Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(5); 2017 > Article
-
Original Article
Diverse Immunoprofile of Ductal Adenocarcinoma of the Prostate with an Emphasis on the Prognostic Factors - Se Un Jeong, Anuja Kashikar Kekatpure, Ja-Min Park1, Minkyu Han2, Hee Sang Hwang, Hui Jeong Jeong, Heounjeong Go, Yong Mee Cho,
-
Journal of Pathology and Translational Medicine 2017;51(5):471-481.
DOI: https://doi.org/10.4132/jptm.2017.06.02
Published online: August 9, 2017
Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
1Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding Author Yong Mee Cho, MD, PhD Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-5965 Fax: +82-2-3010-7898 E-mail: yongcho@amc.seoul.kr
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Ductal adenocarcinoma (DAC) of the prostate is an uncommon histologic subtype whose prognostic factors and immunoprofile have not been fully defined.
-
Methods
- To define its prognostic factors and immunoprofile, the clinicopathological features, including biochemical recurrence (BCR), of 61 cases of DAC were analyzed. Immunohistochemistry was performed on tissue microarray constructs to assess the expression of prostate cancer-related and mammalian target of rapamycin (mTOR) signaling-related proteins.
-
Results
- During the median follow-up period of 19.3 months, BCR occurred in 26 cases (42.6%). DAC demonstrated a wide expression range of prostate cancer-related proteins, including nine cases (14.8%) that were totally negative for pan-cytokeratin (PanCK) immunostaining. The mTOR signaling-related proteins also showed diverse expression. On univariate analysis, BCR was associated with high preoperative serum levels of prostate-specific antigen (PSA), large tumor volume, predominant ductal component, high Gleason score (GS), comedo-necrosis, high tumor stage (pT), lymphovascular invasion, and positive surgical margin. High expressions of phospho-mTOR (p-mTOR) as well as low expressions of PSA, phospho-S6 ribosomal protein (pS6) and PanCK were associated with BCR. On multivariable analysis, GS, pT, and immunohistochemical expressions of PanCK and p-mTOR remained independent prognostic factors for BCR.
-
Conclusions
- These results suggest GS, pT, and immunohistochemical expressions of PanCK and p-mTOR as independent prognostic factors for BCR in DAC. Since DAC showed diverse expression of prostate cancer–related proteins, this should be recognized in interpreting the immunoprofile of DAC. The diverse expression of mTOR-related proteins implicates their potential utility as predictive markers for mTOR targeted therapy.
- Study samples
- This retrospective study initially included 87 cases that underwent radical prostatectomy for clinically localized prostate cancer and were pathologically diagnosed as DAC between January 1995 and December 2015 at Asan Medical Center (Seoul, Republic of Korea). None of these cases were treated with neoadjuvant androgen deprivation therapy. A total of 26 cases were excluded for the following reasons: 16 cases were reassessed as AAC during retrospective review; nine cases were excluded either because the tumor tissue was too small to construct two representative cores of tissue microarray (TMA) or because formalin-fixed paraffin-embedded tissue blocks were unavailable; and one case was excluded because clinical follow-up data was not available. As such, 61 cases of DAC were included in the final analysis.
- Patients’ clinicopathological information was obtained from electronic medical records and surgical pathology reports. BCR was defined as a serum prostate-specific antigen (PSA) level ≥0.2 ng/mL on two consecutive occasions after achieving undetectable PSA following radical prostatectomy [7]. All pathologic materials were reviewed for diagnostic reassessment according to the 2016 World Health Organization Tumor Classification [1]. Gleason score (GS) and pathologic tumor stage (pT) were assigned according to the 2015 modified Gleason grading system and the American Joint Committee on Cancer Staging System, seventh edition, respectively [1,8]. This study was approved by the Institutional Review Board of Asan Medical Center with a waiver of informed consent (2011-0499).
- TMA construction
- A TMA construct of 2-mm-diameter cores was generated from the 10% neutrally buffered formalin-fixed, paraffin-embedded tissue blocks of radical prostatectomy specimens using a tissue microarrayer (Quick-Ray, Unitma Co. Ltd., Seoul, Korea). Two representative cores from different DAC areas were included for each case.
- Immunohistochemistry
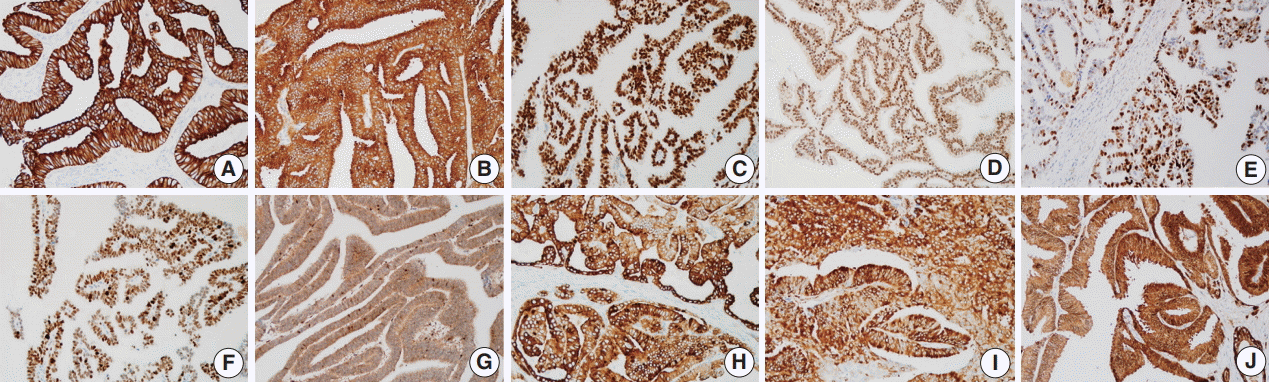
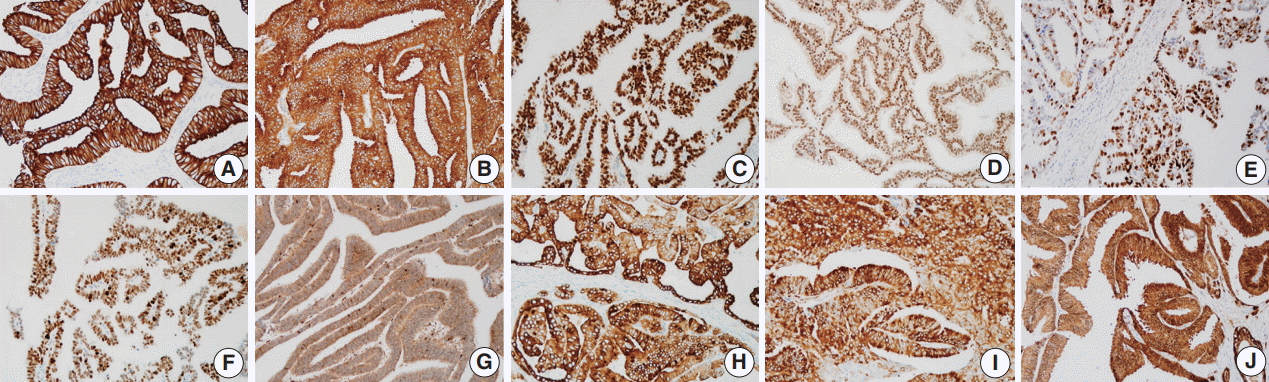
- Prostate cancer–related proteins analyzed in this study included pan-cytokeratin (PanCK), PSA, AR, enhancer of zeste homolog 2 (EZH2), p53, and ETS-related gene (ERG). Phosphatase and tensin homolog (PTEN), phospho-mammalian target of rapamycin (p-mTOR), phospho-S6 ribosomal protein (pS6), and 14-3-3 sigma protein were included as mTOR pathway–related proteins. Immunohistochemical staining was performed using an automated staining system (BenchMark XT, Ventana Medical Systems, Tucson, AZ, USA). The primary antibodies used in this study, their dilutions, and the subcellular location of each antigen are summarized in Table 1. Nuclei were counterstained with hematoxylin. Representative expression patterns of these proteins are presented in Fig. 1.
- The immunohistochemical staining results were assessed in the DAC component only by two pathologists (S.U.J. and A. K.K.), both of whom were blinded to the associated clinicopathological information. The staining intensity of the antibodies was initially scored as negative, weak, moderate, or strong. Cases with moderate to strong intensity were regarded as positive, and then the average percentage of positive cells in all cores was recorded.
- Immunohistochemistry on whole section
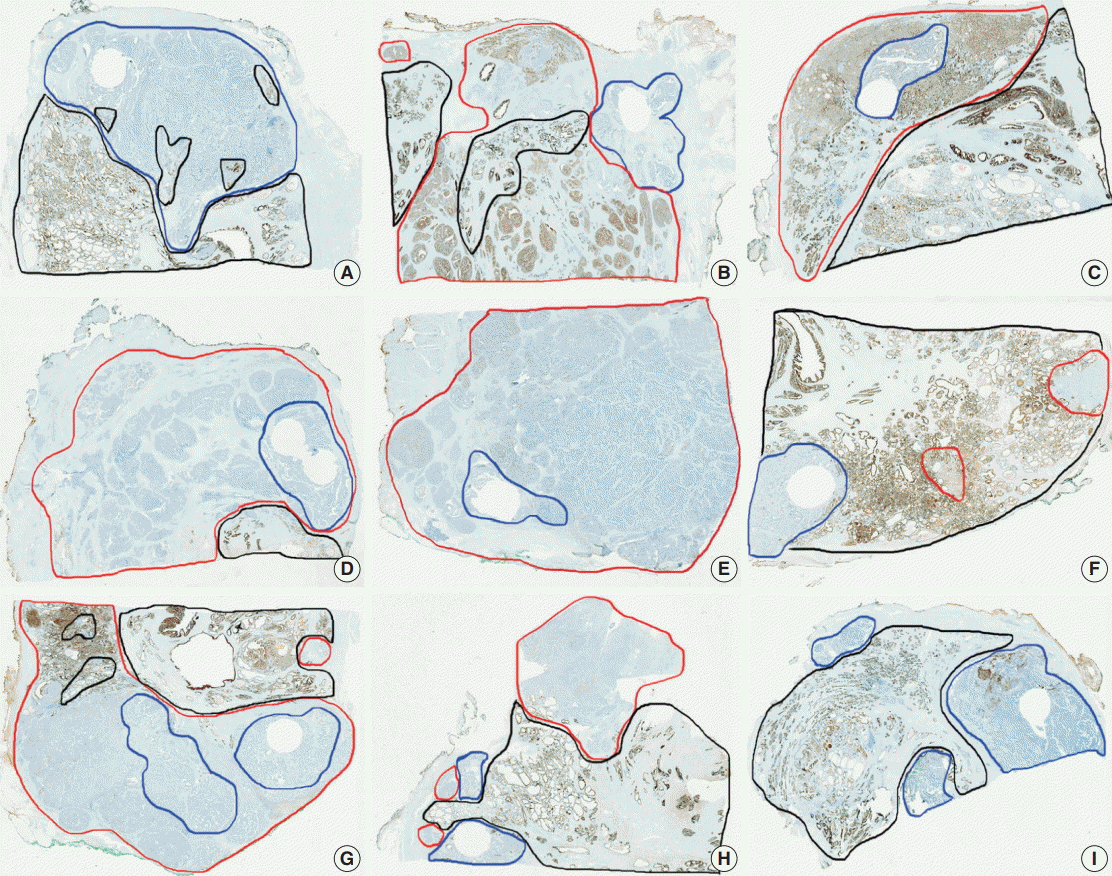
- To exclude the issues of intratumoral heterogeneity, immunohistochemistry was performed on whole sections of one negative case, one intermediate case, and one positive case for each antibody. In addition, to exclude technical problems, such as poor formalin-fixation of radical prostatectomy specimens, immunohistochemistry for PanCK was performed on whole sections of all PanCK-negative cases.
- Statistical analysis
- For descriptive statistics and univariate analyses, all continuous data were expressed as mean±standard deviation and were compared using Student’s t tests. The optimal cut-off value of the protein expression was calculated from the receiver operating characteristic (ROC) curve analysis. Categorical data were compared with the chi-square test. BCR was estimated using the Kaplan-Meier method and the resulting curves were compared by log-rank test. In order to minimize the exclusion of variables that are important in this study, all variables with p-values of <.1 in the univariate analysis were included in the multivariate analysis, for which the Cox proportional hazards model was used. The overlapping variables were excluded in the multivariate analysis. Independent variables were chosen by the stepwise method. p-values of <.05 were considered statistically significant.
MATERIALS AND METHODS
- Clinicopathological features of DAC
- The clinicopathological features of the 61 DAC cases are summarized in Table 2. The median age at the time of radical prostatectomy was 68 years (range, 51 to 77 years), with a median preoperative serum PSA level of 11.7 ng/mL (range, 0.6 to 66.4 ng/mL). The mean total tumor volume was 28.5% (range, 2% to 95%), in which the DAC component occupied 48.3% on average (range, 5% to 100%). Four cases (6.5%) were pure DAC. Among histologic DAC patterns, the papillary pattern was the most common (48 cases, 78.7%), followed by cribriform pattern (nine cases, 14.7%) and prostatic intraepithelial neoplasia-like pattern (four cases, 6.6%). A significant proportion of the cases were of high grade (GS ≥8: 41 cases, 67.2%) with accompanying comedo-necrosis in 17 cases (27.9%). The majority of the cases were of high stage (pT3: 44 cases, 72.1%) with frequent extraprostatic extension (42 cases, 68.9%), lymphovascular invasion (26 cases, 42.6%), positive surgical margin (41 cases, 67.2%), and seminal vesicle involvement (15 cases, 24.6%).
- During the median follow-up period of 19.3 months (range, 1 to 70 months), BCR occurred in 26 cases (42.6%) at a median of 10.5 months (range, 1 to 44 months) after the surgery. Two patients (3.3%) died, and one died of prostate cancer (1.6%).
- Expression of prostate cancer-related proteins in DAC
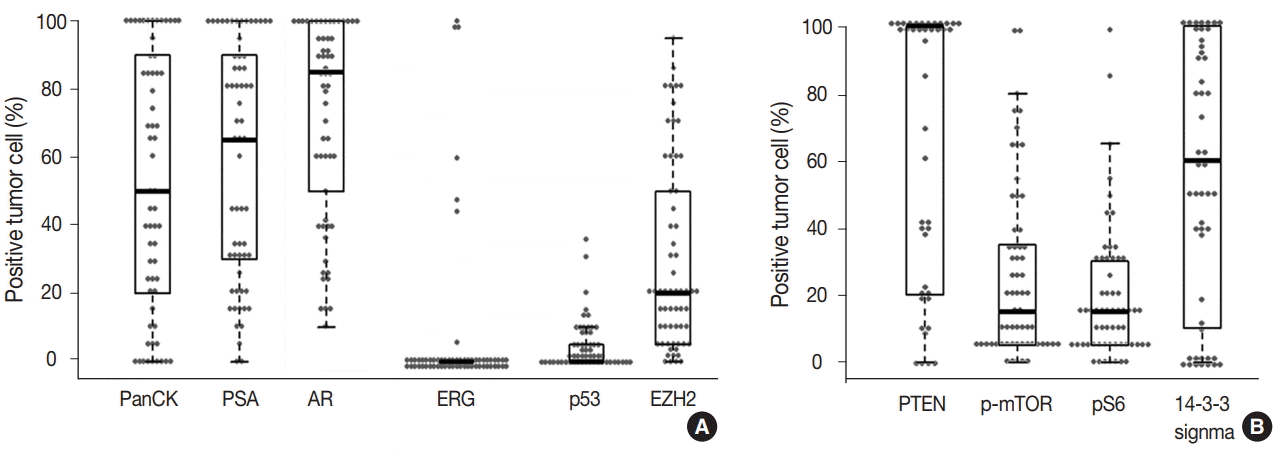
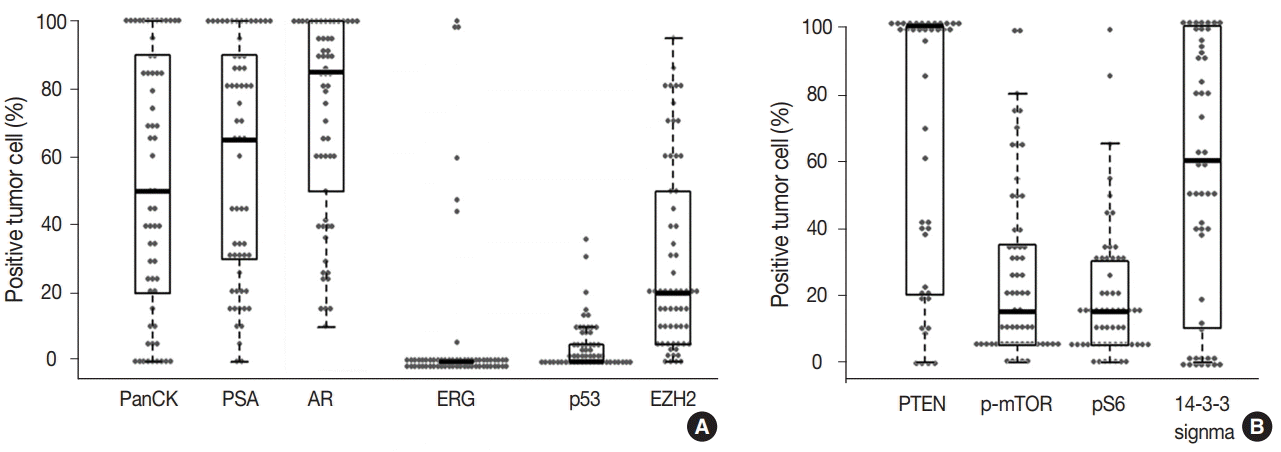
- The prostate cancer–related proteins showed diverse expressions in DAC as shown in Fig. 2A. PanCK and PSA were heterogeneously expressed with a median value of 50% and 65%, respectively (range, 0% to 100%). Furthermore, nine cases (14.8%) were negative for PanCK and and two cases (3.3%) for PSA. AR was expressed in all DAC cases with a heterogeneous pattern and a median value of 85% (range, 10% to 100%). ERG expression was not observed in most cases (54 cases, 88.5%) and only seven cases (11.5%) showed focal or diffuse positivity. p53 and EZH2 were expressed at median values of 17.5% (range, 0% to 35%) and 20% (range, 0% to 95%), respectively.
- Expression of mTOR signaling–related proteins in DAC
- The mTOR signaling-related proteins also showed diverse expressions in DAC, as shown in Fig. 2B. DAC cases showed a high expression of PTEN (median, 100%) and a low expression of p-mTOR (median, 15%) and pS6 (median, 15%). Eight cases (13.1%) showed no immunoreactivity for PTEN. 14-3-3 sigma was also expressed variably with a median value of 60% (range, 0% to 100%).
- Immunohistochemistry on whole section
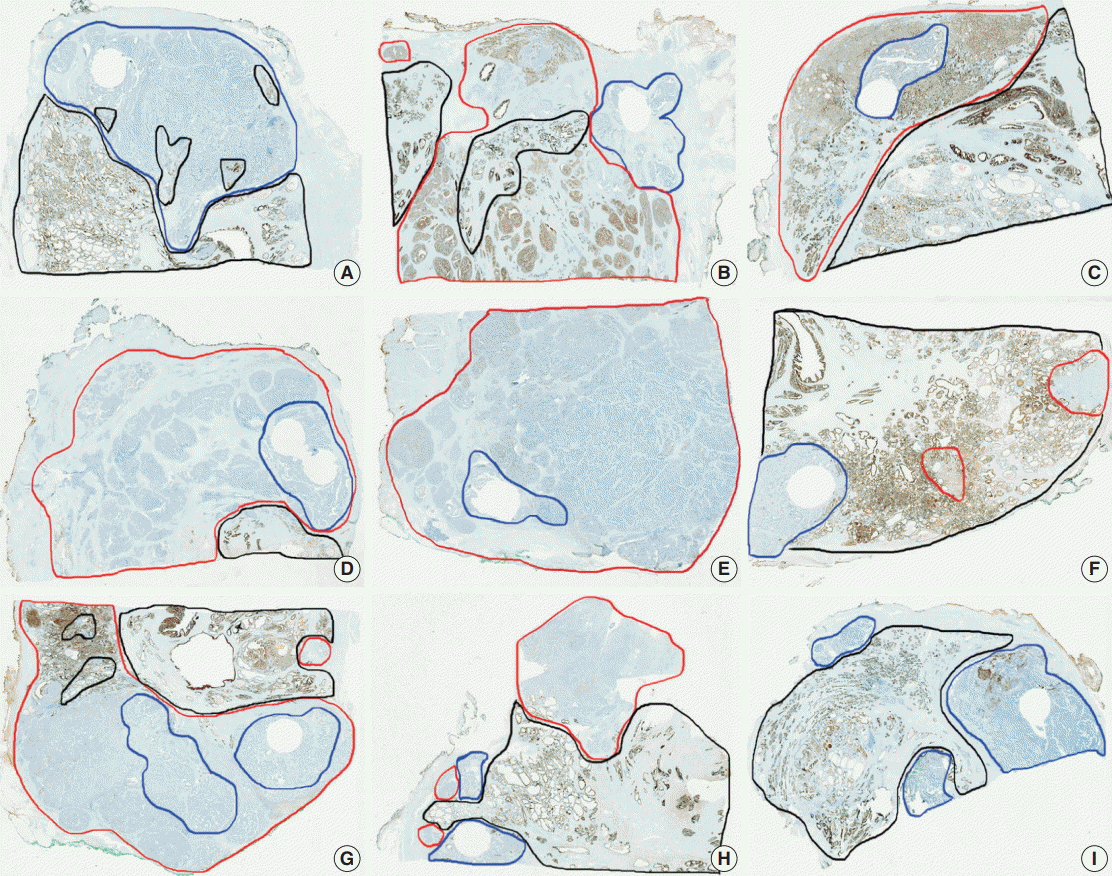
- Tumor heterogeneity was evaluated by immunohistochemistry using whole sections of one negative case, one intermediate case, and one positive case for each antibody. Although there was a slight variation in the cases of intermediate expression, a great degree of similarity was observed in all cases, especially in negative cases and entirely positive cases (data not shown). PanCK immunohistochemistry on whole sections of all PanCK-negative cases on the TMA construct showed immunopositivity in normal prostatic glands and AAC areas as shown in Fig. 3. However, PanCK was negative in eight cases among the nine cases; one case showed focal (15%) immunopositivity on the whole section. Therefore, technical problems were not an issue and the immunohistochemical data using the TMA construct were confirmed to be representative.
- Correlation of protein expression with clinicopathological features
- To define the prognostic significance of the prostate cancer-related proteins and mTOR signaling–related proteins, a cut-off expression value of each protein was determined according to the ROC curve analysis for BCR (Tables 3, 4). The correlation between expressions of prostate cancer-related proteins or mTOR signaling–related proteins and clinicopathological features are summarized in Tables 3 and 4, respectively.
- Low expression of PanCK was associated with high pT (p=.044), whereas high GS was associated with low expressions of PSA and AR (p=.006 and p=.025, respectively) and high expression of p53 (p=.015). DAC cases with lymphovascular invasion showed high expressions of ERG, p53, and EZH2 (p=.039, p=.030, and p=.025, respectively) and low expression of PSA (p=.041).
- Prognostic factors for BCR in DAC
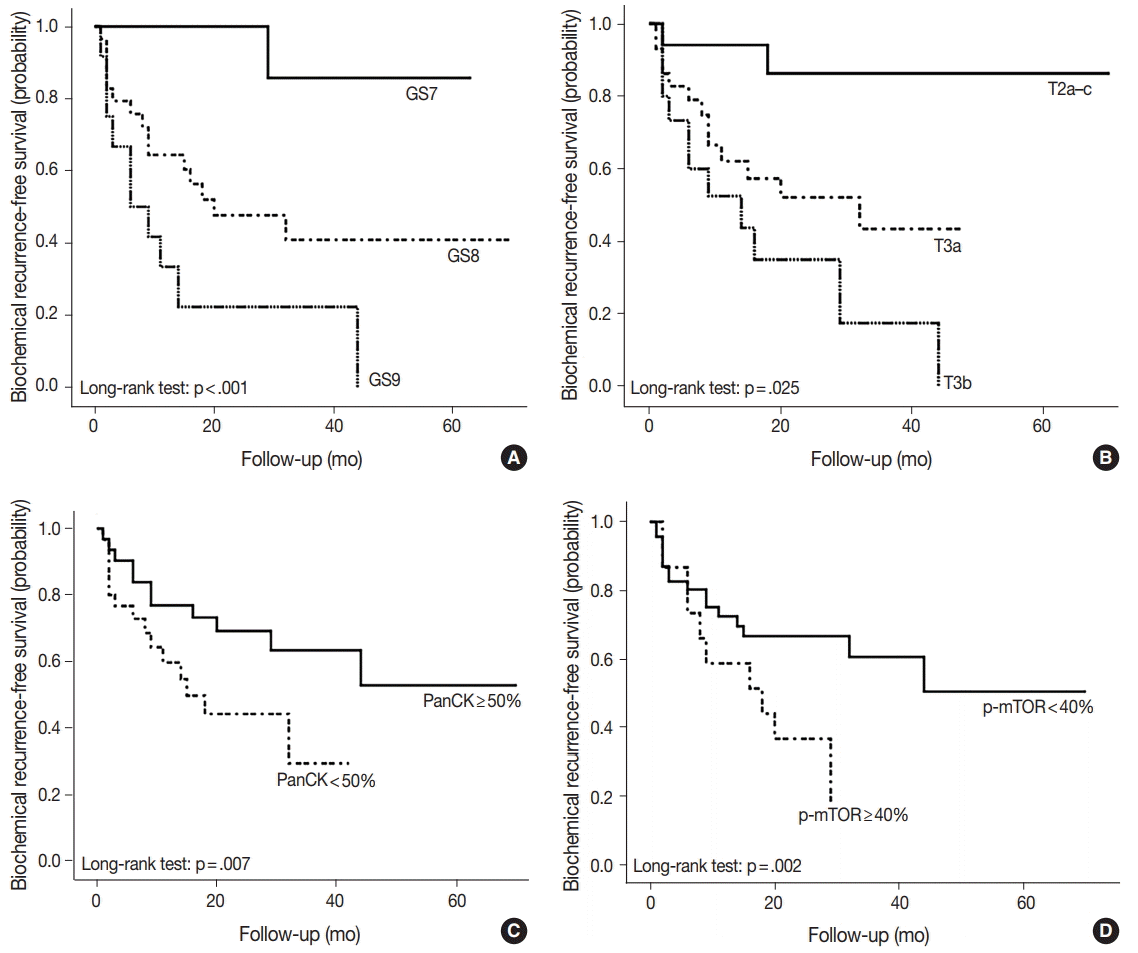
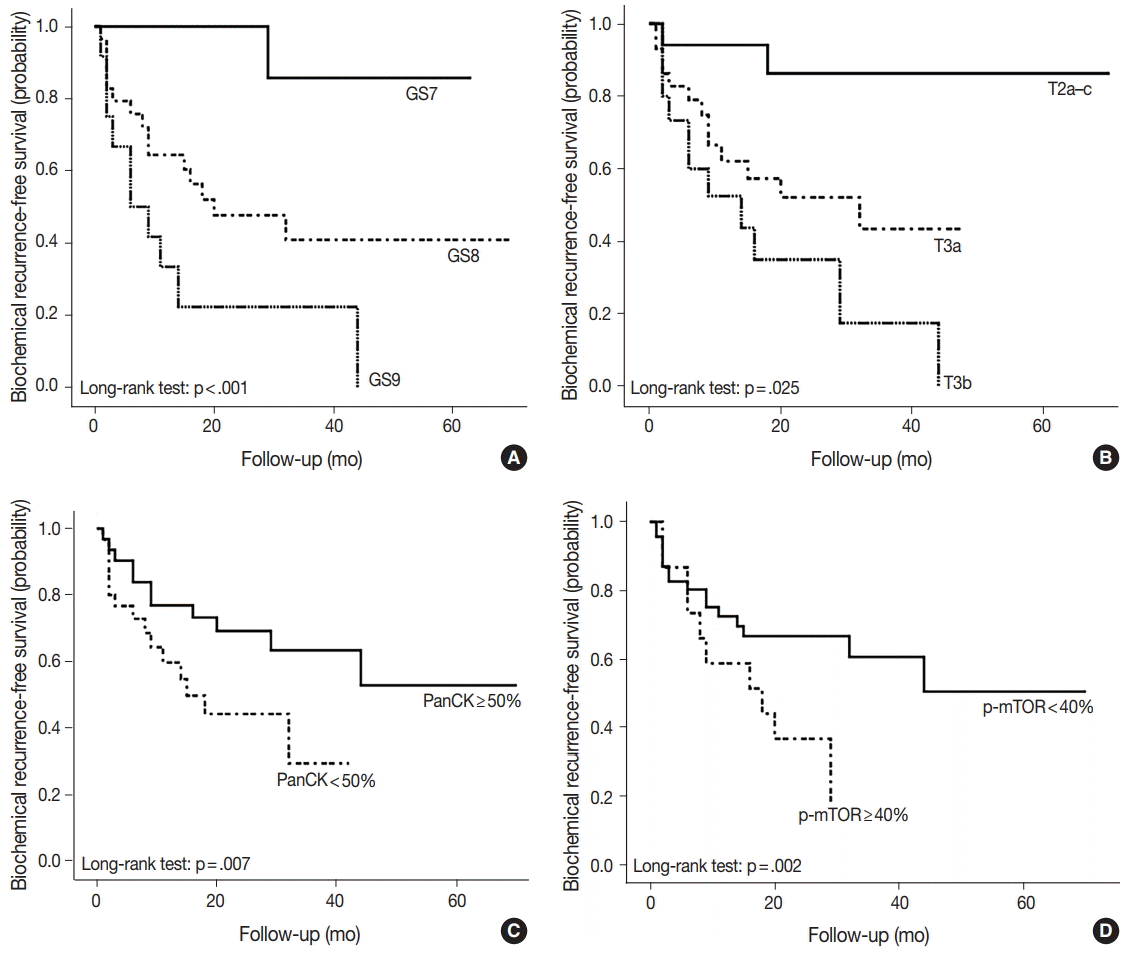
- As shown in Table 5, among the clinicopathological features, the univariate analysis showed that BCR was associated with high preoperative serum PSA level (p<.001), large tumor volume (p<.001), predominant ductal component (p=.021), high GS (p=.004), comedo-necrosis (p=0.015), high pT (p=.010), lympho- vascular invasion (p=.002), and positive surgical margin (p=.015). Among the protein expressions, high expressions of p-mTOR and low expression of PSA and pS6 were associated with BCR (p=.049, p=.022, and p=.033, respectively). Low expression of PanCK showed borderline significance (p=.055). On multivariable analysis, high GS (p<.001), high pT (p=.025), low expression of PanCK (p=.007), and high expression of p-mTOR (p=.002) remained independent prognostic factors for BCR. The Kaplan-Meier survival curves of these four independent prognostic factors are shown in Fig. 4.
RESULTS
- Herein, we analyzed the clinicopathological features and immunoprofile of 61 cases of DAC. The results suggest GS, pT stage, and immunohistochemical expressions of PanCK and p-mTOR as independent prognostic factors for BCR. DAC demonstrated wide expression ranges of prostate cancer–related proteins, which should be recognized during interpretation of immunohistochemical results of DAC. Since DAC demonstrated diverse expression of mTOR-related proteins, these results cautiously suggest their potential utility as predictive markers for mTOR-targeted therapy.
- Although previous studies regarding the immunohistochemical expression of DAC exist, they analyzed a small number of cases and mostly focused on PSA and a few other prostate cancer-related proteins [9-17]. Furthermore, they mostly presented the results as positive, focally positive, or negative without an accurate range of expression. One recent study analyzed a large number of cases (n=60) and showed high expressions of AR, PSA, and PTEN and low expression of ERG in 100%, 100%, 70.2%, and 38.3% of DAC cases, respectively [18]. Nevertheless, this present study is significant because of the detailed description of the expression range of each protein and the assessment of mTOR pathway–associated protein in DAC for the first time. Furthermore, we identified independent prognostic factors for BCR in DAC: GS, pT, and immunohistochemical expressions of PanCK and p-mTOR.
- On light microscopic examination, histologic differences are apparent between DAC and AAC, but it appears that they are similar at the molecular level as assessed by gene expression profile [19]. In line with this notion, DAC cases in this study showed high expression of AR and low expression of p53, similar to AAC [20-22]. PanCK and PSA are drawing special attention among the prostate cancer-related proteins. Even though PanCK and PSA have been proven useful as an epithelial marker and a prostate lineage marker, respectively, the present study showed that they were expressed heterogeneously, including nine cases (14.8%) of PanCK-negative ones and two cases (3.3%) of PSA-negative ones. Previous studies showed that AAC was also focally positive or even negative for PanCK and PSA in a few cases (3.4% and 2%–7%, respectively), similar to DAC in present study [23-27]. Therefore, it is worth noting that both DAC and AAC could be focally positive and even negative for PanCK and PSA, especially in metastatic disease.
- In AAC, fusions between the androgen-regulated transmembrane protease serine 2 gene (TMPRSS2) and the ERGestingly, Japanese population gene are present in approximately 40%–50% of cases, where ERG immunohistochemistry correlates well with fusion-positive cancer [24,26]. In the Korean population, the ERG-positive rate by immunohistochemistry was 24.4%, which is lower than those of Western population-based studies [23,27]. Interestingly, Japanese population-based studies also showed low ERG-positive rates, similar to Korean population. These findings suggest that geographic variation may contribute to the lower rates of ERG-positive cases in Eastern Asian prostate cancer patients [27]. Since only seven cases (11%) were positive for ERG in the present study, it appears that the ERG-positive rate is even lower in DAC than in AAC.
- EZH2 is the catalytic subunit of the polycomb repressive complex (PRC2) responsible for conducting histone methylation. It is important in cell cycle regulation and has a role in tumor cell proliferation and invasive growth [28]. High expression of EZH2 in AAC has been associated with aggressive clinicopathological features, such as GS ≥8, extraprostatic extension, positive surgical margins, and BCR [29]. In contrast to AAC, EZH2 expression was not associated with BCR in DAC by univariate and multivariable analyses, although it was correlated with poor prognostic clinicopathological features, such as comedo-necrosis, high pT, and lymphovascular invasion.
- The mTOR pathway responds to diverse environmental cues, such as amino acids, stress, oxygen, energy, and growth factors, and it controls many biologic processes that generate or use large amounts of energy and nutrients [30]. mTOR signaling impacts most major cellular functions, giving it an important role in regulating basic cellular behaviors, such as cellular growth and proliferation [30]. Overactivation of mTOR signaling contributes to the initiation and development of many types of cancers, including prostate cancer, suggesting that mTOR inhibitors, such as sirolimus, everolimus, and temsirolimus, might lead to an improved patient survival [31]. However, the identification of biomarkers that predict which tumors will respond to mTOR inhibitors remains an unmet need.
- As one of the diverse upstream regulators of the mTOR pathway, PTEN encodes a phosphatase that dephosphorylates phosphatidylinositol-3,4,5-trisphosphate (PIP3), a second messenger in the PI3K–protein kinase B (PKB) signaling pathway [32]. By negatively regulating the PI3K/PKB signaling pathway, it functions as a tumor suppressor. PTEN loss activates PI3K, which then activates not only mTOR complex 1 (mTORC1) by activating AKT, but also mTORC2 directly [33]. The mTORC1 and mTORC2 complexes are composed of mTOR and several common and unique proteins, allowing those different sensitivities to upstream regulators and diverse downstream output [30]. Many stresses, including low energy and oxygen levels and DNA damage, act through tuberous sclerosis 1 (TSC1) and 2 (TSC2), which are key upstream regulators of mTORC1. Adenosine monophosphate-activated protein kinase (AMPK), in response to hypoxia or a low energy state, phosphorylates TSC2 and communicates directly with mTORC1, leading to 14-3-3 binding [30]. The binding of 14-3-3 proteins, including 14-3-3 sigma on mTORC1, may promote mTORC1 signaling under growth factors, but also contributes to the regulatory mechanisms that suppress mTORC1 activity under conditions of cell stress [34]. mTORC1 also directly phosphorylates and activates ribosomal S6 kinase 1 (S6K1), of which the target substrate is the S6 ribosomal protein (pS6), which has been used as a surrogate for mTORC1 activity [35]. Phosphorylation of S6 induces protein synthesis in the ribosome [30].
- Few studies have been conducted on the mTOR signaling pathway as a predictive marker in prostate cancer. In a clinical study to evaluate everolimus in castration-resistant prostate cancer, probably AAC type, deletion of PTEN assessed by fluorescence in situ hybridization was found in seven of 23 tumor samples and associated with longer progression-free survival and response. However, they argued that immunohistochemical expressions of PTEN, pS6, p-mTOR, and ERG were not predictive [36]. To the best of our knowledge, the present study is the first one to assess mTOR pathway-associated proteins in DAC where mTOR-related proteins are diversely expressed. Therefore, it would be interesting to define the usefulness of these proteins as predictive markers of mTOR inhibitors in DAC.
- Although our present study examined a relatively large number of DAC cases, it had some limitations, including its retrospective design and the fact that all patients came from a single institution. Most cases were combined with AAC but the AAC component was not evaluated for immunohistochemical expression of prostate cancer- and mTOR signaling–related proteins. Since this present study showed GS, pT stage, and immunohistochemical expressions of PanCK and p-mTOR as independent prognostic factors, multi-institutional studies are necessary to validate the clinical utility of the results. Furthermore, remarkable advances in investigative tools, such as genomic microarray technologies and next-generation sequencing, may help find novel prognostic and predictive biomarkers. Therefore, efforts should be made to identify more accurate markers by integrating newly discovered biomarkers. Although mTOR-related proteins were cautiously suggested as immunohistochemical predictive markers for mTOR inhibitors, this result should be confirmed by immunohistochemical staining on whole section. It is also obvious that the assumption is still premature and should be investigated through a prospective clinical study.
DISCUSSION
Acknowledgments
Values are presented as median (range) or number (%).
Cut-off for high expression of each protein is ≥ 50% for PanCK, 100 % for ERG, ≥ 10% for p53, 100% for AR, ≥ 25% for EZH2, and ≥ 70% for PSA.
DAC, ductal adenocarcinoma; PanCK, pan-cytokeratin; ERG, ETS-related gene; AR, androgen receptor; EZH2, enhancer of zeste homolog 2; PSA, prostate-specific antigen; GS, Gleason score; pT, pathologic tumor stage; LVI, lymphovascular invasion; RM, resection margin; LN, lymph node.
Values are presented as number (%) or median (range).
Cut-off for each protein is as follows: ≥40% for high expression of p-mTOR, < 80% for loss of 14-3-3 sigma, ≥ 10% for high expression of pS6, and < 85% for loss of PTEN.
mTOR, mammalian target of rapamycin; DAC, ductal adenocarcinoma; p-mTOR, phospho-mammalian target of rapamycin; pS6, phospho-S6 ribosomal protein; PTEN, phosphatase and tensin homolog; PSA, prostate-specific antigen; GS, Gleason score; pT, pathologic tumor stage; LVI, lymphovascular invasion; RM, resection margin; LN, lymph node.
HR, hazard ratio estimated by Cox proportional hazards regression model; CI, confidence interval of the estimated HR; PSA, prostate-specific antigen; PanCK, pan-cytokeratin; ERG, ETS-related gene; AR, androgen receptor; EZH2, enhancer of zeste homolog 2; p-mTOR, phospho-mammalian target of rapamycin; pS6, phospho-S6 ribosomal protein; PTEN, phosphatase and tensin homolog.
- 1. Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO classification of tumours of the urinary system and male genital organs. 4th ed. Lyon: International Agency for Research on Cancer, 2016.
- 2. Melicow MM, Pachter M. Endometrial carcinoma of proxtatic utricle (uterus masculinus). Cancer 1967; 20: 1715-22. ArticlePubMed
- 3. Tarjan M, Lenngren A, Hellberg D, Tot T. Immunohistochemical verification of ductal differentiation in prostate cancer. APMIS 2012; 120: 510-8. ArticlePubMed
- 4. Meeks JJ, Zhao LC, Cashy J, Kundu S. Incidence and outcomes of ductal carcinoma of the prostate in the USA: analysis of data from the Surveillance, Epidemiology, and End Results program. BJU Int 2012; 109: 831-4. ArticlePubMed
- 5. Tu SM, Lopez A, Leibovici D, et al. Ductal adenocarcinoma of the prostate: clinical features and implications after local therapy. Cancer 2009; 115: 2872-80. ArticlePubMed
- 6. Statz CM, Patterson SE, Mockus SM. mTOR inhibitors in castration-resistant prostate cancer: a systematic review. Target Oncol 2017; 12: 47-59. ArticlePubMedPDF
- 7. Jung WY, Sung CO, Han SH, et al. AZGP-1 immunohistochemical marker in prostate cancer: potential predictive marker of biochemical recurrence in post radical prostatectomy specimens. Appl Immunohistochem Mol Morphol 2014; 22: 652-7. PubMed
- 8. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471-4. ArticlePubMedPDF
- 9. Bostwick DG, Kindrachuk RW, Rouse RV. Prostatic adenocarcinoma with endometrioid features: clinical, pathologic, and ultrastructural findings. Am J Surg Pathol 1985; 9: 595-609. PubMed
- 10. Ro JY, Ayala AG, Wishnow KI, Ordóñez NG. Prostatic duct adenocarcinoma with endometrioid features: immunohistochemical and electron microscopic study. Semin Diagn Pathol 1988; 5: 301-11. PubMed
- 11. Gong Y, Caraway N, Stewart J, Staerkel G. Metastatic ductal adenocarcinoma of the prostate: cytologic features and clinical findings. Am J Clin Pathol 2006; 126: 302-9. ArticlePubMedPDF
- 12. Leite KR, Mitteldorf CA, Srougi M, et al. Cdx2, cytokeratin 20, thyroid transcription factor 1, and prostate-specific antigen expression in unusual subtypes of prostate cancer. Ann Diagn Pathol 2008; 12: 260-6. ArticlePubMed
- 13. Copeland JN, Amin MB, Humphrey PA, Tamboli P, Ro JY, Gal AA. The morphologic spectrum of metastatic prostatic adenocarcinoma to the lung: special emphasis on histologic features overlapping with other pulmonary neoplasms. Am J Clin Pathol 2002; 117: 552-7. ArticlePubMedPDF
- 14. Oxley JD, Abbott CD, Gillatt DA, MacIver AG. Ductal carcinomas of the prostate: a clinicopathological and immunohistochemical study. Br J Urol 1998; 81: 109-15. Article
- 15. Lee SS. Endometrioid adenocarcinoma of the prostate: a clinicopathologic and immunohistochemical study. J Surg Oncol 1994; 55: 235-8. ArticlePubMed
- 16. Millar EK, Sharma NK, Lessells AM. Ductal (endometrioid) adenocarcinoma of the prostate: a clinicopathological study of 16 cases. Histopathology 1996; 29: 11-9. ArticlePubMedPDF
- 17. Tulunay O, Orhan D, Baltaci S, Gögüş C, Müftüoglu YZ. Prostatic ductal adenocarcinoma showing Bcl-2 expression. Int J Urol 2004; 11: 805-8. ArticlePubMed
- 18. Seipel AH, Samaratunga H, Delahunt B, Wiklund P, Clements M, Egevad L. Immunohistochemistry of ductal adenocarcinoma of the prostate and adenocarcinomas of non-prostatic origin: a comparative study. APMIS 2016; 124: 263-70. ArticlePubMed
- 19. Sanati S, Watson MA, Salavaggione AL, Humphrey PA. Gene expression profiles of ductal versus acinar adenocarcinoma of the prostate. Mod Pathol 2009; 22: 1273-9. ArticlePubMedPDF
- 20. Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol 1994; 144: 735-46. PubMedPMC
- 21. Henshall SM, Quinn DI, Lee CS, et al. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res 2001; 61: 423-7. PubMed
- 22. Schlomm T, Iwers L, Kirstein P, et al. Clinical significance of p53 alterations in surgically treated prostate cancers. Mod Pathol 2008; 21: 1371-8. ArticlePubMedPDF
- 23. Lee K, Chae JY, Kwak C, Ku JH, Moon KC. TMPRSS2-ERG gene fusion and clinicopathologic characteristics of Korean prostate cancer patients. Urology 2010; 76: 1268e7-13. Article
- 24. Epstein JI, Egevad L, Humphrey PA, Montironi R, Members of the IIiDUPG. Best practices recommendations in the application of immunohistochemistry in the prostate: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol 2014; 38: e6-19. PubMed
- 25. Berner A, Harvei S, Tretli S, Fosså SD, Nesland JM. Prostatic carcinoma: a multivariate analysis of prognostic factors. Br J Cancer 1994; 69: 924-30. ArticlePubMedPMCPDF
- 26. Morais CL, Herawi M, Toubaji A, et al. PTEN loss and ERG protein expression are infrequent in prostatic ductal adenocarcinomas and concurrent acinar carcinomas. Prostate 2015; 75: 1610-9. ArticlePubMedPMCPDF
- 27. Suh JH, Park JW, Lee C, Moon KC. ERG immunohistochemistry and clinicopathologic characteristics in Korean prostate adenocarcinoma patients. Korean J Pathol 2012; 46: 423-8. ArticlePubMedPMC
- 28. Bachmann IM, Halvorsen OJ, Collett K, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol 2006; 24: 268-73. ArticlePubMed
- 29. van Leenders GJ, Dukers D, Hessels D, et al. Polycomb-group oncogenes EZH2, BMI1, and RING1 are overexpressed in prostate cancer with adverse pathologic and clinical features. Eur Urol 2007; 52: 455-63. ArticlePubMed
- 30. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149: 274-93. ArticlePubMedPMC
- 31. Xu K, Liu P, Wei W. mTOR signaling in tumorigenesis. Biochim Biophys Acta 2014; 1846: 638-54. ArticlePubMedPMC
- 32. Giannico GA, Arnold SA, Gellert LL. New and emerging diagnostic and prognostic immunohistochemical biomarkers in prostate pathology. Adv Anat Pathol 2017; 24: 35-44. ArticlePubMedPMC
- 33. Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 2006; 6: 729-34. ArticlePubMedPDF
- 34. Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol 2009; 19: 16-23. ArticlePubMed
- 35. Templeton AJ, Dutoit V, Cathomas R, et al. Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08). Eur Urol 2013; 64: 150-8. ArticlePubMed
- 36. Vaishampayan U, Shevrin D, Stein M, et al. Phase II trial of carboplatin, everolimus, and prednisone in metastatic castration-resistant prostate cancer pretreated with docetaxel chemotherapy: a prostate cancer clinical trial consortium study. Urology 2015; 86: 1206-11. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Intermediate risk prostate tumors contain lethal subtypes
William L. Harryman, James P. Hinton, Rafael Sainz, Jaime M. C. Gard, John M. Ryniawec, Gregory C. Rogers, Noel A. Warfel, Beatrice S. Knudsen, Raymond B. Nagle, Juan J. Chipollini, Benjamin R. Lee, Belinda L. Sun, Anne E. Cress
Frontiers in Urology.2025;[Epub] CrossRef - High GLUT1 membrane expression and low PSMA membrane expression in Ductal Adenocarcinoma and Intraductal Carcinoma of the prostate
Xingming Wang, Li Zhou, Lin Qi, Ye Zhang, Hongling Yin, Yu Gan, Xiaomei Gao, Yi Cai
Prostate Cancer and Prostatic Diseases.2024; 27(4): 720. CrossRef - Association of Lymphovascular Invasion with Biochemical Recurrence and Adverse Pathological Characteristics of Prostate Cancer: A Systematic Review and Meta-analysis
Jakub Karwacki, Marcel Stodolak, Andrzej Dłubak, Łukasz Nowak, Adam Gurwin, Kamil Kowalczyk, Paweł Kiełb, Nazar Holdun, Wojciech Szlasa, Wojciech Krajewski, Agnieszka Hałoń, Anna Karwacka, Tomasz Szydełko, Bartosz Małkiewicz
European Urology Open Science.2024; 69: 112. CrossRef - Impact of Epithelial Histological Types, Subtypes, and Growth Patterns on Oncological Outcomes for Patients with Nonmetastatic Prostate Cancer Treated with Curative Intent: A Systematic Review
Giancarlo Marra, Geert J.L.H. van Leenders, Fabio Zattoni, Claudia Kesch, Pawel Rajwa, Philip Cornford, Theodorus van der Kwast, Roderick C.N. van den Bergh, Erik Briers, Thomas Van den Broeck, Gert De Meerleer, Maria De Santis, Daniel Eberli, Andrea Faro
European Urology.2023; 84(1): 65. CrossRef - Impact of comedonecrosis on prostate cancer outcome: a systematic review
Kaveri T S Aiyer, Lisa J Kroon, Geert J L H van Leenders
Histopathology.2023; 83(3): 339. CrossRef - Survival after radical prostatectomy vs. radiation therapy in ductal carcinoma of the prostate
Francesco Chierigo, Marco Borghesi, Christoph Würnschimmel, Rocco Simone Flammia, Benedikt Horlemann, Gabriele Sorce, Benedikt Höh, Zhe Tian, Fred Saad, Markus Graefen, Michele Gallucci, Alberto Briganti, Francesco Montorsi, Felix K. H. Chun, Shahrokh F.
International Urology and Nephrology.2022; 54(1): 89. CrossRef - Defining Diagnostic Criteria for Prostatic Ductal Adenocarcinoma at Multiparametric MRI
Weranja K. B. Ranasinghe, Patricia Troncoso, Devaki Shilpa Surasi, Juan José Ibarra Rovira, Priya Bhosale, Janio Szklaruk, Andrea Kokorovic, Xuemei Wang, Mohamed Elsheshtawi, Miao Zhang, Ana Aparicio, Brian F. Chapin, Tharakeswara K. Bathala
Radiology.2022; 303(1): 110. CrossRef - Oncological outcomes of patients with ductal adenocarcinoma of the prostate receiving radical prostatectomy or radiotherapy
Mengzhu Liu, Kun Jin, Shi Qiu, Pengyong Xu, Mingming Zhang, Wufeng Cai, Xiaonan Zheng, Lu Yang, Qiang Wei
Asian Journal of Urology.2021; 8(2): 227. CrossRef - Ductal Prostate Cancers Demonstrate Poor Outcomes with Conventional Therapies
Weranja Ranasinghe, Daniel D. Shapiro, Hyunsoo Hwang, Xuemei Wang, Chad A. Reichard, Mohamed Elsheshtawi, Mary F. Achim, Tharakeswara Bathala, Chad Tang, Ana Aparicio, Shi-Ming Tu, Nora Navone, Timothy C. Thompson, Louis Pisters, Patricia Troncoso, John W
European Urology.2021; 79(2): 298. CrossRef - Optimizing the diagnosis and management of ductal prostate cancer
Weranja Ranasinghe, Daniel D. Shapiro, Miao Zhang, Tharakeswara Bathala, Nora Navone, Timothy C. Thompson, Bradley Broom, Ana Aparicio, Shi-Ming Tu, Chad Tang, John W. Davis, Louis Pisters, Brian F. Chapin
Nature Reviews Urology.2021; 18(6): 337. CrossRef - A first case of ductal adenocarcinoma of the prostate having characteristics of neuroendocrine phenotype with PTEN, RB1 and TP53 alterations
Hiroaki Kobayashi, Takeo Kosaka, Kohei Nakamura, Kazunori Shojo, Hiroshi Hongo, Shuji Mikami, Hiroshi Nishihara, Mototsugu Oya
BMC Medical Genomics.2021;[Epub] CrossRef - Knowing what’s growing: Why ductal and intraductal prostate cancer matter
Mitchell G. Lawrence, Laura H. Porter, David Clouston, Declan G. Murphy, Mark Frydenberg, Renea A. Taylor, Gail P. Risbridger
Science Translational Medicine.2020;[Epub] CrossRef - Integrative Genomic Analysis of Coincident Cancer Foci Implicates CTNNB1 and PTEN Alterations in Ductal Prostate Cancer
Marc Gillard, Justin Lack, Andrea Pontier, Divya Gandla, David Hatcher, Adam G. Sowalsky, Jose Rodriguez-Nieves, Donald Vander Griend, Gladell Paner, David VanderWeele
European Urology Focus.2019; 5(3): 433. CrossRef - Genomic Characterization of Prostatic Ductal Adenocarcinoma Identifies a High Prevalence of DNA Repair Gene Mutations
Michael T. Schweizer, Emmanuel S. Antonarakis, Tarek A. Bismar, Liana B. Guedes, Heather H. Cheng, Maria S. Tretiakova, Funda Vakar-Lopez, Nola Klemfuss, Eric Q. Konnick, Elahe A. Mostaghel, Andrew C. Hsieh, Peter S. Nelson, Evan Y. Yu, R. Bruce Montgomer
JCO Precision Oncology.2019; (3): 1. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
| Antibody | Dilution | Company | Subcellular location |
|---|---|---|---|
| PanCK | 1:400 | Leica, Newcastle, UK | Cytoplasm |
| PSA | 1:200 | Dako Corp., Carpinteria, CA | Cytoplasm |
| AR | 1:200 | Cell Marque, Rocklin, CA | Nucleus |
| ERG | 1:100 | Epitomics, Burlingame, CA | Nucleus |
| p53 | 1:1500 | Dako Corp., Carpinteria, CA | Nucleus |
| EZH2 | 1:25 | Cell Signal Technology, Beverly, MA | Nucleus |
| PTEN | 1:100 | Cell Signal Technology, Beverly, MA | Cytoplasm/nucleus |
| p-mTOR | 1:100 | Cell Signal Technology, Beverly, MA | Cytoplasm |
| pS6 | 1:100 | Cell Signal Technology, Beverly, MA | Cytoplasm |
| 14-3-3 sigma | 1:200 | Sigma, St. Louis, MO | Cytoplasm |
| Variable | Value |
|---|---|
| Age (yr) | 68.0 ± 5.6 |
| Preoperative PSA (ng/mL) | 11.7 ± 10.3 |
| Total tumor volume (%) | 28.5 ± 21.5 |
| DAC component (%) | 48.3 ± 32.5 |
| Predominant component | |
| Ductal | 32 (52.5) |
| Acinar | 29 (47.5) |
| Predominant DAC pattern | |
| Papillary | 48 (78.7) |
| Cribriform | 9 (14.7) |
| PIN-like | 4 (6.6) |
| Gleason score | |
| 7 | 20 (32.8) |
| 8 | 29 (47.5) |
| 9 | 12 (19.7) |
| Pathologic tumor stage | |
| pT2a-c | 17 (27.9) |
| pT3a | 29 (47.5) |
| pT3b | 15 (24.6) |
| Tertiary grade 5 | 12 (19.7) |
| Comedonecrosis | 17 (27.9) |
| Extraprostatic extension | 42 (68.9) |
| Lymphovascular invasion | 26 (42.6) |
| Perineural invasion | 52 (85.2) |
| Positive surgical margin | 41 (67.2) |
| Seminal vesicle involvement | 15 (24.6) |
| Lymph node metastasis | 3 (4.9) |
| Biochemical recurrence | 26 (42.6) |
| Death | 2 (3.3) |
| PanCK |
ERG |
p53 |
AR |
EZH2 |
PSA |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | p-value | Low | High | p-value | Low | High | p-value | Low | High | p-value | Low | High | p-value | Low | High | p-value | |
| No. of cases | 30 (49.2) | 31 (50.8) | 58 (95.1) | 3 (4.9) | 46 (75.4) | 15 (24.6) | 42 (68.9) | 19 (31.1) | 38 (62.3) | 23 (37.7) | 33 (54.1) | 28 (45.9) | ||||||
| Age (yr) | 68.3 | 67.7 | .700 | 67.9 | 70.0 | .526 | 67.5 | 69.3 | .285 | 67.3 | 69.5 | .163 | 67.7 | 68.4 | .661 | 67.9 | 68.1 | .875 |
| (55–75) | (51–77) | (51–77) | (65–75) | (51–77) | (53–76) | (51–77) | (59–76) | (51–77) | (57–76) | (54–77) | (51–76) | |||||||
| PSA (ng/mL) | 14.0 | 9.5 | .363 | 11.8 | 9.0 | .648 | 11.3 | 13 | .573 | 12.7 | 9.5 | .269 | 13.1 | 9.3 | .160 | 11.0 | 12.5 | .565 |
| (3.1–66.4) | (0.6–24.1) | (0.6–66.4) | (5.9–13.1) | (0.6–32.8) | (3.4–66.4) | (1.8–66.4) | (0.6–31.3) | (1.8–66.4) | (0.6–30.3) | (1.8–32.8) | (0.6–66.4) | |||||||
| GS | .731 | .175 | .015 | .025 | .246 | .006 | ||||||||||||
| 7 | 10 (33.3) | 10 (32.3) | 20 (34.5) | 0 | 19 (41.3) | 1 (6.7) | 11 (26.2) | 9 (47.4) | 14 (36.8) | 6 (26.1) | 5 (15.2) | 15 (53.6) | ||||||
| 8 | 13 (43.3) | 16 (51.6) | 26 (44.8) | 3 (100) | 21 (45.7) | 8 (53.3) | 19 (45.2) | 10 (52.6) | 19 (50.0) | 10 (43.5) | 20 (60.6) | 9 (32.1) | ||||||
| 9 | 7 (23.3) | 5 (16.1) | 12 (20.7) | 0 | 6 (13.0) | 6 (40.0) | 12 (28.6) | 0 | 5 (13.2) | 7 (30.4) | 8 (24.2) | 4 (14.3) | ||||||
| pT | .044 | .543 | .072 | .350 | .004 | .348 | ||||||||||||
| T2a-c | 5 (16.7) | 12 (38.7) | 17 (29.3) | 0 | 16 (34.8) | 1 (6.7) | 14 (33.3) | 3 (15.8) | 16 (42.1) | 1 (4.3) | 7 (21.2) | 10 (35.7) | ||||||
| T3a | 19 (63.3) | 10 (32.3) | 27 (46.6) | 2 (66.7) | 21 (45.7) | 8 (53.3) | 18 (42.9) | 11 (57.9) | 13 (34.2) | 16 (69.6) | 16 (48.5) | 13 (46.4) | ||||||
| T3b | 6 (20.0) | 9 (29.0) | 14 (24.1) | 1 (33.3) | 9 (19.6) | 6 (40.0) | 10 (23.8) | 5 (26.3) | 9 (23.7) | 6 (26.1) | 10 (30.3) | 5 (17.9) | ||||||
| LVI | .912 | .039 | .030 | .539 | .025 | .041 | ||||||||||||
| Absent | 17 (56.7) | 18(58.1) | 35 (60.3) | 0 | 30 (65.2) | 5 (33.3) | 23 (54.8) | 12 (63.2) | 26 (68.4) | 9 (39.1) | 15 (45.5) | 20 (71.4) | ||||||
| Present | 13 (43.3) | 13 (41.9) | 23 (39.7) | 3 (100) | 16 (34.8) | 10 (66.7) | 19 (45.2) | 7 (36.8) | 12 (31.6) | 14 (60.9) | 18 (54.5) | 8 (28.6) | ||||||
| Comedo-necrosis | .715 | .829 | .011 | .222 | .034 | .301 | ||||||||||||
| Absent | 21 (70.0) | 23 (74.2) | 42 (72.4) | 2 (66.7) | 37 (80.4) | 7 (46.7) | 28 (66.7) | 16 (84.2) | 31 (81.6) | 13 (56.5) | 22 (66.7) | 22 (78.6) | ||||||
| Present | 9 (30.0) | 8 (25.8) | 16 (27.6) | 1 (33.3) | 9 (19.6) | 8 (53.3) | 14 (33.3) | 3 (15.8) | 7 (18.4) | 10 (43.5) | 11 (33.3) | 6 (21.4) | ||||||
| Positive RM | .122 | .215 | .959 | .297 | .761 | .921 | ||||||||||||
| Absent | 7 (23.3) | 13 (41.9) | 20 (34.5) | 0 | 15 (32.6) | 5 (33.3) | 12 (28.6) | 8 (42.1) | 13 (34.2) | 7 (30.4) | 11 (33.3) | 9 (32.1) | ||||||
| Present | 23 (76.7) | 18 (58.1) | 38 (65.5) | 3 (100) | 31 (67.4) | 10 (66.7) | 30 (71.4) | 11 (57.9) | 25 (65.8) | 16 (69.6) | 22 (66.7) | 19 (67.9) | ||||||
| LN metastasis | .573 | .686 | .310 | .232 | .873 | .102 | ||||||||||||
| Absent | 29 (96.7) | 29 (93.5) | 55 (94.8) | 3 (100) | 43 (93.5) | 15 (100) | 39 (92.9) | 19 (100) | 36 (94.8) | 22 (95.7) | 30 (90.9) | 28 (100) | ||||||
| Present | 1 (1.0) | 2 (6.5) | 3 (5.2) | 0 | 3 (6.5) | 0 | 3 (7.1) | 0 | 2 (5.2) | 1 (4.3) | 3 (9.1) | 0 | ||||||
| p-mTOR |
14-3-3 sigma |
pS6 |
PTEN |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | p-value | Low | High | p-value | Low | High | p-value | Intact | Loss | p-value | |
| No. of cases | 46 (75.4) | 15 (24.6) | 25 (41.0) | 36 (59.0) | 39 (63.9) | 22 (36.1) | 37 (60.7) | 24 (39.3) | ||||
| Age (yr) | 67.8 | 68.7 | .589 | 69.6 | 66.9 | .066 | 66.5 | 68.9 | .109 | 68.7 | 66.8 | .197 |
| (51–77) | (59–76) | (54–77) | (51–76) | (54–76) | (51–77) | (59–77) | (51–75) | |||||
| PSA (ng/mL) | 11.6 | 12.0 | .883 | 14.7 | 9.6 | .088 | 14.1 | 10.3 | .166 | 13.4 | 9.1 | .106 |
| (0.6–66.4) | (3.7–31.3) | (0.6–66.4) | (1.8–32.8) | (0.6–66.4) | (0.3–31.3) | (3.4–66.4) | (0.6–30.3) | |||||
| GS | .307 | .298 | .105 | .747 | ||||||||
| 7 | 15 (32.6) | 5 (33.3) | 11 (44.0) | 9 (25.0) | 12 (30.8) | 8 (36.4) | 11 (29.7) | 9 (37.5) | ||||
| 8 | 20 (43.5) | 9 (60.0)) | 10 (40.0) | 19 (52.8) | 22 (56.4) | 7 (31.8) | 19 (51.4) | 10 (41.7) | ||||
| 9 | 11 (23.9) | 1 (6.7) | 4 (16.0) | 8 (22.2) | 5 (12.8) | 7 (31.8) | 7 (18.9) | 5 (20.8) | ||||
| pT | .138 | .426 | .790 | .051 | ||||||||
| T2a–c | 12 (26.1) | 5 (33.3) | 8 (32.0) | 9 (25.0) | 12 (30.8) | 5 (22.7) | 8 (21.6) | 9 (37.5) | ||||
| T3a | 25 (54.3) | 4 (26.7) | 13 (52.0) | 16 (44.4) | 18 (46.2) | 11 (50.0) | 16 (43.2) | 13 (54.2) | ||||
| T3b | 9 (19.6) | 6 (40.0) | 4 (16.0) | 11 (30.6) | 9 (23.1) | 6 (27.3) | 13 (35.1) | 2 (8.3) | ||||
| LVI | .813 | .730 | .737 | .903 | ||||||||
| Absent | 26 (56.5) | 9 (60.0) | 15 (60.0) | 20 (55.6) | 23 (59.0) | 12 (54.5) | 21 (56.8) | 14 (58.3) | ||||
| Present | 20 (43.5) | 6 (40.0) | 10 (40.0) | 16 (44.4) | 16 (41.0) | 10 (45.5) | 16 (43.2) | 10 (41.7) | ||||
| Comedonecrosis | .905 | .574 | .605 | .687 | ||||||||
| Absent | 33 (71.7) | 11 (73.3) | 19 (76.0) | 25 (69.4) | 29 (74.4) | 15 (68.2) | 26 (70.3) | 18 (75.0) | ||||
| Present | 13 (28.3) | 4 (26.7) | 6 (24.0) | 11 (30.6) | 10 (25.6) | 7 (31.8) | 11 (29.7) | 6 (25.0) | ||||
| Positive RM | .224 | .076 | .904 | .942 | ||||||||
| Absent | 17 (37.0) | 3 (20.0) | 5 (20.0) | 15 (41.7) | 13 (33.3) | 7 (31.8) | 12 (32.4) | 8 (33.3) | ||||
| Present | 29 (63.0) | 12 (80.0) | 20 (80.0) | 21 (58.3) | 26 (66.7) | 15 (68.2) | 25 (67.6) | 16 (66.7) | ||||
| LN metastasis | .310 | .782 | .919 | .320 | ||||||||
| Absent | 43 (93.5) | 15 (100) | 24 (96.0) | 34 (94.4) | 37 (94.9) | 21 (95.5) | 36 (97.3) | 22 (91.7) | ||||
| Present | 3 (6.5) | 0 | 1 (4.0) | 2 (5.6) | 2 (5.1) | 1 (4.5) | 1 (2.7) | 2 (8.3) | ||||
| Variable | Univariate analysis Variable |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (yr) | 1.010 | 0.941–1.083 | .783 | - | - | - |
| Preoperative PSA (ng/mL) | 1.066 | 1.038–1.094 | < .001 | - | - | - |
| Total tumor volume (%) | 1.029 | 1.013–1.047 | < .001 | - | - | - |
| Predominant component (ductal) | 2.793 | 1.171–6.664 | .021 | - | - | - |
| Gleason score | .004 | < .001 | ||||
| 7 | 1 | 1 | ||||
| 8 | 12.04 | 1.588–91.21 | .016 | 15.020 | 1.946–115.941 | .009 |
| 9 | 26.79 | 3.406–210.66 | .002 | 26.937 | 3.227–224.851 | .002 |
| Pathologic tumor stage | .010 | .025 | ||||
| T2a–c | 1 | 1 | ||||
| T3a | 5.400 | 1.205–24.190 | .028 | 4.270 | 0.890–20.487 | .070 |
| T3b | 10.190 | 2.237–46.400 | .003 | 8.288 | 1.617–42.494 | .011 |
| Comedonecrosis | 2.618 | 1.202–5.702 | .015 | - | - | - |
| Lymphovascular invasion | 3.728 | 1.615–8.605 | .002 | - | - | - |
| Positive surgical margin | 4.473 | 1.341–14.930 | .015 | - | - | - |
| Lymph node metastasis | 0.616 | 0.082–4.624 | .637 | - | - | - |
| PanCK (high expression) | 0.453 | 0.202–1.016 | .055 | 0.274 | 0.108–0.700 | .007 |
| ERG (high expression) | 1.626 | 0.382–6.931 | .511 | - | - | - |
| p53 (high expression) | 2.082 | 0.938–4.621 | .072 | - | - | - |
| AR (high expression) | 0.728 | 0.291–1.821 | .498 | - | - | - |
| EZH2 (high expression) | 2.012 | 0.901–4.490 | .088 | - | - | - |
| PSA (high expression) | 0.360 | 0.151–0.861 | .022 | - | - | - |
| p-mTOR (high expression) | 2.266 | 1.004–5.117 | .049 | 5.184 | 1.829–14.704 | .002 |
| 14-3-3 sigma (loss of expression) | 1.457 | 0.633–3.356 | .376 | - | - | - |
| pS6 (high expression) | 0.431 | 0.199–0.935 | .033 | - | - | - |
| PTEN (loss of expression) | 0.680 | 0.302–1.532 | .352 | - | - | - |
PanCK, pan-cytokeratin; PSA, prostate-specific antigen; AR, androgen receptor; ERG, ETS-related gene; EZH2, enhancer of zeste Homolog2; PTEN, phosphatase and tensin homolog; p-mTOR, phospho-mammalian target of rapamycin; pS6, phospho-S6 ribosomal protein.
Values are presented as mean ± SD or number (%). PSA, prostate-specific antigen; DAC, ductal adenocarcinoma; PIN-like, prostatic intraepithelial neoplasia-like; SD, standard deviation.
Values are presented as median (range) or number (%). Cut-off for high expression of each protein is ≥ 50% for PanCK, 100 % for ERG, ≥ 10% for p53, 100% for AR, ≥ 25% for EZH2, and ≥ 70% for PSA. DAC, ductal adenocarcinoma; PanCK, pan-cytokeratin; ERG, ETS-related gene; AR, androgen receptor; EZH2, enhancer of zeste homolog 2; PSA, prostate-specific antigen; GS, Gleason score; pT, pathologic tumor stage; LVI, lymphovascular invasion; RM, resection margin; LN, lymph node.
Values are presented as number (%) or median (range). Cut-off for each protein is as follows: ≥40% for high expression of p-mTOR, < 80% for loss of 14-3-3 sigma, ≥ 10% for high expression of pS6, and < 85% for loss of PTEN. mTOR, mammalian target of rapamycin; DAC, ductal adenocarcinoma; p-mTOR, phospho-mammalian target of rapamycin; pS6, phospho-S6 ribosomal protein; PTEN, phosphatase and tensin homolog; PSA, prostate-specific antigen; GS, Gleason score; pT, pathologic tumor stage; LVI, lymphovascular invasion; RM, resection margin; LN, lymph node.
HR, hazard ratio estimated by Cox proportional hazards regression model; CI, confidence interval of the estimated HR; PSA, prostate-specific antigen; PanCK, pan-cytokeratin; ERG, ETS-related gene; AR, androgen receptor; EZH2, enhancer of zeste homolog 2; p-mTOR, phospho-mammalian target of rapamycin; pS6, phospho-S6 ribosomal protein; PTEN, phosphatase and tensin homolog.

 E-submission
E-submission