14-Year-Old Girl
Elif Iltar, Isin Ureyen, Tayfun Toptas, Melike Savas, Sema Çekiç, Aysel Uysal
Journal of Adolescent and Young Adult Oncology.2018; 7(1): 134. CrossRef - Proliferative Highly Differentiated Follicular Carcinoma Of Ovarian Origin (Hdfco) Presenting Long After Bilateral Oophorectomy
Natalie M. Liu, Neda Moatamed, Racquel S. Bueno, Wendy L. Sacks
AACE Clinical Case Reports.2017; 3(3): e264. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Follicular Proliferative Lesion Arising in Struma Ovarii

Fig. 1. Peritoneal nodules found intraoperatively (A) and the microscopic findings (B, C). HBME1 positivity of microfollicles of the first case (D) is observed and the initial ovarian lesion of the first case (E) shows vascular invasion (E, inset). The gross features of the second case (F) and the histological findings (G, H) are suggestive of malignancy, and HBME1 status is positive only in the cytoplasm (I). In the third case, the solid regions are tiny and scattered (J). Microscopically, microfollicles are predominant (K), and cyclin D1 expression is increased (L).
Fig. 1.
Follicular Proliferative Lesion Arising in Struma Ovarii
| Variable | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age (yr) | 35 | 80 | 58 |
| Tumor diameter (cm) | NA | 20 | 16 |
| Progression | Peritoneal seeding | None | None |
| Follow-up (mo) | 25 | 20 | 18 |
| Histologic feature | Micro-/macrofollicles | Micro-/macrofollicles, necrosis | Predominantly micro-follicles |
| Invasion of the ovarian stroma | − | − | − |
| Vascular invasion | + | − | − |
| Galectin-3/HBME1/cyclin D1 | f+/f+/f+ | –/f+ (cytoplasm)/f+ | f+/–/f+ |
Table 1. Summary of patient clinicopathological features
NA, not applicable; f+, focal positive; –, negative.

 E-submission
E-submission