Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 52(1); 2018 > Article
-
Original Article
Programmed Death-Ligand 1 Expression and Its Correlation with Lymph Node Metastasis in Papillary Thyroid Carcinoma - Hyo Jung An1, Gyung Hyuck Ko2,3,4, Jeong-Hee Lee2,3,4, Jong Sil Lee2,3,4, Dong Chul Kim2,3,4, Jung Wook Yang4, Min Hye Kim4, Jin Pyeong Kim2,3,5, Eun Jung Jung2,3,6, Dae Hyun Song,1,2,3
-
Journal of Pathology and Translational Medicine 2018;52(1):9-13.
DOI: https://doi.org/10.4132/jptm.2017.07.26
Published online: October 3, 2017
1Department of Pathology, Gyeongsang National University Changwon Hospital, Changwon, Korea
2Gyeongsang National University School of Medicine, Jinju, Korea
3Gyeongsang Institute of Health Science, Jinju, Korea
4Department of Pathology, Gyeongsang National University Hospital, Jinju, Korea
5Deparment of Otorhinolaryngology, Gyeongsang National University Changwon Hospital, Changwon, Korea
6Department of Surgery, Gyeongsang National University Changwon Hospital, Changwon, Korea
- Corresponding Author Dae Hyun Song, MD Department of Pathology, Gyeongsang National University School of Medicine, 15 Jinju-daero 816beon-gil, Jinju 52727, Korea Tel: +82-55-214-3150 Fax: +82-55-214-3174 E-mail: golgy@hanmail.net
© 2018 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The immunotherapeutic role of programmed death-ligand 1 (PD-L1) in life expectancy in many cancers has been highlighted. However, data regarding PD-L1 expression in papillary thyroid carcinoma (PTC) are limited. In this study, we describe the PD-L1 and programmed cell death protein 1 (PD-1) expressions in PTC and analyze their correlation with lymph node (LN) metastasis.
-
Methods
- Clinicopathological data were obtained from 116 patients with PTC who were treated in Gyeongsang National University Hospital, Jinju, Korea in 2009. Tissue microarray blocks were made using representative paraffin blocks of classical PTCs excluding follicular variants. Two pathologists graded the proportion and intensity of PD-L1 and PD-1 expression in both tumor and inflammatory cells. According to their proportions, positive PTC cells were scored as negative (0%), grade 1 (1%–50%), and grade 2 (51%–100%). Similarly, positive inflammatory cells were graded as negative (0%), grade 1 (1%–10%), and grade 2 (11%–20%). The intensity of each protein expression was simplified as positive or negative.
-
Results
- A statistically significant correlation exists between the proportions of PD-1 and PD-L1 expression both in papillary carcinoma (p=.001) and peritumoral lymphoid cells in the thyroid (p<.001). In addition, the proportion of PD-L1 expression in PTC cells was closely related to metastatic LNs (p=.036).
-
Conclusions
- PD-L1 is a valuable predictive marker for LN metastasis in PTC. Immunomodulating therapies that inhibit PD-L1 might be an option for patients with LN metastasis.
- Case selection
- By reviewing electronic clinical charts, we obtained clinicopathologic data of PTC patients treated at Gyeongsang National University Hospital, Jinju, Korea in 2009. A total of 116 patients with classical PTCs, excluding follicular or encapsulated follicular variants, were enrolled [15]. Total thyroidectomy or lobectomy with lymph node (LN) dissection was performed. The tumor stage of each patient was assessed via the American Joint Committee on Cancer (AJCC) 7th ed. Cancer Staging system [16].
- Two pathologists reviewed the hematoxylin and eosin–stained glass slides. This study was approved by the Institutional Review Board of Gyeongsang National University Hospital with a waiver of informed consent (GNUH-10-026).
- Tissue microarray
- Surgically resected specimens were fixed overnight in buffered neutral formalin (20%). The samples were embedded in paraffin blocks and examined. One or two representative glass slides and matched blocks were selected via microscopic review. One core of 2-mm representative tissue was collected from each paraffin block and transplanted onto new recipient tissue microarray (TMA) blocks.
- Immunohistochemical analysis
- Primary antibody for PD-L1 (1:200, E1L3N, Cell Signaling Technology, Danvers, MA, USA) and PD-1 (1:100, ab52587, Abcam, Cambridge, UK) was used to investigate protein expression. The immunohistochemical (IHC) method was described in our previous report [17]. Fibrotic stromal area was used as negative internal control. IHC results were scored in both tumor and inflammatory cells by two pathologists. Delicate membranous or distinct cytoplasmic staining without nonspecific stromal staining was considered positive. The number of positive cells was analyzed in two categories, PTC cells and inflammatory cells. According to their proportion, PTC cells stained positive in each core of TMA block were scored as negative, grade 1 (1%–50%), and grade 2 (51%–100%). Similarly, positive inflammatory cells were graded as negative, grade 1 (1%–10%), and grade 2 (11%–20%). The intensity of each protein expression was simplified as negative or positive to increase reproducibility.
- Statistical analysis
- The correlation of each factor was analyzed using chi-square tests. The results were considered statistically significant with p-value less than .05. SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis.
MATERIALS AND METHODS
- Clinicopathological features of 116 PTC patients
- The clinicopathological features of the 116 PTC patients are summarized in Table 1. The mean age of the PTC patients was 49.5 years (range, 25 to 88 years). A total of 54 (46.6%), three (2.6%), and 59 (50.9%) patients were evaluated as T stage 1, 2, and 3, respectively. LN metastasis was diagnosed in 24 patients (20.7%).
- Correlation of PD-L1 and PD-1 expression in PTC cells and inflammatory cells
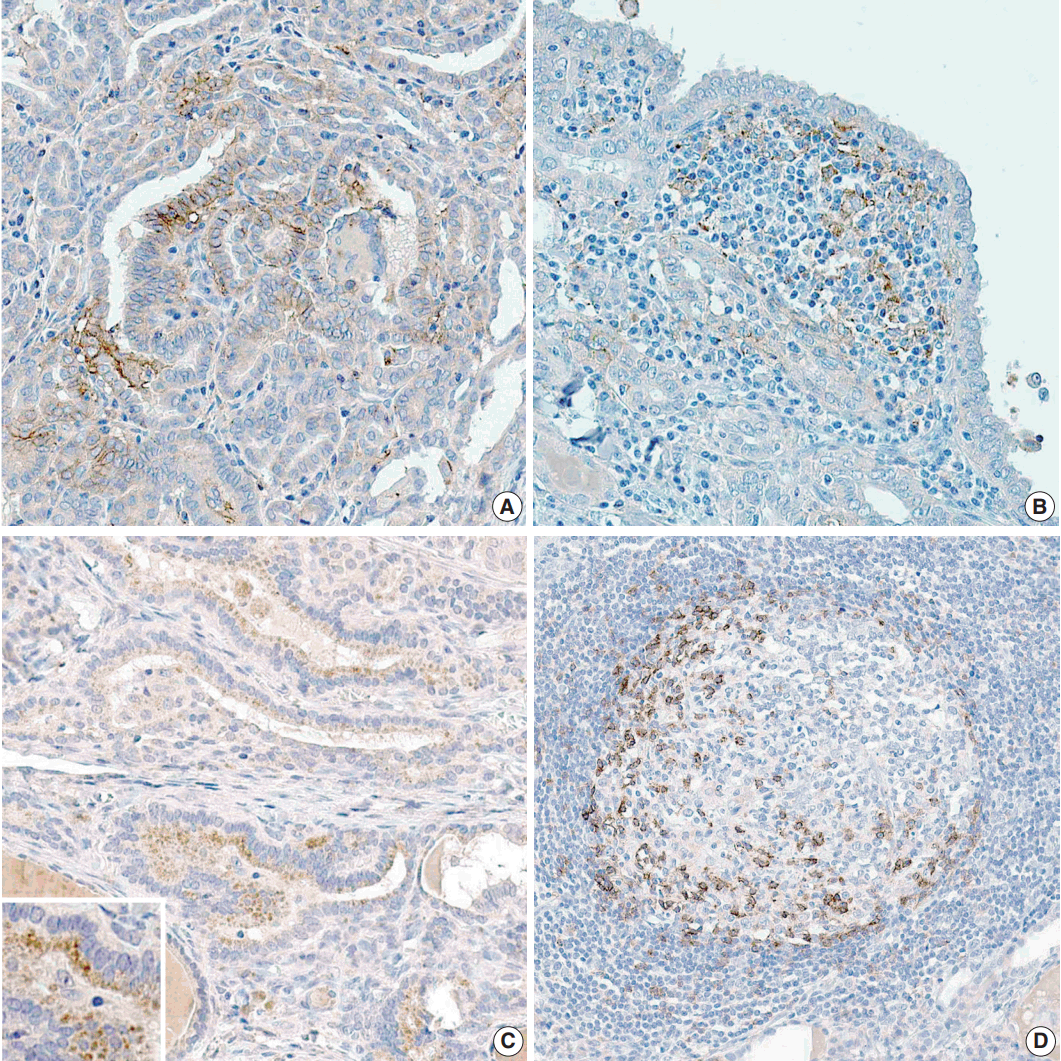
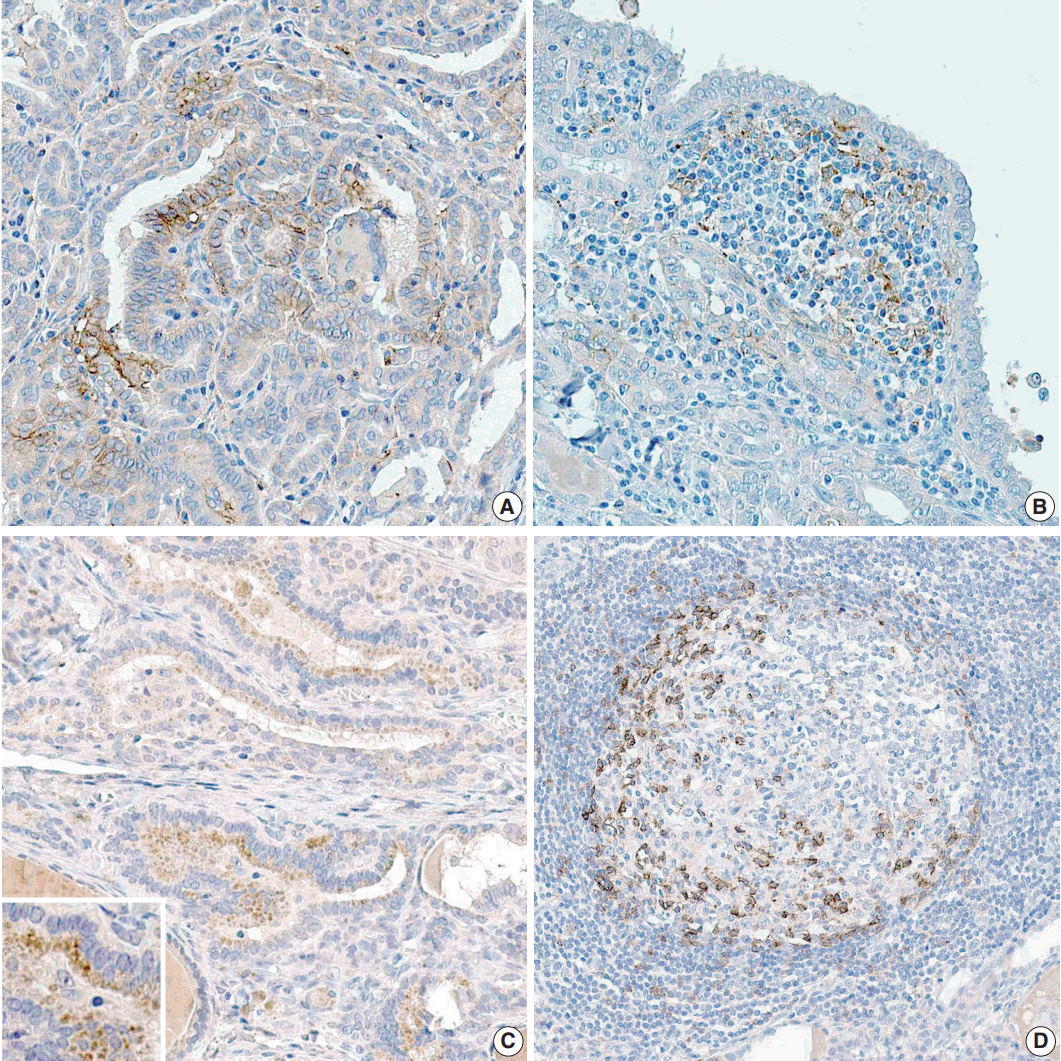
- The proportion of positive cells is summarized in Table 2. For PTC cells, 39 (33.6%) patients exhibited PD-L1 expression, and five (8.3%) were positive for PD-1 protein (Fig. 1). Meanwhile, for inflammatory cells, seven (6%) and six (5.2%) patients were positive for PD-L1 and PD-1 expressions, respectively.
- Correlation among PD-L1 and PD-1 expressions and clinicopathological data
- The correlation between PD-L1 and PD-1 expressions is shown in Table 3. The increased proportion of PD-L1–positive PTC cells (PDL1-PTC) was significantly correlated with increased proportion of PD-1–positive PTC cells (p = .001) and PD-1–positive inflammatory cells (PD1-IC) (p < .001). The increased proportion of PD-L1–positive inflammatory cells was closely related with the increased proportion of PD1-IC (p < .001).
- The relationship between the collected clinicopathological data (age, sex, T stage, and N stage) and the expressions of PDL1 and PD-1 in PTC cells and inflammatory cells was analyzed. The results indicated no significant correlation, except for the proportion of PDL1-PTC and LN metastasis (p = .036) (Table 4).
RESULTS
- In the control of T-cell immunity, positive and negative signals regulated within the T cells make balance between immune cell responses [18]. The receptor and ligand interactions of T cells can support not only positive signals to improve T-cell activation but also negative signals to eliminate T cells [19]. Among the negative signals, immune checkpoint proteins, PD-1 or PD-L1 are crucial to keep self-tolerance to avoid autoimmunity, through the interaction between T cells and antigen-presenting cells [20]. Ligation of PD-1 by PD-L1 inhibits proliferation of lymphocytes and cytokine production by activated cytotoxic T cells [21]. The PD-1/PD-L1 checkpoint pathway regulates not only virally infected T cells in the peripheral tissue [21] but also transformed cells within the tumor microenvironment [22,23]. Tumor cells co-work with immune checkpoints to avoid being destructed by host immune system.
- Activated T lymphocytes normally induce PD-L1 expression on macrophages or host cells. Besides, there are some tumor cells that erratically express PD-L1 [19]. PD-L1, which is called B7-H1 or CD27, is one of the B7 gene family members expressed on the surface of the tumor cells. When effector T cells are activated, PD-1 is highly expressed on the regulatory T cells (Treg cells). The immune responses of Treg cells remain to be discovered completely. By producing inhibitory cytokines, they have an essential role for immune resistance in many tumors [20]. Meanwhile, T cell exhaustion was first reported with chronic lymphocytic choriomeningitis virus infection of mice during which activated virusspecific T cells without effector function persist [24]. Exhausted T cells overexpress multiple cell-surface inhibitory receptors, including PD-1, LAG-3, and 2B4 [25]. These cells are not able to reduce PD-L1 expression, which is normally suppressed when T cells are activated [25].
- Tumors with PD-1 positive exhausted T cells and Treg cells may show high proportion of PD-L1–positive tumor cells. In this study, a statistically significant association existed between the expression of PD-1 and PD-L1 markers both in papillary carcinoma (p = .001) and lymphoid cells (p < .001). Moreover, the proportion of PD-L1 expression in papillary carcinoma cells was closely associated with metastatic LNs (p = .036). Similarly, PD-L1 was high (p = .0443) in patients with nodal metastases in a previous study [26]. In summary, our clinical data of PTC with LN metastasis was significantly associated with PD-1/PD-L1 pathway. Although 12 out of 24 cases were negative for PD-L1 expression in PTC with metastatic LNs, the proportion of its expression and the predictive value are still meaningful. Exhausted T cells change the expression of molecules related to cell adhesion, migration, and chemotaxis during chronic viral infection [25]. We assumed that PD-1–positive exhausted T cells must have altered the expression of molecules, probably modifying migratory characters in the tumor microenvironment along the metastatic cascade.
- In previous studies regarding regionally metastatic differentiated thyroid carcinomas, PD-1–positive T cells and Treg cells were higher in tumor-involved LNs compared with those not involved and were related to recurrent disease [26]. The association between elevated Treg cell levels and recurrent disease supports the hypothesis that Treg cells are induced by the residual metastatic tumor to avoid immune responses [23]. Because tumors with high levels of infiltrating Treg cells may suppress immune responses, inhibition of the PD-1/PD-L1 pathway may improve antitumor immune responses by reducing the number of or suppressing Treg cells [20].
- Our data support the existence of PD-L1 blockades in patients with thyroid carcinoma with metastatic LNs and its predictive value as a biomarker for metastatic LNs. Immunomodulating therapies that suppress PD-L1 might be used for patients with persistent disease because regionally metastatic thyroid carcinomas are not eliminated by the host immune system. The prognostic role of PD-L1 expression associated with LN metastasis was elucidated in this study.
DISCUSSION
Acknowledgments
| PD-L1 | PD-1 | |
|---|---|---|
| PTC (n = 116) | ||
| Negative | 77 | 111 |
| 1%–50% positive | 34 | 3 |
| 51%–100% positive | 5 | 2 |
| IC (n = 116) | ||
| Negative | 109 | 110 |
| 1%–10% positive | 7 | 5 |
| 11%–20% positive | 0 | 1 |
|
PTC |
IC |
|||
|---|---|---|---|---|
| PD-L1 | PD-1 | PD-L1 | PD-1 | |
| PTC | ||||
| PD-L1 | - | 0.001 | 0.071 | < .001 |
| PD-1 | 0.001 | - | 0.126 | 0.172 |
| IC | ||||
| PD-L1 | 0.071 | 0.126 | - | < .001 |
| PD-1 | < .001 | 0.172 | < .001 | - |
| Proportion of PD-L1 expression in PTC cells (%) |
LN metastasis |
||
|---|---|---|---|
| No | Yes | Total | |
| 0 | 65 | 12 | 77 |
| 1–50 | 25 | 9 | 34 |
| 51–100 | 2 | 3 | 5 |
| Total | 92 | 24 | p = .036 |
- 1. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540-50. ArticlePubMed
- 2. Juárez-Salcedo LM, Sandoval-Sus J, Sokol L, Chavez JC, Dalia S. The role of anti-PD-1 and anti-PD-L1 agents in the treatment of diffuse large B-cell lymphoma: The future is now. Crit Rev Oncol Hematol 2017; 113: 52-62. ArticlePubMed
- 3. Motoshima T, Komohara Y, Ma C, et al. PD-L1 expression in papillary renal cell carcinoma. BMC Urol 2017; 17: 8.ArticlePubMedPMCPDF
- 4. Fontugne J, Augustin J, Pujals A, et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget 2017; 8: 24644-51. ArticlePubMedPMC
- 5. Jiang Y, Lo AW, Wong A, et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget 2017; 8: 30175-89. ArticlePubMedPMC
- 6. Ness N, Andersen S, Khanehkenari MR, et al. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. Oncotarget 2017; 8: 26789-801. ArticlePubMedPMC
- 7. Park IH, Yang HN, Lee KJ, et al. Tumor-derived IL-18 induces PD-1 expression on immunosuppressive NK cells in triple-negative breast cancer. Oncotarget 2017; 8: 32722-30. ArticlePubMedPMC
- 8. Jin S, Xu B, Yu L, et al. The PD-1, PD-L1 expression and CD3+ T cell infiltration in relation to outcome in advanced gastric signet-ring cell carcinoma, representing a potential biomarker for immunotherapy. Oncotarget 2017; 8: 38850-62. ArticlePubMedPMC
- 9. Gravelle P, Burroni B, Péricart S, et al. Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: a summary of immunohistochemical studies. Oncotarget 2017; 8: 44960-75. ArticlePubMedPMC
- 10. Zhou Y, Miao J, Wu H, et al. PD-1 and PD-L1 expression in 132 recurrent nasopharyngeal carcinoma: the correlation with anemia and outcomes. Oncotarget 2017; 8: 51210-23. ArticlePubMedPMC
- 11. Chen N, Fang W, Lin Z, et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother 2017; 66: 1175-87. ArticlePubMedPMCPDF
- 12. Chovanec M, Cierna Z, Miskovska V, et al. Prognostic role of programmed-death ligand 1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular germ cell tumors. Oncotarget 2017; 8: 21794-805. ArticlePubMedPMC
- 13. Botti G, Collina F, Scognamiglio G, et al. Programmed death ligand 1 (PD-L1) tumor expression is associated with a better prognosis and diabetic disease in triple negative breast cancer patients. Int J Mol Sci 2017; 18: E459.Article
- 14. Han J, Hong Y, Lee YS. PD-L1 expression and combined status of PD-L1/PD-1-positive tumor infiltrating mononuclear cell density predict prognosis in glioblastoma patients. J Pathol Transl Med 2017; 51: 40-8. ArticlePubMedPDF
- 15. Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016; 2: 1023-9. ArticlePubMedPMC
- 16. Nixon IJ, Wang LY, Migliacci JC, et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid 2016; 26: 373-80. ArticlePubMedPMC
- 17. Song DH, Ko GH, Lee JH, et al. Myoferlin expression in non-small cell lung cancer: Prognostic role and correlation with VEGFR-2 expression. Oncol Lett 2016; 11: 998-1006. ArticlePubMed
- 18. Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004; 4: 336-47. ArticlePubMed
- 19. Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res 2007; 13(2 Pt 2):709s-15s. ArticlePubMed
- 20. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252-64. ArticlePubMedPMCPDF
- 21. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192: 1027-34. ArticlePubMedPMC
- 22. Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793-800. ArticlePubMedPDF
- 23. French JD, Kotnis GR, Said S, et al. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab 2012; 97: E934-43. ArticlePubMedPMCPDF
- 24. Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998; 188: 2205-13. ArticlePubMedPMC
- 25. Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007; 27: 670-84. ArticlePubMed
- 26. Bastman JJ, Serracino HS, Zhu Y, et al. Tumor-infiltrating T cells and the PD-1 checkpoint pathway in advanced differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab 2016; 101: 2863-73. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Chronic Lymphocytic Thyroiditis with Oncocytic Metaplasia Influences PD-L1 Expression in Papillary Thyroid Carcinoma
Vitor Barreto Santana, Vitória Machado Krüger, Maria Cristina Yunes Abrahão, Pietru Lentz Martins Cantú, Rosicler Luzia Brackmann, Gisele Moroni Pandolfi, Liane Scheffler Marisco, Gabriela Remonatto, Luciana Adolfo Ferreira, Marcia Silveira Graudenz
Head and Neck Pathology.2024;[Epub] CrossRef - Exploring markers of immunoresponsiveness in papillary thyroid carcinoma and future treatment strategies
Atish Mohanty, Michelle Afkhami, Amanda Reyes, Rebecca Pharaon, Holly Yin, Haiqing Li, Dana Do, Diana Bell, Arin Nam, Sue Chang, Thomas Gernon, Robert Kang, Arya Amini, Sagus Sampath, Prakash Kulkarni, Raju Pillai, Vicky Villaflor, Ravi Salgia, Ellie Magh
Journal for ImmunoTherapy of Cancer.2024; 12(7): e008505. CrossRef - Update regarding the role of PD-L1 in oncocytic thyroid lesions on cytological samples
Marco Dell'Aquila, Pietro Tralongo, Alessia Granitto, Maurizio Martini, Sara Capodimonti, Mariangela Curatolo, Vincenzo Fiorentino, Alfredo Pontecorvi, Guido Fadda, Celestino Pio Lombardi, Maco Raffaelli, Liron Pantanowitz, Luigi Maria Larocca, Esther Dia
Journal of Clinical Pathology.2023; 76(10): 671. CrossRef - Analysis of anti‐apoptotic PVT1 oncogene and apoptosis‐related proteins (p53, Bcl2, PD‐1, and PD‐L1) expression in thyroid carcinoma
Afaf T. Ibrahiem, Amin K. Makhdoom, Khalid S. Alanazi, Abdulaziz M. Alanazi, Abdulaziz M. Mukhlef, Saad H. Elshafey, Eman A. Toraih, Manal S. Fawzy
Journal of Clinical Laboratory Analysis.2022;[Epub] CrossRef - EphA10 drives tumor progression and immune evasion by regulating the MAPK/ERK cascade in lung adenocarcinoma
Wenyue Zhao, Lu Liu, Xuehao Li, Shun Xu
International Immunopharmacology.2022; 110: 109031. CrossRef - Hashimoto’s Thyroiditis Minimizes Lymph Node Metastasis in BRAF Mutant Papillary Thyroid Carcinomas
Peter P. Issa, Mahmoud Omar, Yusef Buti, Chad P. Issa, Bert Chabot, Christopher J. Carnabatu, Ruhul Munshi, Mohammad Hussein, Mohamed Aboueisha, Mohamed Shama, Ralph L. Corsetti, Eman Toraih, Emad Kandil
Biomedicines.2022; 10(8): 2051. CrossRef - Expression of β-Catenin in Thyroid Neoplasms (Histopathological and Immunohistochemical Study)
Mohamed Sherif Ismail, Amr Mousa Abdel Gawad Mousa, Mohammed Faisal Darwish, M. Mostafa Salem, Randa Said
Open Access Macedonian Journal of Medical Sciences.2022; 10(A): 1565. CrossRef - Identification and validation of an immune-related prognostic signature and key gene in papillary thyroid carcinoma
Rujia Qin, Chunyan Li, Xuemin Wang, Zhaoming Zhong, Chuanzheng Sun
Cancer Cell International.2021;[Epub] CrossRef - PD‐L1 and thyroid cytology: A possible diagnostic and prognostic marker
Marco Dell’Aquila, Alessia Granitto, Maurizio Martini, Sara Capodimonti, Alessandra Cocomazzi, Teresa Musarra, Vincenzo Fiorentino, Alfredo Pontecorvi, Celestino Pio Lombardi, Guido Fadda, Liron Pantanowitz, Luigi Maria Larocca, Esther Diana Rossi
Cancer Cytopathology.2020; 128(3): 177. CrossRef - Programmed Death-Ligand 1 (PD-L1) Is a Potential Biomarker of Disease-Free Survival in Papillary Thyroid Carcinoma: a Systematic Review and Meta-Analysis of PD-L1 Immunoexpression in Follicular Epithelial Derived Thyroid Carcinoma
Ilaria Girolami, Liron Pantanowitz, Ozgur Mete, Matteo Brunelli, Stefano Marletta, Chiara Colato, Pierpaolo Trimboli, Anna Crescenzi, Massimo Bongiovanni, Mattia Barbareschi, Albino Eccher
Endocrine Pathology.2020; 31(3): 291. CrossRef - Regression of Papillary Thyroid Cancer during Nivolumab for Renal Cell Cancer
Andrea Palermo, Andrea Napolitano, Daria Maggi, Anda Mihaela Naciu, Gaia Tabacco, Silvia Manfrini, Anna Crescenzi, Chiara Taffon, Francesco Pantano, Bruno Vincenzi, Guiseppe Tonini, Daniele Santini
European Thyroid Journal.2020; 9(3): 157. CrossRef - A potential biomarker hsa-miR-200a-5p distinguishing between benign thyroid tumors with papillary hyperplasia and papillary thyroid carcinoma
Xian Wang, Shan Huang, Xiaocan Li, Dongrui Jiang, Hongzhen Yu, Qiang Wu, Chaobing Gao, Zhengsheng Wu, Yi-Hsien Hsieh
PLOS ONE.2018; 13(7): e0200290. CrossRef - Papillary Thyroid Carcinoma Emerging from Hashimoto Thyroiditis Demonstrates Increased PD-L1 Expression, Which Persists with Metastasis
Daniel Lubin, Ezra Baraban, Amanda Lisby, Sahar Jalali-Farahani, Paul Zhang, Virginia Livolsi
Endocrine Pathology.2018; 29(4): 317. CrossRef - Chemotherapeutic Treatments Increase PD-L1 Expression in Esophageal Squamous Cell Carcinoma through EGFR/ERK Activation
Hoi Yan Ng, Jian Li, Lihua Tao, Alfred King-Yin Lam, Kwok Wah Chan, Josephine Mun Yee Ko, Valen Zhuoyou Yu, Michael Wong, Benjamin Li, Maria Li Lung
Translational Oncology.2018; 11(6): 1323. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

Fig. 1.
| Variable | No. (%) |
|---|---|
| Clinical information | |
| Age, mean (range) | 49.5 (25–88) |
| Sex (male:female) | 20:96 |
| Patients number with LND | 51 (44.0) |
| Pathologic information | |
| T stage | |
| 1 | 54 (46.6) |
| 2 | 3 (2.6) |
| 3 | 59 (50.9) |
| N stage | |
| 0 | 92 (79.3) |
| 1 | 24 (20.7) |
| PD-L1 | |
| Positive in PTC | 39 (33.6) |
| Positive in IC | 7 (6.0) |
| PD-1 | |
| Positive in PTC | 5 (8.3) |
| Positive in IC | 6 (5.2) |
| Total | 116 |
| PD-L1 | PD-1 | |
|---|---|---|
| PTC (n = 116) | ||
| Negative | 77 | 111 |
| 1%–50% positive | 34 | 3 |
| 51%–100% positive | 5 | 2 |
| IC (n = 116) | ||
| Negative | 109 | 110 |
| 1%–10% positive | 7 | 5 |
| 11%–20% positive | 0 | 1 |
| PTC |
IC |
|||
|---|---|---|---|---|
| PD-L1 | PD-1 | PD-L1 | PD-1 | |
| PTC | ||||
| PD-L1 | - | 0.001 | 0.071 | < .001 |
| PD-1 | 0.001 | - | 0.126 | 0.172 |
| IC | ||||
| PD-L1 | 0.071 | 0.126 | - | < .001 |
| PD-1 | < .001 | 0.172 | < .001 | - |
| Proportion of PD-L1 expression in PTC cells (%) | LN metastasis |
||
|---|---|---|---|
| No | Yes | Total | |
| 0 | 65 | 12 | 77 |
| 1–50 | 25 | 9 | 34 |
| 51–100 | 2 | 3 | 5 |
| Total | 92 | 24 | p = .036 |
LND, lymph node dissection; PD-L1, programmed death-ligand 1; PTC, papillary thyroid carcinoma; IC, inflammatory cells; PD-1, programmed cell death protein 1.
PD-L1, programmed death-ligand 1; PD-1, programmed cell death protein 1; PTC, papillary thyroid carcinoma; IC, inflammatory cells.
PD-L1, programmed death-ligand 1; PD-1, programmed cell death protein 1; TMA, tissue microarray; PTC, papillary thyroid carcinoma; IC, inflammatory cells.
PD-L1, programmed death-ligand 1; PTC, papillary thyroid carcinoma; LN, lymph node.

 E-submission
E-submission