Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(3); 2017 > Article
-
Original Article
Overexpression of POSTN in Tumor Stroma Is a Poor Prognostic Indicator of Colorectal Cancer - Hyeon Jeong Oh1,2, Jeong Mo Bae2,3, Xian-Yu Wen2, Nam-Yun Cho2, Jung Ho Kim1,2, Gyeong Hoon Kang,1,2
-
Journal of Pathology and Translational Medicine 2017;51(3):306-313.
DOI: https://doi.org/10.4132/jptm.2017.01.19
Published online: April 12, 2017
1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
2Laboratory of Epigenetics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
3Department of Pathology, SMG-SNU Boramae Medical Center, Seoul, Korea
- Corresponding Author Gyeong Hoon Kang, MD Department of Pathology, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-740-8263 Fax: +82-2-765-5600 E-mail: ghkang@snu.ac.kr
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Tumor microenvironment has recently drawn attention in that it is related with tumor prognosis. Cancer-associated fibroblast also plays a critical role in cancer invasiveness and progression in colorectal cancers. Periostin (POSTN), originally identified to be expressed in osteoblasts and osteoblast-derived cells, is expressed in cancer-associated fibroblasts in several tissue types of cancer. Recent studies suggest an association between stromal overexpression of POSTN and poor prognosis of cancer patients.
-
Methods
- We analyzed colorectal cancer cases for their expression status of POSTN in tumor stroma using immunohistochemistry and correlated the expression status with clinicopathological and molecular features.
-
Results
- High level of POSTN expression in tumor stroma was closely associated with tumor location in proximal colon, infiltrative growth pattern, undifferentiated histology, tumor budding, luminal necrosis, and higher TNM stage. High expression status of POSTN in tumor stroma was found to be an independent prognostic parameter implicating poor 5-year cancer-specific survival and 5-year progression-free survival.
-
Conclusions
- Our findings suggest that POSTN overexpression in tumor stroma of colorectal cancers could be a possible candidate marker for predicting poor prognosis in patients with colorectal cancers.
- Tissue samples
- A consecutive series of CRC cases were retrieved from the surgical files of the Department of Pathology, Seoul National University Hospital, Seoul, Korea. Among the patients who underwent surgical resection for primary CRC from 2004 to 2007, we excluded those with neo-adjuvant treatment, non-invasive cancers, familial adenomatous polyposis, multiple or recurrent tumors, and a history of other malignancy within 5 years. Demographic data and clinicopathological information were retrieved from electronic medical records. Two pathologists (J.M.B. and G.H.K.) reviewed hematoxylin and eosin–stained tissue slides for the degree of histologic differentiation which was categorized as well-moderate versus poor (> 50% vs ≤ 50%, respectively). Staging was classified according to the sixth edition guidelines of the AJCC. Hematoxylin and eosin–stained slides were also reviewed for evaluation of tumor budding, which is defined to be present when five or more buddings are present at × 200 magnification. Status of tumor infiltrating lymphocytes was divided into high and low (≥ 8 at × 400 magnification and < 8 at × 400 magnification, respectively). The study was approved by Institutional Ethics Committee of Seoul National University Hospital which waived the requirement to obtain informed consent (approval No. 1502-029-647).
- Evaluation of POSTN expression
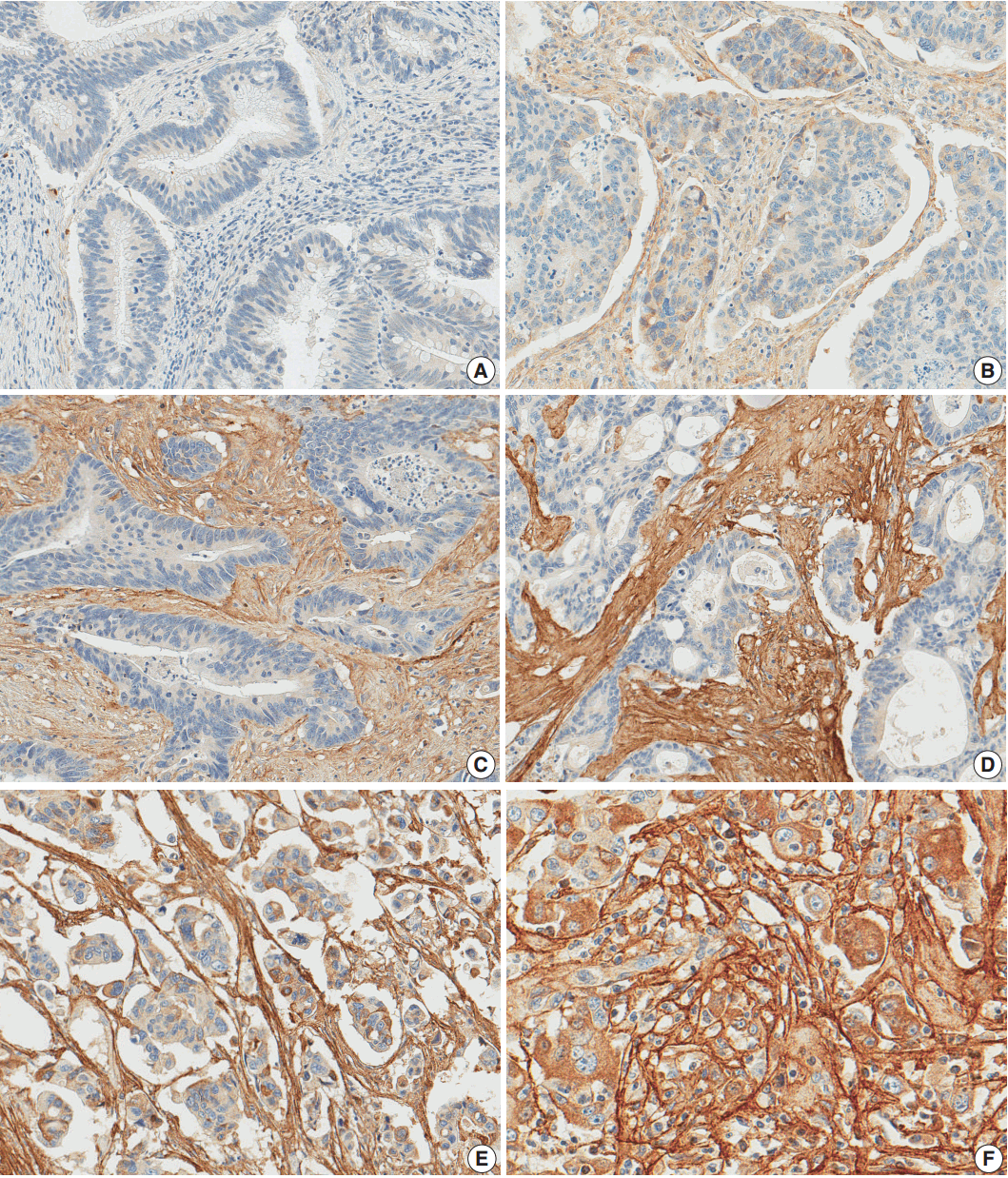
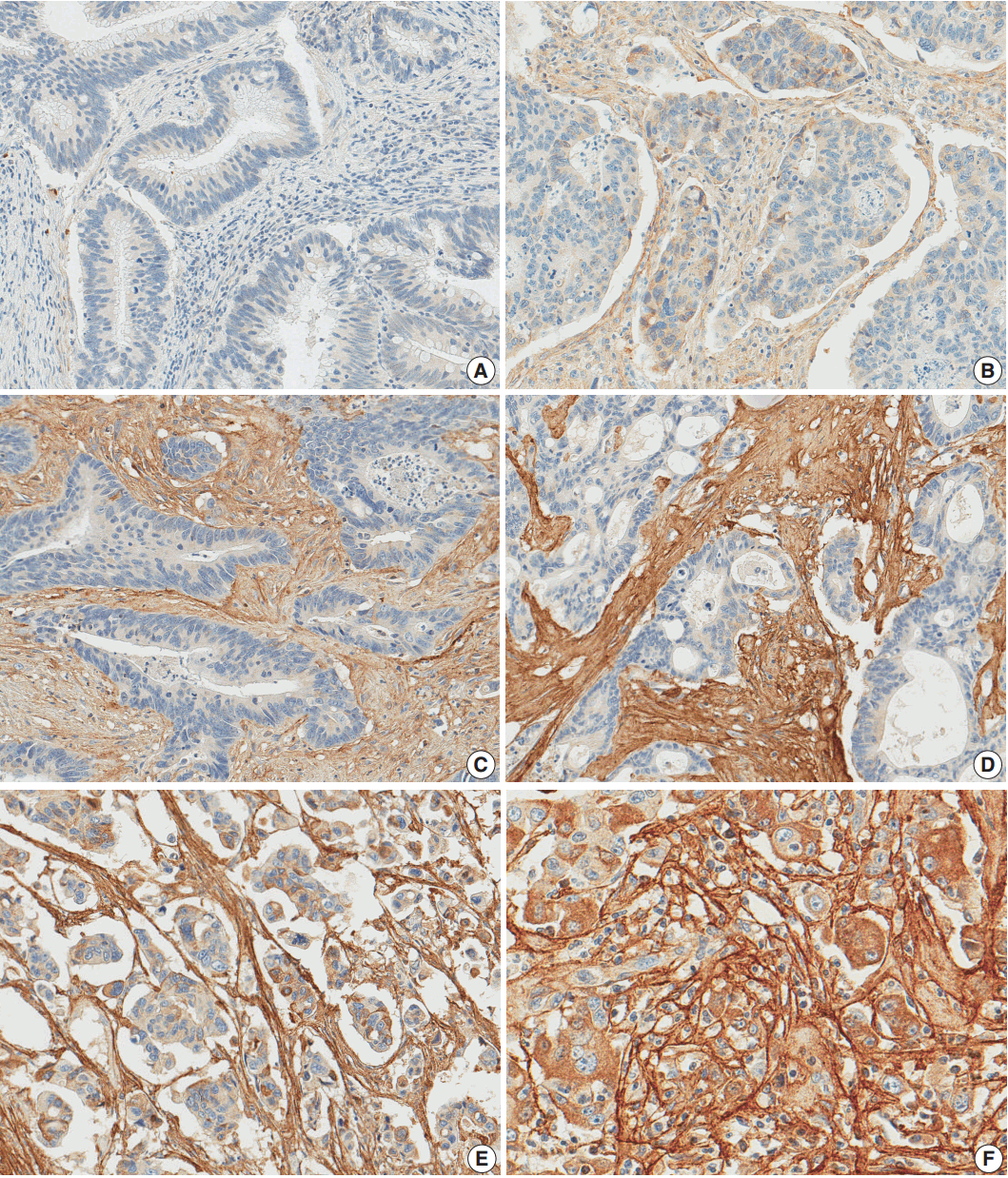
- After reviewing the hematoxylin and eosin tissue slides, representative areas of the tumor invasive front were selected and marked. Two-millimeter-core tissues were harvested from individual paraffin-embedded colon cancer tissues and were arranged in a new recipient paraffin block using a trephine apparatus (Superbiochip Laboratories, Seoul, Korea). Immunohistochemical analysis was performed with commercially available antibody against POSTN (1:200, HPA012306, Sigma, St. Louis, MO, USA). Two pathologists (H.J.O. and J.M.B.) independently evaluated POSTN immunohistochemistry. POSTN expression in tumor stroma showed homogeneous staining intensity and the level of POSTN expression was graded as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong) (Fig. 1). Then, grades 0–2 and 3 were categorized as POSTN-low and POSTN-high, respectively.
- BRAF and KRAS mutation and microsatellite instability analysis
- Through microscopic examination, representative tumor areas in each case were marked and microdissected. The dissected tissues were subject to incubation at 55°C with lysis buffer and proteinase K for 2 days. Allele specific polymerase chain reaction for BRAF codon 600 and direct sequencing of KRAS codons 12 and 13 were performed. The MSI status of each tumor tissue versus normal tissue was determined by five National Cancer Institute markers including BAT25, BAT26, D2S123, D5S346, and D17S250. High MSI status was defined as when tumor DNA had altered alleles compared to normal DNA in two or more markers. Low MSI status was defined as when tumor DNA had altered allele compared to normal DNA in one marker. Microsatellite stable was defined as when no altered allele was present in tumor DNA.
- CIMP analysis
- CIMP status was examined by MethyLight assay. Bisulfite modified DNA was subject to MethyLight assay which was performed as previously described [18]. Methylation statuses of eight CIMP-specific CpG islands (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1) were quantified. CIMP-high was defined as five or more markers methylated, and CIMP-low was defined as four or less markers methylated out of eight. CIMP-0 was defined as no methylated marker.
- Statistical analysis
- SAS software (ver. 9.4 for Microsoft Windows, SAS Institute Inc., Cary, NC, USA) was used for the statistical analysis in our cohort. To compare clinicopathologic characteristics to stromal POSTN expression, we performed Pearson’s chi-square test. The age of CRC group according to stromal POSTN expression was compared using Wilcoxon’s rank-sum test. For the survival analysis, 5-year cancer-specific survival (CSS) and 5-year progression-free survival (PFS) were calculated using the log-rank test with a Kaplan-Meier curve. Hazard ratios (HRs) were calculated using the Cox proportional hazard model. The assumption of the proportional hazards was verified by plotting the log{–log[S(t)]} against the time of the study. In the modeling process, all variables that were associated with PFS with a p < .10 were entered into an initial model; these variables were subsequently reduced by backward elimination. All statistical tests were two-sided, and statistical significance was defined as p < .05.
MATERIALS AND METHODS
- Patient characteristics
- In a total of 1,135 CRC patients, the median age at diagnosis was 62 years (range, 20 to 90 years). The male to female ratio was 1.49:1 (673 males and 452 females). Tumor location was proximal colon (proximal to splenic flexure) in 277 patients (24.6%), distal colon in 438 patients (38.9%), and rectum in 410 patients (36.4%). KRAS mutation and BRAF mutation were observed in 313 (27.8%) and 48 patients (4.3%), respectively. In microsatellite analysis, microsatellite stable, MSI-low, and MSI-high were observed in 964 (85.7%), 73 (6.5%), and 88 (7.8%) patients, respectively. In CIMP analysis, CIMP-0, CIMP-low, and CIMP-high were observed in 510 (45.3%), 553 (49.2%), and 62 (5.5%) patients, respectively. Median follow-up duration was 69.8 months (range, 0.3 to 150.2 months). Seven hundred seventy-nine patients received 5-fluorouracil (5-FU)-based adjuvant chemotherapy.
- Clinicopathological features of CRCs according to stromal POSTN expression
- Of 1,125 CRC cases, 33 (2.9%), 296 (26.3%), 492 (43.7%), and 304 (27.0%) patients showed stromal POSTN expression from grade 0 to 3, respectively. POSTN-low CRCs were 821 (73.0%) and POSTN-high CRCs were 304 (27.0%) (Table 1). CRCs with stromal POSTN-high expression were associated with proximal location (35.2% in POSTN-high group vs 20.7% in POSTN-low group, p < .001), infiltrative growth pattern (44.7% vs 30.7%, p < .001), advanced T, N, M category (p < .001), frequent tumor budding (80.9% vs 68.2%, p < .001) and luminal necrosis (94.4% vs 89.6%, p = .014) compared with CRCs with stromal POSTN-low expression. In molecular aspect, CRCs with stromal POSTN-high expression showed higher frequency of CIMP-high (7.9% vs 4.6%, p = .030) and BRAF mutation (6.3% vs 3.5%, p = .046) compared with CRCs with stromal POSTN-low expression. However, the frequency of MSI-high and KRAS mutation were not statistically associated with stromal POSTN expression.
- Prognostic implication of stromal POSTN expression in CRCs
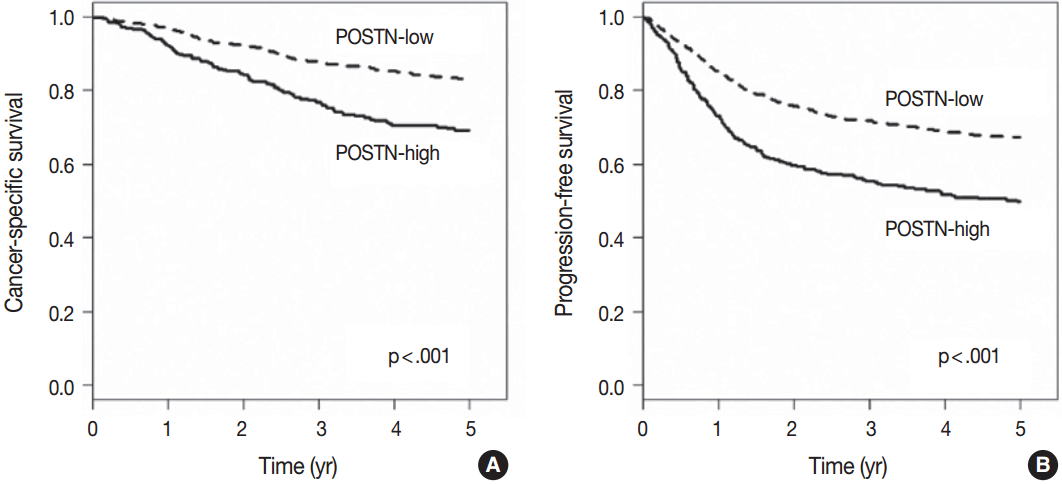
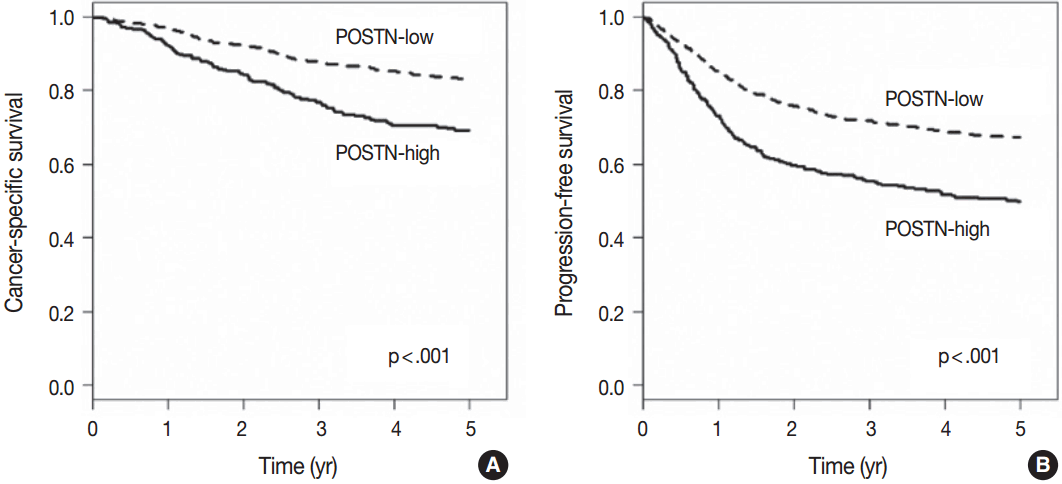
- When we performed univariate survival analysis, CRCs with stromal POSTN-high expression showed worse 5-year PFS (HR, 1.80; 95% CI, 1.47 to 2.20; p < .001) (Fig. 2A) and worse 5-year CSS (HR, 2.00; 95% CI, 1.52 to 2.63; p < .001) (Fig. 2B) compared with CRCs with stromal POSTN-low expression. In multivariate survival analysis, stromal POSTN-high expression was an independent prognostic indicator of poor 5-year CSS (HR, 1.50; 95% CI, 1.13 to 2.00; p = .006) (Table 2) and poor 5-year PFS (HR, 1.38; 95% CI, 1.12 to 1.70; p = .003) (Table 3).
RESULTS
- Recent studies show POSTN is overexpressed in cancer stroma of various malignancies, including colorectal, breast, lung, pancreatic, ovarian, gastric, head and neck, thyroid and prostate cancer as well as in glioblastoma [19]. POSTN is thought to play important roles in tumorigenesis, such as invasiveness, metastasis, angiogenesis, lymphangiogenesis, and chemoresistance [8,15,20-24]. In this study, we found that POSTN is overexpressed in 304 (27.0%) out of 1,125 cases, which is consistent with the results of other studies in several malignancies [19]. Our study demonstrated that high POSTN expression is correlated with several aggressive clinicopathological features of CRCs. First, stromal POSTN expression was higher in CRCs of infiltrative growth pattern than CRCs of fungating growth pattern, which implies that POSTN is involved in infiltrative growth of tumor. Second, POSTN expression was correlated with T, N, or M category. These results suggest that POSTN might participate in tumor progression and nodal or distant metastasis, which is consistent with the results of a recent study that POSTN expression is higher in distant metastatic lesion than in matched primary colorectal lesion [17]. Cell line studies demonstrated that POSTN induces invasive activity and bestows a metastatic potential to HEK 293T cells which are tumorigenic but nonmetastatic [11,17]. These results support the idea that POSTN might contribute to metastasis of tumor cells. Third, high tumor budding status was correlated with high POSTN expression. Several studies addressed that tumor budding should be regarded as a biomarker of poor prognosis, correlated with epithelial mesenchymal transition [25-28]. A cell line study has demonstrated that stable transfection of POSTN induced epithelial-mesenchymal transition and increased invasive activity in HEK 293T cells [11]. However, our present study has several limitations. First, this study is a retrospective study in a single institution. Second, stromal POSTN expression was evaluated in tissue microarray.
- In our study, stromal POSTN expression was associated with CIMP-high and BRAF mutation. Because CIMP-high and BRAF mutation are hallmarks of molecular alterations in serrated neoplasia pathway, we can presume that stromal POSTN overexpression is associated with serrated neoplasia pathway. In a study by De Sousa et al [29]., CCS3 subtype, which is related with serrated neoplasia pathway, showed overexpression of genes associated with epithelial mesenchymal transition and matrix remodeling. Recently, Fessler et al [30]. demonstrated the induction of a mesenchymal phenotype upon transforming growth factor β treatment in a genetically engineered organoid culture carrying BRAF V600E mutation. In serrated neoplasia pathway, three molecular groups are known to be involved. One is BRAF-mutant/CIMP-high/ MSI-high subgroup, another is BRAF-mutant/CIMP-high/microsatellite stable (MSS) subgroup, and the other is KRAS-mutant/CIMP-low/MSS subgroup [31,32]. In this study, stromal POSTN overexpression was related with BRAF-mutant/CIMP-high/MSS subgroup (data not shown). Moreover, some clinicopathological features associated with CIMP, such as right colon preponderance, poor differentiation, high rates of tumor budding, frequent lymphatic, vascular, or perineural invasion and nodal metastasis, were more frequently observed in POSTN-high colon cancer compared with POSTN-low colon cancer. Further molecular test is needed to elucidate the relation between high POSTN stromal expression and serrated neoplasia pathway.
- An accumulating series of study results indicate that cancer+associated fibroblasts contribute to chemo-resistance and poor patient survival [15,17,28]. According to Xu et al., medium to high stromal POSTN expression was an independent predictor of poor disease-free survival and disease-specific survival. They also presented the association of chemo-resistance and stromal POSTN expression in stage II CRCs and stage III CRCs with chemotherapy history. In in vitro study, SW480 and HT29 cells cultured in stromal POSTN-enriched media showed chemoresistance to 5-FU. Recombinant POSTN increased proliferation and phosphorylation of Akt in SW480 and HT29 cells. Furthermore, the proliferation and phosphorylation of Akt were inhibited by phosphoinositide 3-kinase inhibitor LY294002 [17,24]. In our present study, stromal POSTN-high expression was an independent prognostic indicator of poor 5-year CSS and 5-year PFS. However, we could not confirm the chemo-resistance effect of stromal POSTN expression in our cohort (data not shown).
- In conclusion, our findings suggest that immunohistochemical evaluation of stromal POSTN expression could be utilized for the identification of a subset of CRCs with poor prognosis. To determine whether stromal POSTN expression status is a prognostic marker of CRC, further studies are needed to validate this finding with an independent series of large-scaled CRC cases.
DISCUSSION
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359-86. ArticlePubMed
- 2. O’Connell MJ, Campbell ME, Goldberg RM, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol 2008; 26: 2336-41. ArticlePubMed
- 3. Bozóky B, Savchenko A, Csermely P, et al. Novel signatures of cancer-associated fibroblasts. Int J Cancer 2013; 133: 286-93. ArticlePubMed
- 4. Chen WJ, Ho CC, Chang YL, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun 2014; 5: 3472.ArticlePubMedPDF
- 5. Huang L, Xu AM, Liu S, Liu W, Li TJ. Cancer-associated fibroblasts in digestive tumors. World J Gastroenterol 2014; 20: 17804-18. ArticlePubMedPMC
- 6. Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 2008; 68: 918-26. ArticlePubMedPMCPDF
- 7. Pankova D, Chen Y, Terajima M, et al. Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol Cancer Res 2016; 14: 287-95. ArticlePubMedPDF
- 8. Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch 2011; 459: 465-75. ArticlePubMedPMC
- 9. Kikuchi Y, Kashima TG, Nishiyama T, et al. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem 2008; 56: 753-64. ArticlePubMedPMCPDF
- 10. Fukushima N, Kikuchi Y, Nishiyama T, Kudo A, Fukayama M. Periostin deposition in the stroma of invasive and intraductal neoplasms of the pancreas. Mod Pathol 2008; 21: 1044-53. ArticlePubMedPDF
- 11. Yan W, Shao R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation. J Biol Chem 2006; 281: 19700-8. ArticlePubMed
- 12. Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for αVβ3 and αVβ5 integrins and promotes cell motility. Cancer Res 2002; 62: 5358-64. PubMed
- 13. Baril P, Gangeswaran R, Mahon PC, et al. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene 2007; 26: 2082-94. ArticlePubMedPDF
- 14. Bao S, Ouyang G, Bai X, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 2004; 5: 329-39. ArticlePubMed
- 15. Sung PL, Jan YH, Lin SC, et al. Periostin in tumor microenvironment is associated with poor prognosis and platinum resistance in epithelial ovarian carcinoma. Oncotarget 2016; 7: 4036-47. ArticlePubMed
- 16. Underwood TJ, Hayden AL, Derouet M, et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol 2015; 235: 466-77. ArticlePubMedPMCPDF
- 17. Xu X, Chang W, Yuan J, et al. Periostin expression in intra-tumoral stromal cells is prognostic and predictive for colorectal carcinoma via creating a cancer-supportive niche. Oncotarget 2016; 7: 798-813. ArticlePubMed
- 18. Kim JH, Shin SH, Kwon HJ, Cho NY, Kang GH. Prognostic implications of CpG island hypermethylator phenotype in colorectal cancers. Virchows Arch 2009; 455: 485-94. ArticlePubMedPDF
- 19. Ratajczak-Wielgomas K, Dziegiel P. The role of periostin in neoplastic processes. Folia Histochem Cytobiol 2015; 53: 120-32. ArticlePubMed
- 20. Kyutoku M, Taniyama Y, Katsuragi N, et al. Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Mol Med 2011; 28: 181-6. PubMed
- 21. Shao R, Bao S, Bai X, et al. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol 2004; 24: 3992-4003. ArticlePubMedPMCPDF
- 22. Takanami I, Abiko T, Koizumi S. Expression of periostin in patients with non-small cell lung cancer: correlation with angiogenesis and lymphangiogenesis. Int J Biol Markers 2008; 23: 182-6. PubMed
- 23. Wu G, Wang X, Zhang X. Clinical implications of periostin in the liver metastasis of colorectal cancer. Cancer Biother Radiopharm 2013; 28: 298-302. PubMed
- 24. Xiao ZM, Wang XY, Wang AM. Periostin induces chemoresistance in colon cancer cells through activation of the PI3K/Akt/survivin pathway. Biotechnol Appl Biochem 2015; 62: 401-6. PubMed
- 25. Dawson H, Lugli A. Molecular and pathogenetic aspects of tumor budding in colorectal cancer. Front Med (Lausanne) 2015; 2: 11.PubMedPMC
- 26. Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015; 527: 525-30. PubMedPMC
- 27. Koelzer VH, Dawson H, Andersson E, et al. Active immunosurveillance in the tumor microenvironment of colorectal cancer is associated with low frequency tumor budding and improved outcome. Transl Res 2015; 166: 207-17. PubMed
- 28. Ueno H, Shinto E, Shimazaki H, et al. Histologic categorization of desmoplastic reaction: its relevance to the colorectal cancer microenvironment and prognosis. Ann Surg Oncol 2015; 22: 1504-12. PubMed
- 29. De Sousa EM, Wang X, Jansen M, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med 2013; 19: 614-8. PubMed
- 30. Fessler E, Drost J, van Hooff SR, et al. TGFbeta signaling directs serrated adenomas to the mesenchymal colorectal cancer subtype. EMBO Mol Med 2016; 8: 745-60. PubMedPMC
- 31. Kim JH, Bae JM, Cho NY, Kang GH. Distinct features between MLH1-methylated and unmethylated colorectal carcinomas with the CpG island methylator phenotype: implications in the serrated neoplasia pathway. Oncotarget 2016; 7: 14095-111. PubMedPMC
- 32. Tsai JH, Cheng CH, Chen CC, et al. Traditional serrated adenoma with BRAF mutation is associated with synchronous/metachronous BRAF-mutated serrated lesions. Histopathology 2016; 68: 810-8. PubMed
REFERENCES
Figure & Data
References
Citations

- Unmasking a Recessive Allele by a Rare Interstitial Deletion at 10q26.13q26.2: Prenatal Diagnosis of MMP21 ‐Related Disorder and Further Refine INSYN2A Involvement in the Postnatal Cognitive Phenotype
Jiasun Su, Shujie Zhang, Wei Li, Yuan Wei, Fei Lin, Chaofan Zhou, Xianglian Tang, Yueyun Lan, Minpan Huang, Qiang Zhang, Shang Yi, Qi Yang, Sheng Yi, Xunzhao Zhou, Zailong Qin, Peng Huang
Molecular Genetics & Genomic Medicine.2025;[Epub] CrossRef - Periostin from Tumor Stromal Cells Might Be Associated with Malignant Progression of Colorectal Cancer via Smad2/3
Canfeng Fan, Qiang Wang, Saki Kanei, Kyoka Kawabata, Hinano Nishikubo, Rika Aoyama, Zhonglin Zhu, Daiki Imanishi, Takashi Sakuma, Koji Maruo, Gen Tsujio, Yurie Yamamoto, Tatsunari Fukuoka, Masakazu Yashiro
Cancers.2025; 17(3): 551. CrossRef - Electroanalytical Immunotool to Determine Matricellular Protein Periostin, a Stromal Biomarker of Prognosis in Colorectal Cancer
Marina Blázquez‐García, Jennifer Quinchia, Víctor Ruiz‐Valdepeñas Montiel, Rebeca M. Torrente‐Rodríguez, Verónica Serafín, María Garranzo‐Asensio, Ana García‐Romero, Jahir Orozco, Rodrigo Barderas, José M. Pingarrón, Susana Campuzano
ChemElectroChem.2024;[Epub] CrossRef - Simultaneous Expression of CD70 and POSTN in Cancer-Associated Fibroblasts Predicts Worse Survival of Colorectal Cancer Patients
Masayuki Komura, Chengbo Wang, Sunao Ito, Shunsuke Kato, Akane Ueki, Masahide Ebi, Naotaka Ogasawara, Toyonori Tsuzuki, Kenji Kasai, Kunio Kasugai, Shuji Takiguchi, Satoru Takahashi, Shingo Inaguma
International Journal of Molecular Sciences.2024; 25(5): 2537. CrossRef - The combined tumour-based Fascin/Snail and stromal periostin reveals the effective prognosis prediction in colorectal cancer patients
Niphat Jirapongwattana, Suyanee Thongchot, Ananya Pongpaibul, Atthaphorn Trakarnsanga, Jean Quinn, Peti Thuwajit, Chanitra Thuwajit, Joanne Edwards, Peng Zhang
PLOS ONE.2024; 19(6): e0304666. CrossRef - SPOCK1 and POSTN are valuable prognostic biomarkers and correlate with tumor immune infiltrates in colorectal cancer
Caiqin Gan, Mengting Li, Yuanyuan Lu, Ganjing Peng, Wenjie Li, Haizhou Wang, Yanan Peng, Qian Hu, Wanhui Wei, Fan Wang, Lan Liu, Qiu Zhao
BMC Gastroenterology.2023;[Epub] CrossRef - Stromal POSTN Enhances Motility of Both Cancer and Stromal Cells and Predicts Poor Survival in Colorectal Cancer
Akane Ueki, Masayuki Komura, Akira Koshino, Chengbo Wang, Kazuhiro Nagao, Mai Homochi, Yuki Tsukada, Masahide Ebi, Naotaka Ogasawara, Toyonori Tsuzuki, Kenji Kasai, Kunio Kasugai, Satoru Takahashi, Shingo Inaguma
Cancers.2023; 15(3): 606. CrossRef - POSTN Secretion by Extracellular Matrix Cancer-Associated Fibroblasts (eCAFs) Correlates with Poor ICB Response via Macrophage Chemotaxis Activation of Akt Signaling Pathway in Gastric Cancer
Tingting You, Hui Tang, Wenjing Wu, Jingxi Gao, Xuechun Li, Ningning Li, Xiuxiu Xu, Jiazhang Xing, Hui Ge, Yi Xiao, Junchao Guo, Bin Wu, Xiaoyi Li, Liangrui Zhou, Lin Zhao, Chunmei Bai, Qin Han, Zhao Sun, Robert Chunhua Zhao
Aging and disease.2023; 14(6): 2177. CrossRef - A Pan-cancer Analysis Reveals the Tissue Specificity and Prognostic Impact
of Angiogenesis-associated Genes in Human Cancers
Zhenshen Bao, Minzhen Liao, Wanqi Dong, Yanhao Huo, Xianbin Li, Peng Xu, Wenbin Liu
Current Bioinformatics.2023; 18(8): 670. CrossRef - Cancer‐associated stroma reveals prognostic biomarkers and novel insights into the tumour microenvironment of colorectal cancer and colorectal liver metastases
Kai M. Brown, Aiqun Xue, Ross C. Smith, Jaswinder S. Samra, Anthony J. Gill, Thomas J. Hugh
Cancer Medicine.2022; 11(2): 492. CrossRef - Periostin in Angiogenesis and Inflammation in CRC—A Preliminary Observational Study
Agnieszka Kula, Miriam Dawidowicz, Sylwia Mielcarska, Paweł Kiczmer, Magdalena Chrabańska, Magdalena Rynkiewicz, Elżbieta Świętochowska, Dariusz Waniczek
Medicina.2022; 58(1): 96. CrossRef - Periostin promotes the proliferation and metastasis of osteosarcoma by increasing cell survival and activates the PI3K/Akt pathway
Chaojian Xu, Ziyue Wang, Long Zhang, Yi Feng, Jia Lv, Zhuangzhuang Wu, Rong Yang, Taiyong Wu, Jian Li, Ruhao Zhou, Zhi Tian, Junjun Bai, Huadong Zhang, Yanping Lan, Zhi Lv
Cancer Cell International.2022;[Epub] CrossRef - Periostin in lymph node pre-metastatic niches governs lymphatic endothelial cell functions and metastatic colonization
Lionel Gillot, Alizée Lebeau, Louis Baudin, Charles Pottier, Thomas Louis, Tania Durré, Rémi Longuespée, Gabriel Mazzucchelli, Christophe Nizet, Silvia Blacher, Frédéric Kridelka, Agnès Noël
Cellular and Molecular Life Sciences.2022;[Epub] CrossRef - Prognostic impact of stromal periostin expression in upper urinary tract urothelial carcinoma
Kosuke Miyai, Kazuki Kawamura, Keiichi Ito, Susumu Matsukuma, Hitoshi Tsuda
BMC Cancer.2022;[Epub] CrossRef - Periostin‐ and podoplanin‐positive cancer‐associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma
Cindy Neuzillet, Rémy Nicolle, Jérôme Raffenne, Annemilaï Tijeras‐Raballand, Alexia Brunel, Lucile Astorgues‐Xerri, Sophie Vacher, Floriane Arbateraz, Marjorie Fanjul, Marc Hilmi, Rémi Samain, Christophe Klein, Aurélie Perraud, Vinciane Rebours, Muriel Ma
The Journal of Pathology.2022; 258(4): 408. CrossRef - Periostin: biology and function in cancer
Shima Dorafshan, Mahdieh Razmi, Sadegh Safaei, Erica Gentilin, Zahra Madjd, Roya Ghods
Cancer Cell International.2022;[Epub] CrossRef - Periostin as a key molecule defining desmoplastic environment in colorectal cancer
Takahiro Sueyama, Yoshiki Kajiwara, Satsuki Mochizuki, Hideyuki Shimazaki, Eiji Shinto, Kazuo Hase, Hideki Ueno
Virchows Archiv.2021; 478(5): 865. CrossRef - Expression Patterns of Microenvironmental Factors and Tenascin-C at the Invasive Front of Stage II and III Colorectal Cancer: Novel Tumor Prognostic Markers
Mai Hashimoto, Noriyuki Uesugi, Mitsumasa Osakabe, Naoki Yanagawa, Koki Otsuka, Yoshiki Kajiwara, Hideki Ueno, Akira Sasaki, Tamotsu Sugai
Frontiers in Oncology.2021;[Epub] CrossRef - Deregulation of extracellular matrix modeling with molecular prognostic markers revealed by transcriptome sequencing and validations in Oral Tongue squamous cell carcinoma
Soundara Viveka Thangaraj, Vidyarani Shyamsundar, Arvind Krishnamurthy, Vijayalakshmi Ramshankar
Scientific Reports.2021;[Epub] CrossRef - Inhibition of Postn Rescues Myogenesis Defects in Myotonic Dystrophy Type 1 Myoblast Model
Xiaopeng Shen, Zhongxian Liu, Chunguang Wang, Feng Xu, Jingyi Zhang, Meng Li, Yang Lei, Ao Wang, Chao Bi, Guoping Zhu
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Radiographical assessment of tumour stroma and treatment outcomes using deep learning: a retrospective, multicohort study
Yuming Jiang, Xiaokun Liang, Zhen Han, Wei Wang, Sujuan Xi, Tuanjie Li, Chuanli Chen, Qingyu Yuan, Na Li, Jiang Yu, Yaoqin Xie, Yikai Xu, Zhiwei Zhou, George A Poultsides, Guoxin Li, Ruijiang Li
The Lancet Digital Health.2021; 3(6): e371. CrossRef - Periostin expression and its supposed roles in benign and malignant thyroid nodules: an immunohistochemical study of 105 cases
Kimihide Kusafuka, Masaru Yamashita, Tomohiro Iwasaki, Chinatsu Tsuchiya, Aki Kubota, Kazuki Hirata, Akinori Murakami, Aya Muramatsu, Kazumori Arai, Makoto Suzuki
Diagnostic Pathology.2021;[Epub] CrossRef - Cancer-associated fibroblasts in colorectal cancer
S. Kamali Zonouzi, P. S. Pezeshki, S. Razi, N. Rezaei
Clinical and Translational Oncology.2021; 24(5): 757. CrossRef - Prognostic value of periostin in multiple solid cancers: A systematic review with meta‐analysis
Tao Yang, Zhengdong Deng, Zhongya Pan, Yawei Qian, Wei Yao, Jianming Wang
Journal of Cellular Physiology.2020; 235(3): 2800. CrossRef - Serum periostin is associated with cancer mortality but not cancer risk in older home-dwelling men: A 8-year prospective analysis of the STRAMBO study
Jean-Charles Rousseau, Cindy Bertholon, Roland Chapurlat, Pawel Szulc
Bone.2020; 132: 115184. CrossRef - Systematic prediction of key genes for ovarian cancer by co‐expression network analysis
Mingyuan Wang, Jinjin Wang, Jinglan Liu, Lili Zhu, Heng Ma, Jiang Zou, Wei Wu, Kangkai Wang
Journal of Cellular and Molecular Medicine.2020; 24(11): 6298. CrossRef - Upregulation of adipocyte enhancer‐binding protein 1 in endothelial cells promotes tumor angiogenesis in colorectal cancer
Akira Yorozu, Eiichiro Yamamoto, Takeshi Niinuma, Akihiro Tsuyada, Reo Maruyama, Hiroshi Kitajima, Yuto Numata, Masahiro Kai, Gota Sudo, Toshiyuki Kubo, Toshihiko Nishidate, Kenji Okita, Ichiro Takemasa, Hiroshi Nakase, Tamotsu Sugai, Kenichi Takano, Hiro
Cancer Science.2020; 111(5): 1631. CrossRef - Periostin aggravates NLRP3 inflammasome-mediated pyroptosis in myocardial ischemia-reperfusion injury
Lei Yao, Jie Song, Xiao wen Meng, Jian yun Ge, Bo xiang Du, Jun Yu, Fu hai Ji
Molecular and Cellular Probes.2020; 53: 101596. CrossRef - Vitamin K and Kidney Transplantation
Maria Fusaro, Laura Cosmai, Pieter Evenepoel, Thomas L. Nickolas, Angela M. Cheung, Andrea Aghi, Giovanni Tripepi, Mario Plebani, Giorgio Iervasi, Roberto Vettor, Martina Zaninotto, Maura Ravera, Marina Foramitti, Sandro Giannini, Stefania Sella, Maurizio
Nutrients.2020; 12(9): 2717. CrossRef - Periostin regulates autophagy through integrin α5β1 or α6β4 and an AKT‐dependent pathway in colorectal cancer cell migration
Suyanee Thongchot, Ekapot Singsuksawat, Nuttavut Sumransub, Ananya Pongpaibul, Attaporn Trakarnsanga, Peti Thuwajit, Chanitra Thuwajit
Journal of Cellular and Molecular Medicine.2020; 24(21): 12421. CrossRef - Genomic, transcriptomic, and viral integration profiles associated with recurrent/metastatic progression in high‐risk human papillomavirus cervical carcinomas
Jing Jing Liu, Jung Yoon Ho, Jung Eum Lee, Soo Young Hur, Jinseon Yoo, Kyu Ryung Kim, Daeun Ryu, Tae Min Kim, Youn Jin Choi
Cancer Medicine.2020; 9(21): 8243. CrossRef - Periostin Secreted by Carcinoma-Associated Fibroblasts Promotes Ovarian Cancer Cell Platinum Resistance Through the PI3K/Akt Signaling Pathway
Lei Chu, Fangce Wang, Wenjun Zhang, Huai-fang Li, Jun Xu, Xiao-wen Tong
Technology in Cancer Research & Treatment.2020;[Epub] CrossRef - Overexpression of periostin is positively associated with gastric cancer metastasis through promoting tumor metastasis and invasion
Hai Zhong, Xiang Li, Junhua Zhang, Xu Wu
Journal of Cellular Biochemistry.2019; 120(6): 9927. CrossRef - Genes associated with bowel metastases in ovarian cancer
Andrea Mariani, Chen Wang, Ann L. Oberg, Shaun M. Riska, Michelle Torres, Joseph Kumka, Francesco Multinu, Gunisha Sagar, Debarshi Roy, Deok–Beom Jung, Qing Zhang, Tommaso Grassi, Daniel W. Visscher, Vatsal P. Patel, Ling Jin, Julie K. Staub, William A. C
Gynecologic Oncology.2019; 154(3): 495. CrossRef - Periostin: A Matricellular Protein With Multiple Functions in Cancer Development and Progression
Laura González-González, Javier Alonso
Frontiers in Oncology.2018;[Epub] CrossRef - Upregulation of Periostin expression in the pathogenesis of ameloblastoma
Yuanyuan Kang, Jie Liu, Ying Zhang, Yan Sun, Junting Wang, Biying Huang, Ming Zhong
Pathology - Research and Practice.2018; 214(12): 1959. CrossRef - Periostin expression in neoplastic and non-neoplastic diseases of bone and joint
Jennifer M. Brown, Akiro Mantoku, Afsie Sabokbar, Udo Oppermann, A. Bass Hassan, Akiro Kudo, Nick Athanasou
Clinical Sarcoma Research.2018;[Epub] CrossRef - Molecular patterns of cancer colonisation in lymph nodes of breast cancer patients
Gaurav Chatterjee, Trupti Pai, Thomas Hardiman, Kelly Avery-Kiejda, Rodney J. Scott, Jo Spencer, Sarah E. Pinder, Anita Grigoriadis
Breast Cancer Research.2018;[Epub] CrossRef - Multiplicity of Advanced T Category–Tumors Is a Risk Factor for Survival in Patients with Colorectal Carcinoma
Hye Eun Park, Seungyeon Yoo, Jeong Mo Bae, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2018; 52(6): 386. CrossRef - Periostin attenuates tumor growth by inducing apoptosis in colitis-related colorectal cancer
Yusuke Shimoyama, Keiichi Tamai, Rie Shibuya, Mao Nakamura, Mai Mochizuki, Kazunori Yamaguchi, Yoichi Kakuta, Yoshitaka Kinouchi, Ikuro Sato, Akira Kudo, Tooru Shimosegawa, Kennichi Satoh
Oncotarget.2018; 9(28): 20008. CrossRef - Periostin serves an important role in the pathogenesis of oral squamous cell carcinoma
Yuanyuan Kang, Xue Wang, Ying Zhang, Yan Sun
Oncology Letters.2018;[Epub] CrossRef - The prognostic significance of cancer-associated fibroblasts in pancreatic ductal adenocarcinoma
Hyunjin Park, Yangkyu Lee, Hyejung Lee, Jin-Won Kim, Jin-Hyeok Hwang, Jaihwan Kim, Yoo-Seok Yoon, Ho-Seong Han, Haeryoung Kim
Tumor Biology.2017; 39(10): 101042831771840. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| Variable | POSTN-low (n = 821, 73.0%) | POSTN-high (n = 304, 27.0%) | p-value | |
|---|---|---|---|---|
| Age (yr) | 62 (20–87) | 62 (29–90) | .932 | |
| Sex | Male | 500 (60.9) | 173 (56.9) | .225 |
| Female | 321 (39.1) | 131 (43.1) | ||
| Location | Proximal | 170 (20.7) | 107 (35.2) | < .001 |
| Distal | 336 (40.9) | 102 (33.5) | ||
| Rectum | 315 (38.4) | 95 (31.3) | ||
| Growth pattern | Fungating | 569 (69.3) | 168 (55.3) | < .001 |
| Fungating | 252 (30.7) | 136 (44.7) | ||
| T category | T1,2 | 192 (23.4) | 18 (5.9) | < .001 |
| T3,4 | 629 (76.6) | 286 (94.1) | ||
| N category | N0 | 454 (55.3) | 117 (38.5) | < .001 |
| N1,2 | 367 (44.7) | 187 (61.5) | ||
| M category | 0 | 712 (86.7) | 222 (73.0) | < .001 |
| 1 | 109 (13.3) | 82 (27.0) | ||
| Stage | I, II | 432 (52.6) | 104 (34.2) | < .001 |
| III, IV | 109 (13.3) | 200 (65.8) | ||
| Differentiation | Differentiated | 802 (97.7) | 284 (93.4) | .001 |
| Undifferentiated | 19 (2.3) | 20 (6.6) | ||
| Tumor budding | Absent | 261 (31.8) | 58 (19.1) | < .001 |
| Present | 560 (68.2) | 246 (80.9) | ||
| Dirty necrosis | Absent | 85 (10.4) | 17 (5.6) | .014 |
| Present | 736 (89.6) | 287 (94.4) | ||
| Crohn-like reaction | Absent | 703 (85.6) | 251 (82.6) | .204 |
| Present | 118 (14.4) | 53 (17.4) | ||
| Tumor-infiltrating lymphocytes | High (≥ 8/HPF) | 599 (73.0) | 233 (76.6) | .211 |
| Low (< 8/HPF) | 222 (27.0) | 71 (23.4) | ||
| Serration | Absent | 793 (96.6) | 288 (94.7) | .155 |
| Present | 28 (3.4) | 16 (5.3) | ||
| Mucin production | Absent | 727 (88.5) | 265 (87.2) | .525 |
| Present | 94 (11.5) | 39 (12.8) | ||
| CIMP | CIMP-0 | 387 (47.1) | 123 (40.5) | .030 |
| CIMP-low | 396 (48.2) | 157 (51.6) | ||
| CIMP-high | 38 (4.6) | 24 (7.9) | ||
| MSI | MSS | 702 (85.5) | 262 (86.2) | .959 |
| MSI-low | 54 (6.6) | 19 (6.2) | ||
| MSI-high | 65 (7.9) | 23 (7.6) | ||
| KRAS mutation | Wild type | 602 (73.3) | 210 (69.1) | .158 |
| Mutant | 219 (26.7) | 94 (30.9) | ||
| BRAF mutation (n = 1,124) | Wild type | 791 (96.5) | 285 (93.7) | .046 |
| Mutant | 29 (3.5) | 19 (6.3) |
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR | p-value | HR | p-value | |
| Gross (infiltrative/fungating) | 1.87 (1.32–2.66) | < .001 | 1.64 (1.24–2.18) | .001 |
| Stage (III, IV/I, II) | 4.52 (3.26–6.26) | < .001 | 6.30 (4.27–9.29) | < .001 |
| Differentiation (PD/WD, MD) | 3.41 (2.05–5.67) | < .001 | 2.15 (1.27–3.62) | .013 |
| BRAF (mutant/wild type) | 1.92 (1.15–3.19) | .012 | 1.92 (1.11–3.34) | .041 |
| Chemotherapy (treated/not-treated) | 0.94 (0.71–1.25) | .655 | 0.38 (0.28–0.52) | < .001 |
| POSTN (high/low) | 2.00 (1.52–2.64) | < .001 | 1.50 (1.13–2.00) | .006 |
| Tumor location (right/left) | 1.56 (1.19–2.06) | .002 | 1.46 (1.09–1.96) | .011 |
| Tumor-infiltrating lymphocytes (high/low) | 0.69 (0.49–0.97) | .032 | - | .051 |
| Budding (present/absent) | 1.90 (1.36–2.66) | < .001 | - | .129 |
| Crohn-like reaction (present/absent) | 0.71 (0.48–1.06) | .095 | - | .305 |
| Age (≥ 65 yr/< 65 yr) | 1.49 (1.15–1.93) | .003 | - | .053 |
| CIMP (CIMP-H/CIMP-0, L) | 1.91 (1.21–3.02) | .006 | - | .632 |
| Sex (male/female) | 1.04 (0.80–1.36) | .762 | - | - |
| Necrosis (present/absent) | 1.11 (0.70–1.78) | .652 | - | - |
| Serration (present/absent) | 1.42 (0.80–2.54) | .235 | - | - |
| Mucin (present/absent) | 1.32 (0.92–1.90) | .136 | - | - |
| MSI (MSI-H/MSS, MSI-L) | 0.82 (0.48–1.41) | .476 | - | - |
| KRAS (mutant/wild type) | 1.12 (0.84–1.49) | .440 | - | - |
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Gross (infiltrative/fungating) | 1.96 (1.61–2.39) | < .001 | 1.43 (1.17–1.76) | .001 |
| Stage (III, IV/I, II) | 4.72 (3.71–6.02) | < .001 | 4.75 (3.63–6.23) | < .001 |
| Differentiation (PD/WD, MD) | 3.32 (2.28–4.85) | < .001 | 1.85 (1.26–2.71) | .002 |
| BRAF (mutant/wild type) | 1.61 (1.06–2.45) | .027 | 1.72 (1.12–2.63) | .013 |
| Chemotherapy (treated/not-treated) | 1.40 (1.11–1.76) | .004 | 0.59 (0.46–0.76) | < .001 |
| POSTN (high/low) | 1.80 (1.47–2.20) | < .001 | 1.40 (1.08–1.83) | .012 |
| Budding (present/absent) | 2.15 (1.66–2.79) | < .001 | 1.51 (1.15–1.97) | .003 |
| Crohn-like reaction (present/absent) | 0.72 (0.53–0.98) | .034 | - | .302 |
| Tumor-infiltrating lymphocytes (high/low) | 0.70 (0.55–0.89) | .004 | - | .089 |
| Tumor location (right/left) | 1.22 (0.98–1.51) | .080 | - | .773 |
| Age (≥ 6 yr/< 65 yr) | 1.22 (1.00–1.49) | .046 | - | .098 |
| CIMP (CIMP-H/CIMP-0, L) | 1.44 (0.98–2.12) | .061 | - | .277 |
| Sex (male/female) | 0.94 (0.77–1.14) | .514 | - | - |
| Necrosis (present/absent) | 1.14 (0.80–1.62) | .463 | - | - |
| Serration (present/absent) | 1.32 (0.83–2.08) | .244 | - | - |
| Mucin (present/absent) | 1.12 (0.84–1.50) | .448 | - | - |
| MSI (MSI-H/MSS, MSI-L) | 0.80 (0.54–1.20) | .285 | - | - |
| KRAS (mutant/wild type) | 1.05 (0.85–1.31) | .633 | - | - |
Values are presented as median (range) or number (%). POSTN, periostin; HPF, high-power field; CIMP, CpG island methylator phenotype; MSI, microsatellite instability.
HR, hazard ratio; PD, poorly differentiated; WD, well differentiated; MD, moderately differentiated; POSTN, periostin; CIMP, CpG island methylator phenotype; CIMP-H, CIMP-high; CIMP-L, CIMP-low; MSI, microsatellite instability; MSI-H, MSI-high; MSS, microsatellite stable; MSI-L, MSI-low.
HR, hazard ratio; CI, confidence interval; PD, poorly differentiated; WD, well differentiated; MD, moderately differentiated; POSTN, periostin; CIMP, CpG island methylator phenotype; CIMP-H, CIMP-high; MSI, microsatellite instability; MSI-H, MSI-high; MSS, microsatellite stable; MSI-L, MSI-low.

 E-submission
E-submission