Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(4); 2016 > Article
-
Original Article
Aquaporin 1 Is an Independent Marker of Poor Prognosis in Lung Adenocarcinoma - Sumi Yun1,2,*, Ping-Li Sun3,*, Yan Jin4, Hyojin Kim1, Eunhyang Park1, Soo Young Park1, Kyuho Lee1, Kyoungyul Lee5, Jin-Haeng Chung1
-
Journal of Pathology and Translational Medicine 2016;50(4):251-257.
DOI: https://doi.org/10.4132/jptm.2016.03.30
Published online: June 7, 2016
1Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea
2Department of Pathology, Soonchunhyang University Hospital, Seoul, Korea
3Department of Pathology, Second Hospital of Jilin University, Changchun, China
4Department of Pathology, Fudan University, Shanghai Cancer Center, Shanghai, China
5Department of Pathology, Kangwon National University Hospital, Chuncheon, Korea
- Corresponding Author Jin-Haeng Chung, MD, PhD Department of Pathology, Seoul National University Bundang Hospital, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea Tel: +82-31-787-7713 Fax: +82-31-787-4012 E-mail: chungjh@snu.ac.kr
- *Sumi Yun and Ping-Li Sun contributed equally to this work.
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background:
- Aquaporin 1 (AQP1) overexpression has been shown to be associated with uncontrolled cell replication, invasion, migration, and tumor metastasis. We aimed to evaluate AQP1 expression in lung adenocarcinomas and to examine its association with clinicopathological features and prognostic significance. We also investigated the association between AQP1 overexpression and epithelial-mesenchymal transition (EMT) markers.
-
Methods:
- We examined AQP1 expression in 505 cases of surgically resected lung adenocarcinomas acquired at the Seoul National University Bundang Hospital from 2003 to 2012. Expression of AQP1 and EMT-related markers, including Ecadherin and vimentin, were analyzed by immunohistochemistry and tissue microarray.
-
Results:
- AQP1 overexpression was associated with several aggressive pathological parameters, including venous invasion, lymphatic invasion, and tumor recurrence. AQP1 overexpression tended to be associated with higher histological grade, advanced pathological stage, and anaplastic lymphoma kinase (ALK) translocation; however, these differences were not statistically significant. In addition, AQP1 overexpression positively correlated with loss of E-cadherin expression and acquired expression of vimentin. Lung adenocarcinoma patients with AQP1 overexpression showed shorter progression-free survival (PFS, 46.1 months vs. 56.2 months) compared to patients without AQP1 overexpression. Multivariate analysis confirmed that AQP1 overexpression was significantly associated with shorter PFS (hazard ratio, 1.429; 95% confidence interval, 1.033 to 1.977; p=.031).
-
Conclusions:
- AQP1 overexpression was thereby concluded to be an independent factor of poor prognosis associated with shorter PFS in lung adenocarcinoma. These results suggested that AQP1 overexpression might be considered as a prognostic biomarker of lung adenocarcinoma.
- Tissue samples and classification
- Tumor samples were collected from 505 consecutive lung adenocarcinoma patients who underwent curative surgical resection at Seoul National University Bundang Hospital between May 2003 and December 2012. Clinicopathological information and follow-up data were obtained by reviewing the medical and the pathological records of the enrolled patients. Smoking history was defined as smokers who have smoked 100 cigarettes, and never-smokers who have never smoked or smoked less than 100 cigarettes in their life time. Two pathologists (S.Y. and J.-H.C) independently reviewed the hematoxylin and eosin stained slides and classified the diagnosis according to the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) criteria and the 2015 World Health Organization (WHO) classification system [19,20]. Histological grade was based on the predominant histological subtypes as follows: grade 1, lepidic; grade 2, acinar and papillary; and grade 3, solid and micropapillary. Tumors were staged according to the American Joint Committee on Cancer, seventh staging system. Progression-free survival (PFS) was measured from the date of surgery until disease progression or death. Overall survival (OS) was measured from the date of surgery to the time of death or last follow-up visit. The Institutional Review Board of the Seoul National University Bundang Hospital approved this study.
- Tissue microarray
- The most representative areas were obtained for each tumor sample and arranged for tissue microarray (TMA). Tissue cores with a diameter of 2 mm were embedded within TMA blocks, which were sectioned into series of 4-μm-thick slices and then stained with hematoxylin and eosin; immunohistochemical labeling was then performed.
- Immunohistochemical staining and assessment
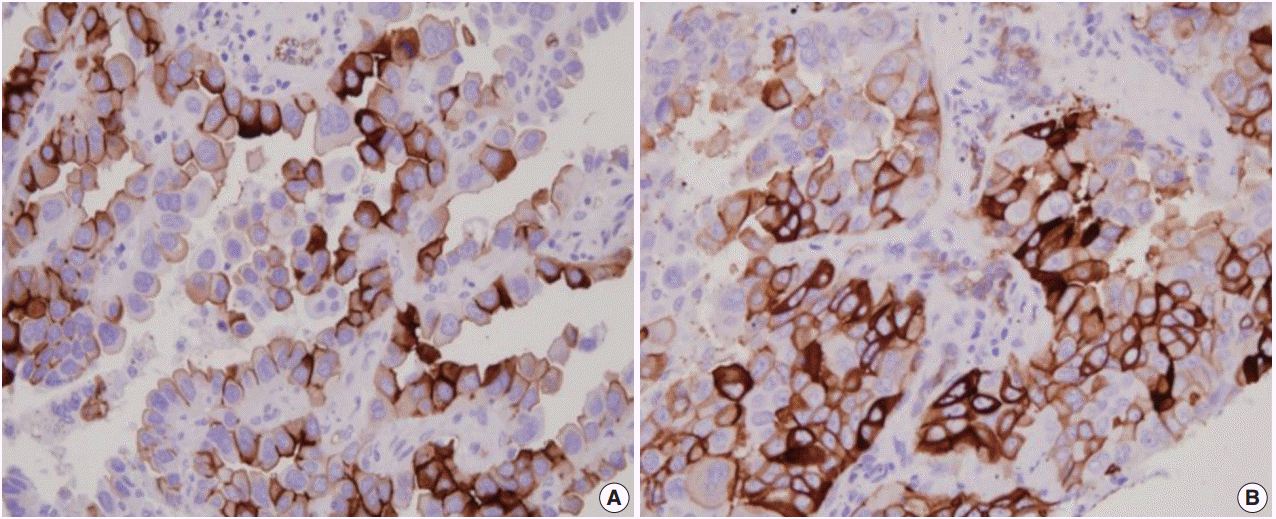
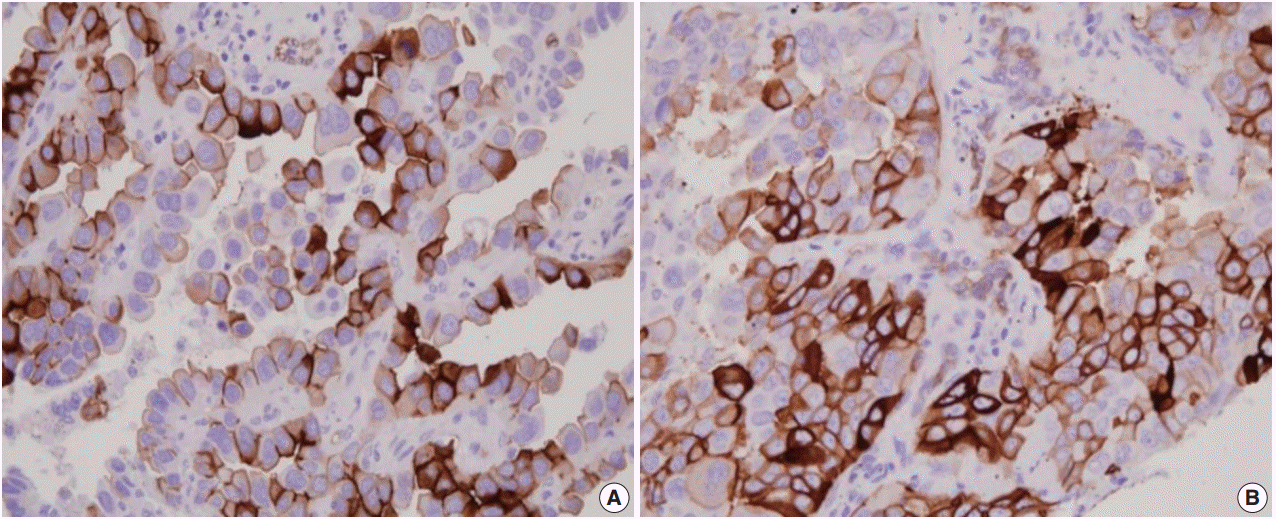
- Immunohistochemistry for AQP1, E-cadherin, and vimentin were performed according to the antibody manufacturer’s instructions. The following antibodies were used: AQP1 (B-11, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), E-cadherin (SPM471, 1:150, Thermo Fisher Scientific, Carlsbad, CA, USA), and vimentin (1:100, 4A4, Zeta Corporation, Arcadia, CA, USA). AQP1 showed both apicolateral staining and complete circumferential membranous staining (Fig. 1). AQP1 overexpression was defined when ≥ 25% showed membranous staining with loss of polarization, as previously reported [21]. Immunohistochemical stains of E-cadherin and vimentin were scored using a semi-quantitative evaluation for each case. The intensity of staining was scored on a four-point scale as follows: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The score was based on the fraction of positive cells (1%–100%). The final score was calculated by multiplying the intensity score to the fraction score, generating a total range of 0–300. Scores of 0–100 and 101–300 were considered as negative and positive, respectively, as previously reported [22].
- Molecular characteristics
- Translocation of anaplastic large cell lymphoma kinase (ALK) was evaluated in 440 cases by fluorescence in situ hybridization analysis using a probe to ALK (Vysis LSI ALK dual color, break-apart rearrangement probe, Abbott Molecular, Des Plaines, IL, USA); translocation was observed in 28 out of 440 cases (6.4%). Epidermal growth factor receptor (EGFR) mutations of exon 18 to 21 and KRAS mutations of codon 12 and 13 were evaluated in 484 and 413 cases using polymerase chain reaction and direct DNA sequencing, as previously described [23]. EGFR and KRAS mutations were identified in 49.0% (237/484) and 6.1% (25/413) of cases, respectively.
- Statistical analysis
- All statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). The association between immunohistochemistry results and clinicopathological variables was assessed by chi-square test, Fisher exact test, or Spearman’s rank correlation test. Kaplan-Meier analysis with log-rank test and multivariate cox regression analysis were performed for survival analysis. Statistical significance was defined as p<.05.
MATERIALS AND METHODS
- Clinicopathological characteristics
- The clinicopathological characteristics of patients are summarized in Table 1. The tumor specimens in this study were from 505 lung adenocarcinoma patients, consisting of 247 male (48.9%) and 258 female (51.1%) patients. The mean age of patients was 62.9 years (range, 21 to 83 years), with 302 non-smokers (59.8%) and 203 smokers (40.2%). With respect to tumor pathology, 274 samples (54.3%) were pathologic stage I, 93 (18.4%) were stage II, 109 (21.6%) were stage III, and 29 (5.7%) were stage IV. According to the new IASLC/ATS/ERS adenocarcinoma classification and the 2015 WHO classification, 298 (59.0%) were acinar, 83 (16.4%) were papillary, 73 (14.5%) were solid, 33 (6.5%) were lepidic, 8 (1.6%) were micropapillary, and 10 (2.0%) were invasive mucinous subtypes. Venous invasion, lymphatic invasion, and perineural invasion were observed in 25.7% (130/505), 50.1% (253/505), and 7.1% (36/505) of samples, respectively.
- AQP1 protein expression by immunohistochemistry
- AQP1 expression was observed in the vascular endothelial cells and the apicolateral surfaces of hyperplastic type II pneumocytes around tumors. AQP1 was also detected in myoepithelial cells of bronchial glands and red blood cells (data not shown).
- Association of AQP1 overexpression with clinicopathological features
- AQP1 overexpression (Fig. 1) was detected in 20.8% of adenocarcinoma cases (105/505). Table 1 shows the association of AQP1 overexpression with clinicopathological variables. There was a significant association of AQP1 overexpression with venous invasion (p=.035) and lymphatic invasion (p=.039). The recurrence rate of patients with AQP1 overexpression was significantly higher than that of patients without AQP1 overexpression (p=.029). AQP1 overexpression was not associated with higher histological grade (p=.097), pleural invasion (p=.131), and other clinicopathological variables or molecular characteristics, such as EGFR and KRAS mutation and ALK rearrangement.
- Association between AQP1 overexpression and EMT-related marker expression
- In total, immunohistochemical analyses of E-cadherin and vimentin were performed for 479 and 471 cases, respectively. Loss of E-cadherin expression was observed in 201 of 479 cases (42.0%), and expression of vimentin was observed in 192 of 471 cases (40.8%). We compared the association of AQP1 overexpression to expression of E-cadherin or vimentin (Table 2) and found that AQP1 overexpression was correlated with loss of E-cadherin expression (p=.011) and expression of vimentin (p<.001).
- Survival analysis according to AQP1 overexpression
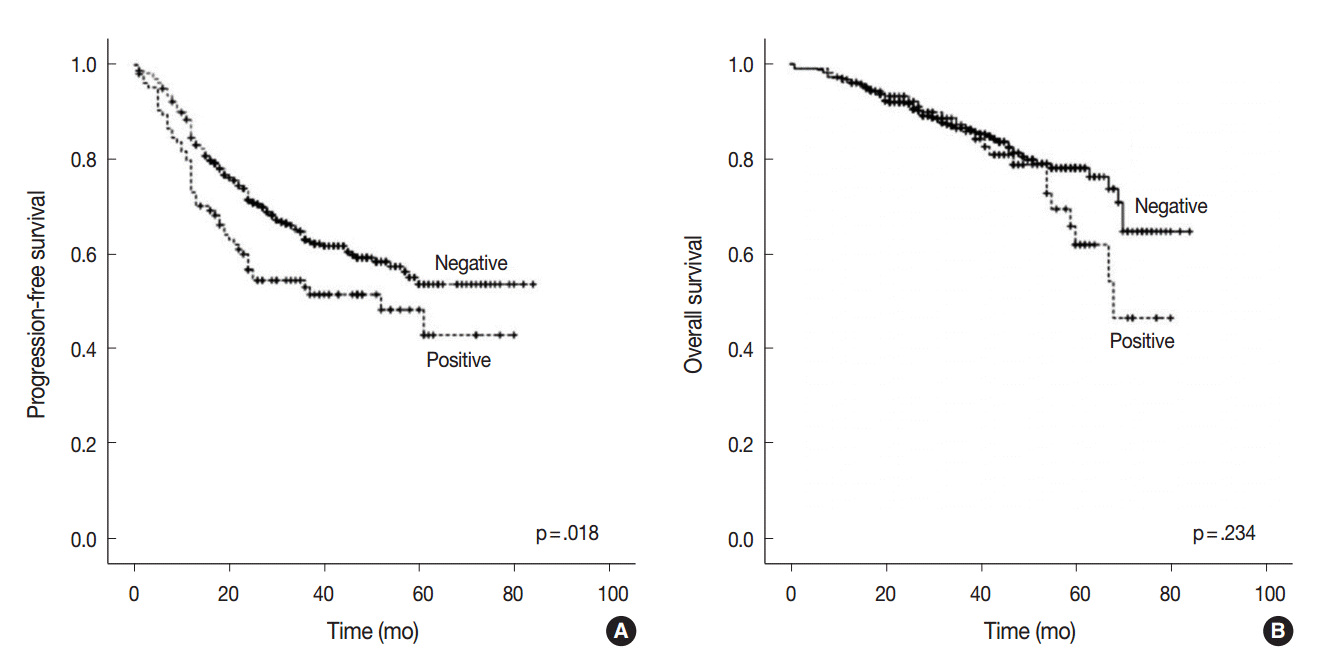
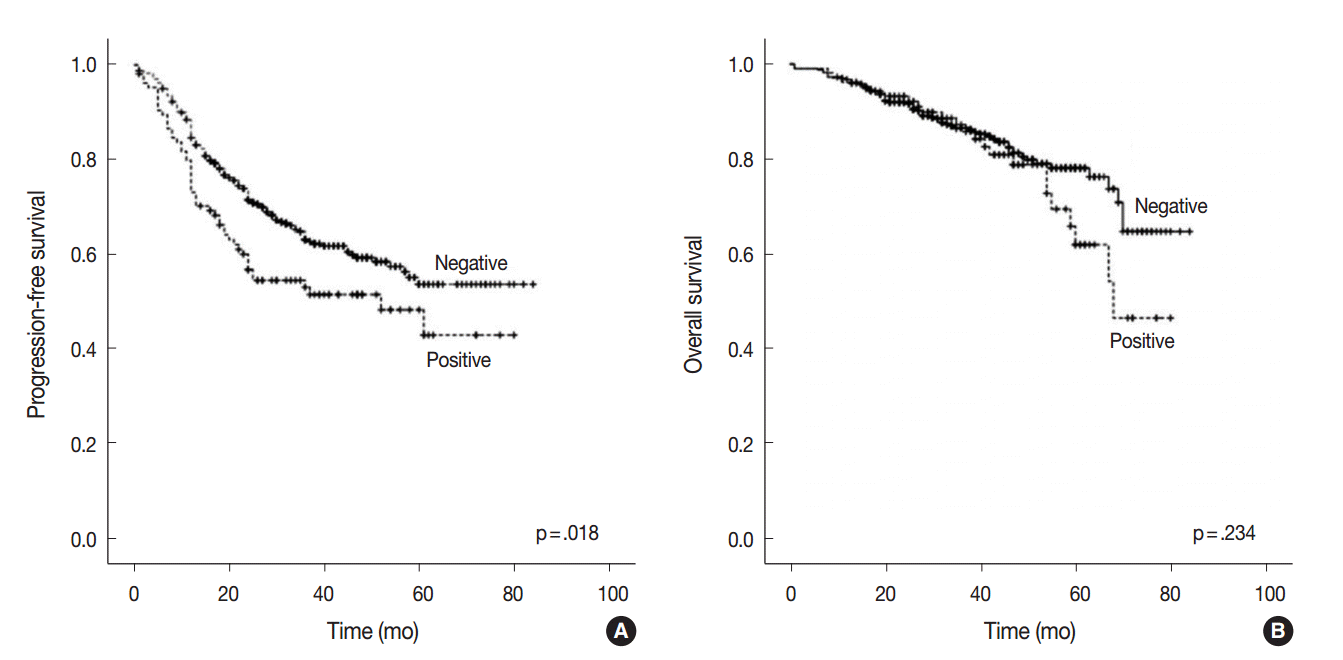
- At the time of analysis, the median PFS was 31.0 months (range, 1 to 84 months) and the median OS was 39 months (range, 1 to 84 months). During this time, 194 patients (38.4%) suffered tumor recurrence and 90 patients (17.8%) died from cancer. Survival analysis using Kaplan-Meier and Cox proportional hazards analyses were performed to evaluate the prognostic impact of AQP1 overexpression. As shown in Fig. 2, Kaplan-Meier revealed that PFS of patients with AQP1 overexpression was significantly shorter than that of the patients without AQP1 overexpression group (p=.018). However, AQP1 overexpression had no prognostic impact on OS (p=.234). Univariate analysis indicated that larger tumor size (p<.001), higher histological grade (p=.032), pleural invasion (p<.001), venous invasion (p<.001), lymphatic invasion (p<.001), perineural invasion (p=.043), pTNM stage (p<.001), and AQP1 overexpression (46.1 months vs. 56.2 months, p=.020) were associated with shorter PFS (Fig. 2A). Multivariate cox regression analysis demonstrated AQP1 overexpression to be an independent factor indicating poor prognosis with regard to PFS in patients with lung adenocarcinoma (hazard ratio [HR], 1.429; 95% confidence interval [CI], 1.033 to 1.977; p=.031). Larger tumor size (HR, 1.797; 95% CI, 1.336 to 2.418; p<.001), pleural invasion (HR, 1.372; 95% CI, 1.007 to 1.871; p=.045), lymphatic invasion (HR, 1.547; 95% CI, 1.113 to 2.151; p=.009), and pTNM stage (HR, 2.179; 95% CI, 1.586 to 2.995; p<.001) were also independent prognostic factors associated with shorter PFS in lung adenocarcinoma. For OS, larger tumor size (p<.001), higher histological grade (p=.021), pleural invasion (p<.001), venous invasion (p<.001), lymphatic invasion (p<.001), perineural invasion (p=.001), vimentin expression (p=.045), and pTNM stage (p<.001) reached statistical significance by univariate analysis. AQP1 overexpression was not associated with OS (63.2 months vs. 70.1 months, p=.237). In multivariate analysis, larger tumor size (HR, 1.775; 95% CI, 1.137 to 2.771; p=.012), venous invasion (HR, 2.129; 95% CI, 1.352 to 3.354; p=.001), and pTNM stage (HR, 4.789; 95% CI, 3.026 to 7.578; p<.001) were statistically significant.
RESULTS
- In the present study, we assessed the expression of AQP1 in 505 lung adenocarcinomas and evaluated the relationship between AQP1 overexpression and various clinicopathological factors and molecular characteristics, as well as the expression of EMT-related markers. Our study showed that AQP1 overexpression significantly correlated with several aggressive pathological factors and can be used as an independent prognostic factor for PFS in lung adenocarcinoma.
- AQP1 is a plasma membrane channel involved in transepithelial water transport [5,7]. Recently, the functional roles of AQP1 protein expression have been studied in various cancers. Previous studies have demonstrated that AQP1 is upregulated in several cancer tissues in vitro and in vivo, and AQP1 overexpression is associated with poor prognosis [11,13,16,17,24]. Hoque et al. [8] reported that upregulated AQP1 in lung cancer may play a role in cancer cell proliferation by resisting apoptosis. Machida et al. [10] showed that AQP1 overexpression with a loss of polarization is associated with invasive growth and poor prognosis in lung adenocarcinomas. Consistent with previous observations, our study showed that AQP1 overexpression tended to be more frequently observed in the high grade histological subtypes of adenocarcinomas although it was not statistically significant.
- We also analyzed the association of AQP1 overexpression with EMT-related markers (E-cadherin and vimentin). The loss of E-cadherin and increased expression of vimentin, both hallmarks of a mesenchymal phenotype, were frequently observed in tumors with AQP1 overexpression. We hypothesized that AQP1 may participate in tumor progression through EMT (loss of E-cadherin and vimentin expression) in lung adenocarcinoma. EMT is implicated in tumor progression, invasion, metastasis, and poor prognosis in lung cancer [6,13,25]. Recently, several reports have suggested that AQP1 overexpression is associated with cancer cell invasion. AQP1 has been suggested to function as a linker molecule to promote EMT, or to stabilize the cytoskeletal complex [8,10,26,27]. Similar results were reported in various cancers including colorectal cancer, breast cancer, and brain tumors [13,17,28], which is in line with our results. The exact biological and functional mechanism of AQP1 to promote EMT needed to be clarifiied by further studies. Of note, some studies demonstrated that tumor tissue showed intratumoral heterogeneity of AQP1 expression in brain tumor [24,29]. Thus, it is necessary to clarify the intratumoral heterogenous distribution of AQP1 overexpression in cancer and its clinical significance.
- In conclusion, we demonstrated that overexpression of AQP1 was significantly associated with venous invasion, lymphatic invasion, higher pathological stage, and cancer recurrence in lung adenocarcinomas. AQP1 was also deemed an independent marker of poor prognosis with regard to PFS. In particular, increased expression of AQP1 protein was associated with the expression of vimentin and loss of E-cadherin expression, suggesting that AQP1 overexpression may be involved in tumor cell invasion through facilitating EMT.
DISCUSSION
Acknowledgments
| Variable | Total |
AQP1 overexpression |
||
|---|---|---|---|---|
| Negative | Positive | p-value | ||
| Sex | .938 | |||
| Male | 247 | 196 (79.4) | 51 (20.6) | |
| Female | 258 | 204 (79.1) | 54 (20.9) | |
| Age (yr) | .092 | |||
| ≤ 60 | 181 | 136 (75.1) | 45 (24.9) | |
| > 60 | 324 | 264 (81.5) | 60 (18.5) | |
| Smoking history | .347 | |||
| Non-smoker | 302 | 235 (77.8) | 67 (22.2) | |
| Smoker | 203 | 165 (81.3) | 38 (18.7) | |
| Tumor size (cm) | .145 | |||
| ≤ 3 | 276 | 212 (76.8) | 64 (23.2) | |
| > 3 | 229 | 188 (82.1) | 41 (17.9) | |
| Histological grade | .097 | |||
| G1 | 34 | 30 (88.2) | 4 (11.8) | |
| G2 | 390 | 312 (80.0) | 78 (20.0) | |
| G3 | 81 | 58 (71.6) | 23 (28.4) | |
| Acinar predominant | .368 | |||
| No | 207 | 168 (81.2) | 39 (18.8) | |
| Yes | 298 | 232 (77.9) | 66 (22.1) | |
| Papillary predominant | .120 | |||
| No | 422 | 339 (78.0) | 93 (22.0) | |
| Yes | 83 | 71 (85.5) | 12 (14.5) | |
| Lepidic predominant | .087 | |||
| No | 472 | 370 (78.4) | 102 (21.6) | |
| Yes | 33 | 30 (90.9) | 3 (9.1) | |
| Solid predominant | .233 | |||
| No | 432 | 346 (80.1) | 86 (19.9) | |
| Yes | 73 | 54 (74.0) | 19 (26.0) | |
| Othersa | .552 | |||
| No | 487 | 387 (79.5) | 100 (20.5) | |
| Yes | 18 | 13 (72.2) | 5 (27.8) | |
| Pleural invasion | .131 | |||
| Absent | 283 | 231 (81.6) | 52 (18.4) | |
| Present | 222 | 169 (76.1) | 53 (23.9) | |
| Venous invasion | .035b | |||
| Absent | 377 | 307 (81.4) | 70 (18.6) | |
| Present | 130 | 93 (72.7) | 35 (27.3) | |
| Lymphatic invasion | .039b | |||
| Absent | 252 | 209 (82.9) | 43 (17.1) | |
| Present | 253 | 193 (75.5) | 62 (24.5) | |
| Perineural invasion | .290 | |||
| Absent | 469 | 369 (78.7) | 100 (21.2) | |
| Present | 36 | 31 (86.1) | 5 (13.9) | |
| pTNM stage | .072 | |||
| I–II | 367 | 298 (81.2) | 69 (18.8) | |
| III–IV | 138 | 102 (73.9) | 36 (26.1) | |
| Recurrence | .029b | |||
| No | 311 | 256 (82.3) | 55 (17.7) | |
| Yes | 194 | 144 (74.2) | 50 (25.8) | |
| Death | .130 | |||
| No | 415 | 334 (80.5) | 81 (19.5) | |
| Yes | 90 | 66 (73.3) | 24 (26.7) | |
| EGFR mutation (n = 484) | .583 | |||
| Wild type | 247 | 193 (78.1) | 54 (21.9) | |
| Mutant type | 237 | 190 (80.2) | 47 (19.8) | |
| KRAS mutation (n = 413) | .326 | |||
| Wild type | 388 | 311 (80.2) | 77 (19.8) | |
| Mutant type | 25 | 18 (72.0) | 7 (28.0) | |
| ALK translocation (n = 440) | .089 | |||
| Wild type | 412 | 334 (81.1) | 79 (18.9) | |
| Mutant type | 28 | 19 (67.9) | 9 (32.1) | |
| Variable | Total |
AQP1 overexpression |
||
|---|---|---|---|---|
| Negative | Positive | p-value | ||
| E-Cadherin expression (n = 479) | .011a | |||
| Decreased | 201 | 147 (73.1) | 54 (26.9) | |
| Preserved | 278 | 230 (82.7) | 48 (17.3) | |
| Vimentin expression (n = 471) | < .001a | |||
| Negative | 279 | 238 (85.3) | 41 (14.7) | |
| Positive | 192 | 133 (69.3) | 59 (30.7) | |
- 1. Kim JE, Kim H, Choe JY, Sun P, Jheon S, Chung JH. High expression of Sonic hedgehog signaling proteins is related to the favorable outcome, EGFR mutation, and lepidic predominant subtype in primary lung adenocarcinoma. Ann Surg Oncol 2013; 20 Suppl 3: S570-6. ArticlePDF
- 2. Seo AN, Yang JM, Kim H, et al. Clinicopathologic and prognostic significance of c-MYC copy number gain in lung adenocarcinomas. Br J Cancer 2014; 110: 2688-99. ArticlePubMedPMCPDF
- 3. Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011; 12: 175-80. ArticlePubMed
- 4. Cuddapah VA, Sontheimer H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am J Physiol Cell Physiol 2011; 301: C541-9. PubMedPMC
- 5. Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci 2005; 118(Pt 15):3225-32. ArticlePubMedPDF
- 6. Wang J, Feng L, Zhu Z, et al. Aquaporins as diagnostic and therapeutic targets in cancer: how far we are? J Transl Med 2015; 13: 96.ArticlePubMedPMCPDF
- 7. Nico B, Ribatti D. Aquaporins in tumor growth and angiogenesis. Cancer Lett 2010; 294: 135-8. ArticlePubMed
- 8. Hoque MO, Soria JC, Woo J, et al. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am J Pathol 2006; 168: 1345-53. ArticlePubMedPMC
- 9. López-Campos JL, Sánchez Silva R, Gómez Izquierdo L, et al. Overexpression of aquaporin-1 in lung adenocarcinomas and pleural mesotheliomas. Histol Histopathol 2011; 26: 451-9. PubMed
- 10. Machida Y, Ueda Y, Shimasaki M, et al. Relationship of aquaporin 1, 3, and 5 expression in lung cancer cells to cellular differentiation, invasive growth, and metastasis potential. Hum Pathol 2011; 42: 669-78. ArticlePubMed
- 11. Xie Y, Wen X, Jiang Z, Fu HQ, Han H, Dai L. Aquaporin 1 and aquaporin 4 are involved in invasion of lung cancer cells. Clin Lab 2012; 58: 75-80. PubMed
- 12. Wei X, Dong J. Aquaporin 1 promotes the proliferation and migration of lung cancer cell in vitro. Oncol Rep 2015; 34: 1440-8. ArticlePubMed
- 13. Sato M, Shames DS, Hasegawa Y. Emerging evidence of epithelial-to-mesenchymal transition in lung carcinogenesis. Respirology 2012; 17: 1048-59. ArticlePubMed
- 14. Kase S, Sugio K, Yamazaki K, Okamoto T, Yano T, Sugimachi K. Expression of E-cadherin and beta-catenin in human non-small cell lung cancer and the clinical significance. Clin Cancer Res 2000; 6: 4789-96. PubMed
- 15. Shi Y, Wu H, Zhang M, Ding L, Meng F, Fan X. Expression of the epithelial-mesenchymal transition-related proteins and their clinical significance in lung adenocarcinoma. Diagn Pathol 2013; 8: 89.ArticlePubMedPMCPDF
- 16. Yoshida T, Hojo S, Sekine S, et al. Expression of aquaporin-1 is a poor prognostic factor for stage II and III colon cancer. Mol Clin Oncol 2013; 1: 953-8. ArticlePubMedPMC
- 17. Jiang Y. Aquaporin-1 activity of plasma membrane affects HT20 colon cancer cell migration. IUBMB Life 2009; 61: 1001-9. ArticlePubMed
- 18. Yin T, Yu S, Xiao L, Zhang J, Liu C, Lu Y. Correlation between the expression of aquaporin 1 and hypoxia-inducible factor 1 in breast cancer tissues. J Huazhong Univ Sci Technolog Med Sci 2008; 28: 346-8. ArticlePubMedPDF
- 19. Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244-85. PubMedPMC
- 20. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press, 2015.
- 21. Li XQ, Yang XL, Zhang G, et al. Nuclear beta-catenin accumulation is associated with increased expression of Nanog protein and predicts poor prognosis of non-small cell lung cancer. J Transl Med 2013; 11: 114.PubMedPMC
- 22. Kim H, Yoo SB, Sun P, et al. Alteration of the E-cadherin/beta-catenin complex is an independent poor prognostic factor in lung adenocarcinoma. Korean J Pathol 2013; 47: 44-51. ArticlePubMedPMC
- 23. Chung JH, Choe G, Jheon S, et al. Epidermal growth factor receptor mutation and pathologic-radiologic correlation between multiple lung nodules with ground-glass opacity differentiates multicentric origin from intrapulmonary spread. J Thorac Oncol 2009; 4: 1490-5. ArticlePubMed
- 24. Deb P, Pal S, Dutta V, Boruah D, Chandran VM, Bhatoe HS. Correlation of expression pattern of aquaporin-1 in primary central nervous system tumors with tumor type, grade, proliferation, microvessel density, contrast-enhancement and perilesional edema. J Cancer Res Ther 2012; 8: 571-7. ArticlePubMed
- 25. Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax 2014; 69: 760-5. ArticlePubMed
- 26. Monzani E, Bazzotti R, Perego C, La Porta CA. AQP1 is not only a water channel: it contributes to cell migration through Lin7/beta-catenin. PLoS One 2009; 4: e6167. Article
- 27. Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J 2006; 20: 1892-4. ArticlePubMedPDF
- 28. Johnson MD, O’Connell M. Na-K-2Cl cotransporter and aquaporin 1 in arachnoid granulations, meningiomas, and meningiomas invading dura. Hum Pathol 2013; 44: 1118-24. ArticlePubMed
- 29. Oshio K, Binder DK, Liang Y, et al. Expression of the aquaporin-1 water channel in human glial tumors. Neurosurgery 2005; 56: 375-81. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability
Catarina Pimpão, Inês V. da Silva, Graça Soveral
International Journal of Molecular Sciences.2025; 26(3): 1330. CrossRef - The comprehensive potential of AQP1 as a tumor biomarker: evidence from kidney neoplasm cohorts, cell experiments and pan-cancer analysis
Yifan Liu, Donghao Lyu, Yuntao Yao, Jinming Cui, Jiangui Liu, Zikuan Bai, Zihui Zhao, Yuanan Li, Bingnan Lu, Keqin Dong, Xiuwu Pan
Human Genomics.2025;[Epub] CrossRef - The Association of Aquaporins with MAPK Signaling Pathway Unveils Potential Prognostic Biomarkers for Pancreatic Cancer: A Transcriptomics Approach
Inês V. da Silva, Paula A. Lopes, Elisabete Fonseca, Emanuel Vigia, Jorge Paulino, Graça Soveral
Biomolecules.2025; 15(4): 488. CrossRef - Obesity Impacts Post‐Myocardial Infarction Neovascularization by Downregulating AQP1 Expression via the TRPC5‐NFATc3 Signaling Pathway
Mengru Gao, Jing Han, Yifei Zhu, Xin Wen, Lei Feng, Tingting Zhou
Comprehensive Physiology.2025;[Epub] CrossRef - Aquaporin‐1, aquaporin‐3 and aquaporin‐5 differentially modulate cell biophysical and biomechanical properties, impacting cell stiffness and cell–cell adhesion
Catarina Pimpão, Filomena A. Carvalho, Inês Vieira da Silva, Andreia Barateiro, Nuno C. Santos, Graça Soveral
The FEBS Journal.2025;[Epub] CrossRef - Prognostic Assessment of Aquaporins in Pancreatic Adenocarcinoma: An In Silico Analysis
Vignesh Krishnasamy, Lalhmingliana, Nachimuthu Senthil Kumar
Current Biotechnology.2025; 14(2): 130. CrossRef - Clinical application of cold atmospheric-pressure plasma: mechanisms and irradiation conditions
Eun Ji Jeong, Hyun Min Park, Dong Jae Lee, Jun Lee, Jun Yeong Cho, Kyung Deok Seo, Seokjun Je, Min Hyung Jung, Woo Yeon Hwang, Kyung Sook Kim
Journal of Physics D: Applied Physics.2024; 57(37): 373001. CrossRef - Aquaporins in Cancer Biology
Chul So Moon, David Moon, Sung Koo Kang
Frontiers in Oncology.2022;[Epub] CrossRef - A Comprehensive Prognostic Analysis of Tumor-Related Blood Group Antigens in Pan-Cancers Suggests That SEMA7A as a Novel Biomarker in Kidney Renal Clear Cell Carcinoma
Yange Wang, Chenyang Li, Xinlei Qi, Yafei Yao, Lu Zhang, Guosen Zhang, Longxiang Xie, Qiang Wang, Wan Zhu, Xiangqian Guo
International Journal of Molecular Sciences.2022; 23(15): 8799. CrossRef - Differential modulation of lung aquaporins among other pathophysiological markers in acute (Cl2 gas) and chronic (carbon nanoparticles, cigarette smoke) respiratory toxicity mouse models
Sukanta S. Bhattacharya, Brijesh Yadav, Ekta Yadav, Ariel Hus, Niket Yadav, Perminder Kaur, Lauren Rosen, Roman Jandarov, Jagjit S. Yadav
Frontiers in Physiology.2022;[Epub] CrossRef - Aquaporin water channels as regulators of cell-cell adhesion proteins
Sarannya Edamana, Frédéric H. Login, Soichiro Yamada, Tae-Hwan Kwon, Lene N. Nejsum
American Journal of Physiology-Cell Physiology.2021; 320(5): C771. CrossRef - Targeting Aquaporins in Novel Therapies for Male and Female Breast and Reproductive Cancers
Sidra Khan, Carmela Ricciardelli, Andrea J. Yool
Cells.2021; 10(2): 215. CrossRef - Targeting ion channels for the treatment of lung cancer
Liqin Zhang, Shuya Bing, Mo Dong, Xiaoqiu Lu, Yuancheng Xiong
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2021; 1876(2): 188629. CrossRef - Comprehensive Analysis of Aquaporin Superfamily in Lung Adenocarcinoma
Guofu Lin, Luyang Chen, Lanlan Lin, Hai Lin, Zhifeng Guo, Yingxuan Xu, Chanchan Hu, Jinglan Fu, Qinhui Lin, Wenhan Chen, Yiming Zeng, Yuan Xu
Frontiers in Molecular Biosciences.2021;[Epub] CrossRef - Diagnostic accuracy of urinary aquaporin-1 as a biomarker for renal cell carcinoma
Abhilash Cheriyan, Arun Jose Nellickal, Nirmal Thampi John, Lakshmanan Jeyaseelan, Santosh Kumar, Antony Devasia, Nitin Kekre
Indian Journal of Urology.2021; 37(1): 59. CrossRef - Aquaporin 1, 3, and 5 Patterns in Salivary Gland Mucoepidermoid Carcinoma: Expression in Surgical Specimens and an In Vitro Pilot Study
Mérin Barbara Stamboni, Ágatha Nagli de Mello Gomes, Milena Monteiro de Souza, Katia Klug Oliveira, Claudia Fabiana Joca Arruda, Fernanda de Paula, Barbara Beltrame Bettim, Márcia Martins Marques, Luiz Paulo Kowalski, Clóvis Antônio Lopes Pinto, Victor El
International Journal of Molecular Sciences.2020; 21(4): 1287. CrossRef - Combined Systematic Review and Transcriptomic Analyses of Mammalian Aquaporin Classes 1 to 10 as Biomarkers and Prognostic Indicators in Diverse Cancers
Pak Hin Chow, Joanne Bowen, Andrea J Yool
Cancers.2020; 12(7): 1911. CrossRef - Aquaporins in lung health and disease: Emerging roles, regulation, and clinical implications
Ekta Yadav, Niket Yadav, Ariel Hus, Jagjit S. Yadav
Respiratory Medicine.2020; 174: 106193. CrossRef - Dissecting gene‐environment interactions: A penalized robust approach accounting for hierarchical structures
Cen Wu, Yu Jiang, Jie Ren, Yuehua Cui, Shuangge Ma
Statistics in Medicine.2018; 37(3): 437. CrossRef - Immunohistochemical Expression of Aquaporin-1 in Fluoro-Edenite-Induced Malignant Mesothelioma: A Preliminary Report
Giuseppe Angelico, Rosario Caltabiano, Carla Loreto, Antonio Ieni, Giovanni Tuccari, Caterina Ledda, Venerando Rapisarda
International Journal of Molecular Sciences.2018; 19(3): 685. CrossRef - Mechanisms of Aquaporin-Facilitated Cancer Invasion and Metastasis
Michael L. De Ieso, Andrea J. Yool
Frontiers in Chemistry.2018;[Epub] CrossRef - Aquaporin 1 suppresses apoptosis and affects prognosis in esophageal squamous cell carcinoma
Yuzo Yamazato, Atsushi Shiozaki, Daisuke Ichikawa, Toshiyuki Kosuga, Katsutoshi Shoda, Tomohiro Arita, Hirotaka Konishi, Shuhei Komatsu, Takeshi Kubota, Hitoshi Fujiwara, Kazuma Okamoto, Mitsuo Kishimoto, Eiichi Konishi, Yoshinori Marunaka, Eigo Otsuji
Oncotarget.2018; 9(52): 29957. CrossRef - Aquaporin 1 expression is associated with response to adjuvant chemotherapy in stage�II and III colorectal cancer
Hideko Imaizumi, Keiichiro Ishibashi, Seiichi Takenoshita, Hideyuki Ishida
Oncology Letters.2018;[Epub] CrossRef - Aquaporin 3 facilitates tumor growth in pancreatic cancer by modulating mTOR signaling
Xunwei Huang, Li Huang, Minhua Shao
Biochemical and Biophysical Research Communications.2017; 486(4): 1097. CrossRef - Prognostic implication of aquaporin 1 overexpression in resected lung adenocarcinoma†
Guido Bellezza, Jacopo Vannucci, Fortunato Bianconi, Giulio Metro, Rachele Del Sordo, Marco Andolfi, Ivana Ferri, Paola Siccu, Vienna Ludovini, Francesco Puma, Angelo Sidoni, Lucio Cagini
Interactive CardioVascular and Thoracic Surgery.2017; 25(6): 856. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| Variable | Total | AQP1 overexpression |
||
|---|---|---|---|---|
| Negative | Positive | p-value | ||
| Sex | .938 | |||
| Male | 247 | 196 (79.4) | 51 (20.6) | |
| Female | 258 | 204 (79.1) | 54 (20.9) | |
| Age (yr) | .092 | |||
| ≤ 60 | 181 | 136 (75.1) | 45 (24.9) | |
| > 60 | 324 | 264 (81.5) | 60 (18.5) | |
| Smoking history | .347 | |||
| Non-smoker | 302 | 235 (77.8) | 67 (22.2) | |
| Smoker | 203 | 165 (81.3) | 38 (18.7) | |
| Tumor size (cm) | .145 | |||
| ≤ 3 | 276 | 212 (76.8) | 64 (23.2) | |
| > 3 | 229 | 188 (82.1) | 41 (17.9) | |
| Histological grade | .097 | |||
| G1 | 34 | 30 (88.2) | 4 (11.8) | |
| G2 | 390 | 312 (80.0) | 78 (20.0) | |
| G3 | 81 | 58 (71.6) | 23 (28.4) | |
| Acinar predominant | .368 | |||
| No | 207 | 168 (81.2) | 39 (18.8) | |
| Yes | 298 | 232 (77.9) | 66 (22.1) | |
| Papillary predominant | .120 | |||
| No | 422 | 339 (78.0) | 93 (22.0) | |
| Yes | 83 | 71 (85.5) | 12 (14.5) | |
| Lepidic predominant | .087 | |||
| No | 472 | 370 (78.4) | 102 (21.6) | |
| Yes | 33 | 30 (90.9) | 3 (9.1) | |
| Solid predominant | .233 | |||
| No | 432 | 346 (80.1) | 86 (19.9) | |
| Yes | 73 | 54 (74.0) | 19 (26.0) | |
| Others |
.552 | |||
| No | 487 | 387 (79.5) | 100 (20.5) | |
| Yes | 18 | 13 (72.2) | 5 (27.8) | |
| Pleural invasion | .131 | |||
| Absent | 283 | 231 (81.6) | 52 (18.4) | |
| Present | 222 | 169 (76.1) | 53 (23.9) | |
| Venous invasion | .035 |
|||
| Absent | 377 | 307 (81.4) | 70 (18.6) | |
| Present | 130 | 93 (72.7) | 35 (27.3) | |
| Lymphatic invasion | .039 |
|||
| Absent | 252 | 209 (82.9) | 43 (17.1) | |
| Present | 253 | 193 (75.5) | 62 (24.5) | |
| Perineural invasion | .290 | |||
| Absent | 469 | 369 (78.7) | 100 (21.2) | |
| Present | 36 | 31 (86.1) | 5 (13.9) | |
| pTNM stage | .072 | |||
| I–II | 367 | 298 (81.2) | 69 (18.8) | |
| III–IV | 138 | 102 (73.9) | 36 (26.1) | |
| Recurrence | .029 |
|||
| No | 311 | 256 (82.3) | 55 (17.7) | |
| Yes | 194 | 144 (74.2) | 50 (25.8) | |
| Death | .130 | |||
| No | 415 | 334 (80.5) | 81 (19.5) | |
| Yes | 90 | 66 (73.3) | 24 (26.7) | |
| EGFR mutation (n = 484) | .583 | |||
| Wild type | 247 | 193 (78.1) | 54 (21.9) | |
| Mutant type | 237 | 190 (80.2) | 47 (19.8) | |
| KRAS mutation (n = 413) | .326 | |||
| Wild type | 388 | 311 (80.2) | 77 (19.8) | |
| Mutant type | 25 | 18 (72.0) | 7 (28.0) | |
| ALK translocation (n = 440) | .089 | |||
| Wild type | 412 | 334 (81.1) | 79 (18.9) | |
| Mutant type | 28 | 19 (67.9) | 9 (32.1) | |
| Variable | Total | AQP1 overexpression |
||
|---|---|---|---|---|
| Negative | Positive | p-value | ||
| E-Cadherin expression (n = 479) | .011 |
|||
| Decreased | 201 | 147 (73.1) | 54 (26.9) | |
| Preserved | 278 | 230 (82.7) | 48 (17.3) | |
| Vimentin expression (n = 471) | < .001a | |||
| Negative | 279 | 238 (85.3) | 41 (14.7) | |
| Positive | 192 | 133 (69.3) | 59 (30.7) | |
Values are presented as number (%). AQP1, aquaporin 1; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase. Invasive mucinous and micropapillary subtype; Statistically significant value.
Values are presented as number (%). AQP1, aquaporin 1; EMT, epithelial-mesenchymal transition. Statistically significant value.

 E-submission
E-submission