Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 54(5); 2020 > Article
-
Case Study
Renal intravascular large B cell lymphoma: the first case report in Korea and a review of the literature -
Moonsik Kim1
 , Haerim Chung2
, Haerim Chung2 , Woo Ick Yang1
, Woo Ick Yang1 , Hyeon Joo Jeong,1
, Hyeon Joo Jeong,1
-
Journal of Pathology and Translational Medicine 2020;54(5):426-431.
DOI: https://doi.org/10.4132/jptm.2020.06.18
Published online: August 13, 2020
1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
2Division of Hematology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- Corresponding Author: Hyeon Joo Jeong, MD, Department of Pathology, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea Tel: +82-2-2228-1766, Fax: +82-2-362-0860, E-mail: Jeong10@yuhs.ac
© 2020 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Herein, we describe the first case of renal intravascular large B cell lymphoma in Korea occurring in a 66-year-old female. She presented with mild fever and dyspnea. On physical and laboratory evaluations, hemophagocytic lymphohistiocytosis was suspected, but the bone marrow biopsy results were unremarkable. During the work-up, massive proteinuria developed, which led to a renal biopsy. The renal architecture was relatively well-preserved, but the glomeruli were hypercellular with the infiltration of atypical, large lymphoid cells with increased nucleus-cytoplasm ratio and clumped chromatin. Similar cells were also present in the peritubular capillaries. The tumor cells exhibited membranous staining for CD20 and CD79a. After the diagnosis of intravascular large B cell lymphoma, the patient received rituximab-based chemotherapy under close follow-up.
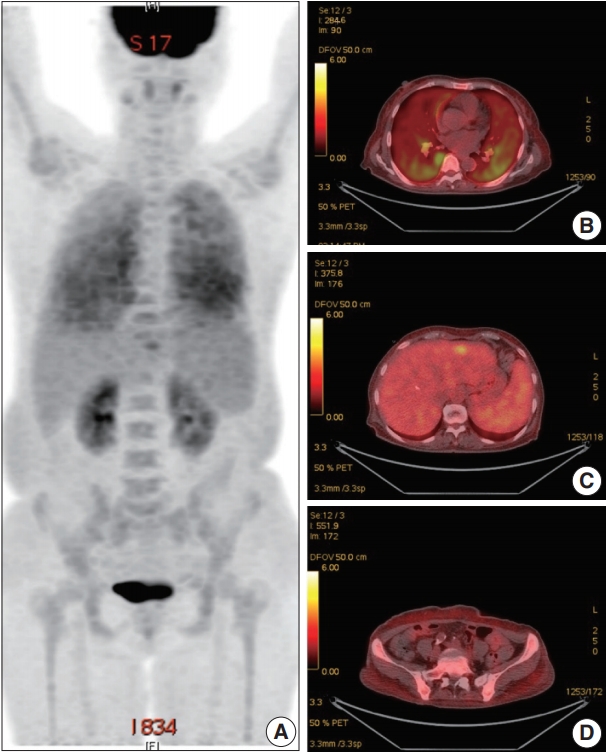
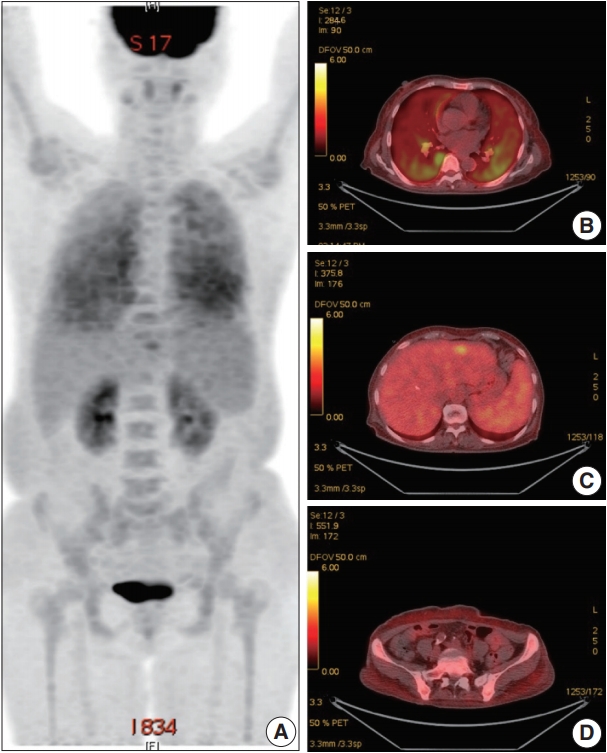
- A 66-year-old female was admitted to the Hematology Division of Internal Medicine with the presentation of a fever of 38°C, cough, non-bloody sputum, and dyspnea, which had started a month previously. Her past medical history was unremarkable. Her blood pressure was 126/67 mm Hg, pulse rate was 79 beats per minute, and respiratory rate was 18 breaths per minute. The physical examination revealed no specific findings. Neither lymphadenopathy nor skin rashes were noted. The analysis of the complete blood cell count revealed anemia (hemoglobin, 7.1 g/dL) and thrombocytopenia (platelet count, 95×103/μL). Ferritin was 1,003.0 ng/mL. Serum haptoglobin level was normal and the Coombs test was negative. The C-reactive protein level was 86.7 mg/L and the lactate dehydrogenase level was 1,779 U/L. Blood urea nitrogen and serum creatinine levels were 2.8 mmol/L and 0.75 mg/dL, respectively. Serum aspartate transaminase was 31 IU/L and alanine transaminase was 10 IU/L. Total bilirubin level was mildly elevated (1.5 mg/dL). Antinuclear antibodies, anti-neutrophil cytoplasmic antibodies, antiglomerular basement antibody, and hepatitis B and C viral markers were all negative. Sputum, blood, and urine cultures revealed all negative results. Chest computed tomography (CT) showed mild bronchiolitis in the right upper lobe field without definite evidence of pulmonary thromboembolism. Abdominal CT revealed mild hepatosplenomegaly. On the positron emission tomography–CT, diffuse 18F-Fluorodeoxyglucose (FDG) uptake on the bilateral lungs, mild FDG uptake on the left lateral segment of the liver, and increased FDG uptake along the axial and appendicular bones were noted (Fig. 1). Clinically, hemophagocytic lymphohistiocytosis was suggested. A subsequent bone marrow biopsy revealed normocellular marrow without abnormal cell infiltration. Liver biopsy showed mild lobular hepatitis with Kupffer cell hypertrophy and increased hemophagocytosis.
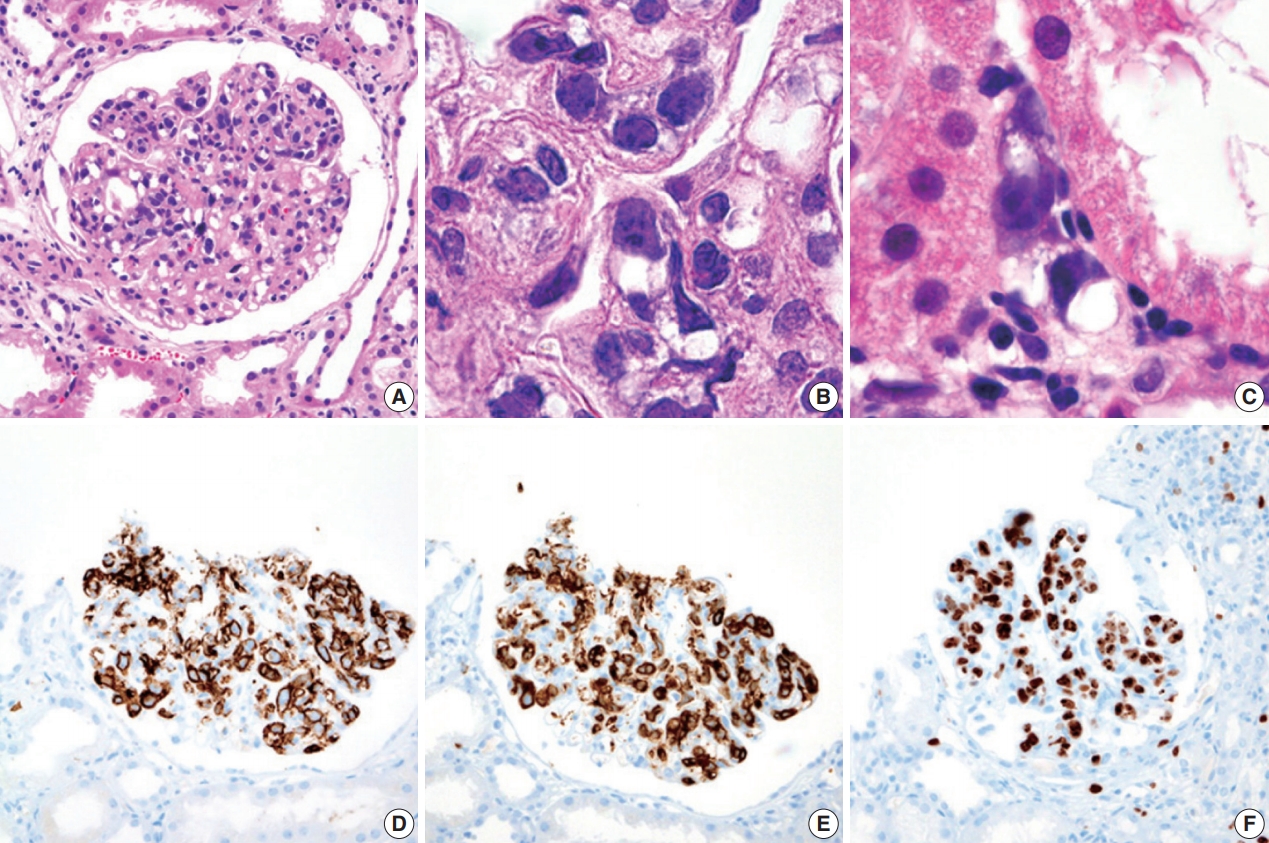
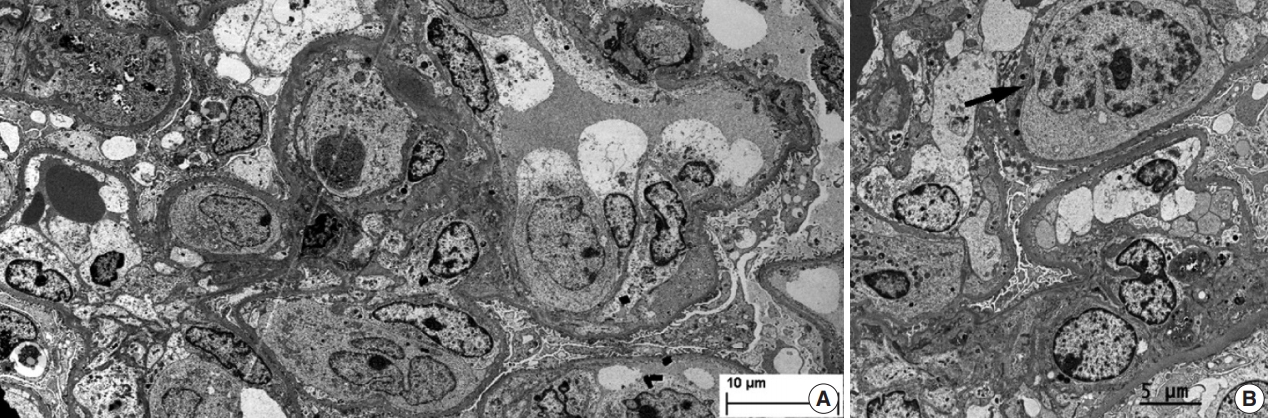
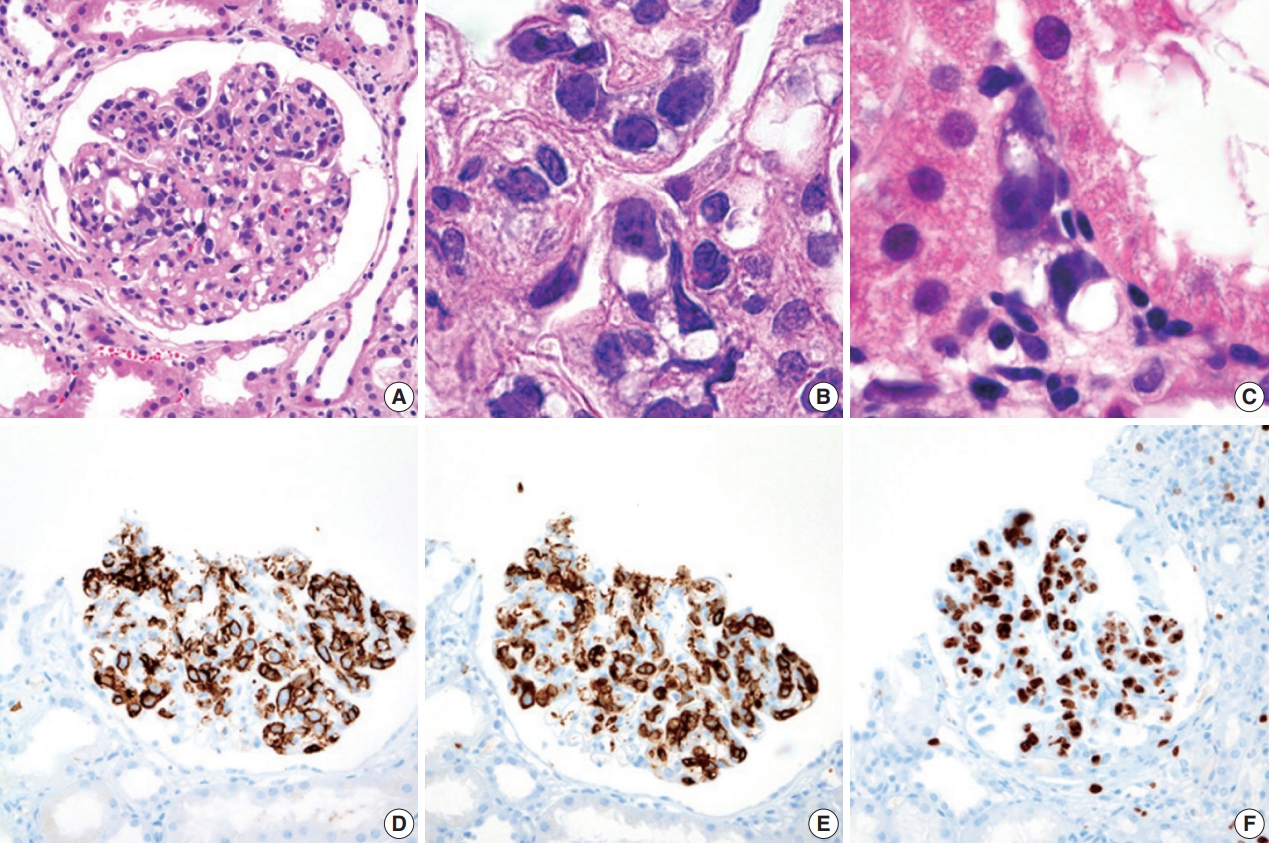
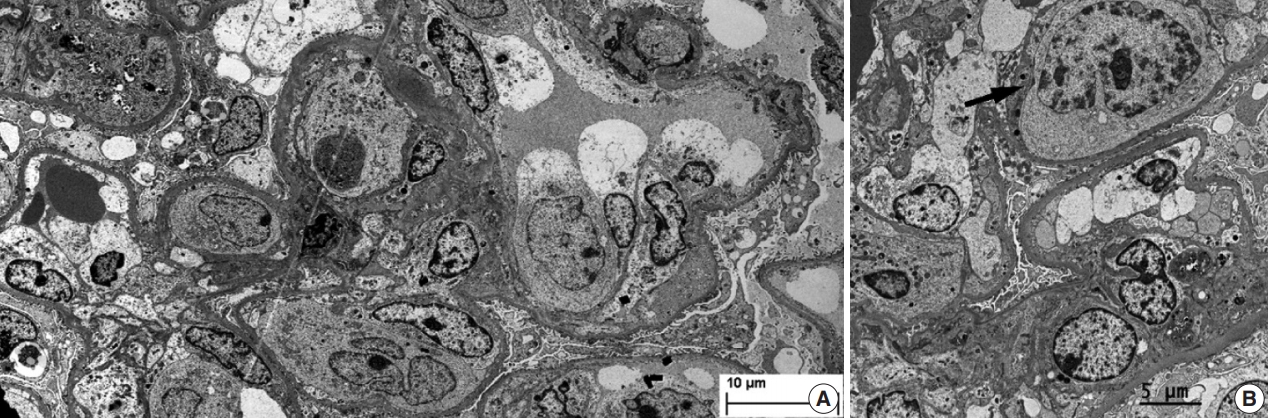
- During further work ups, heavy proteinuria (4 g/day and urine protein-to-creatinine ratio of 4.94) suddenly developed. A renal biopsy was performed on the twenty-fourth hospital day. The biopsy core of the kidney contained nineteen glomeruli. Most of the glomeruli were mildly enlarged and hypercellular with infiltration of atypical large lymphoid cells in the capillary lumina. They exhibited a high nucleus-cytoplasm ratio with size variation, chromatin clumping, and inconspicuous nucleoli. Immunohistochemical stains demonstrated strong CD20 and CD79a membranous positivity with a Ki-67 proliferation index of 80% (Fig. 2). The tubulointerstitium was relatively wellpreserved, but the tumor cells were also present in some peritubular capillaries. On electron microscopy, the glomerular capillary lumen was obliterated by swollen endothelial cells and infiltrating atypical lymphoid cells (Fig. 3). The diagnosis of renal intravascular large B cell lymphoma was confirmed. The patient is currently receiving chemotherapy consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) with close follow-ups for three months.
CASE REPORT
- IVLBL is a rare type of aggressive, non-Hodgkin B cell lymphoma that is confined to small to medium sized vessels. Clinically, two major patterns have been recognized [6]. The first pattern, called the ‘Classic form’, is prevalent in Western populations and usually demonstrates skin and CNS involvement and related symptoms. The second pattern, so called the ‘Asian variant’, is characterized by hemophagocytic syndrome, fever, multiorgan failure, hepatosplenomegaly, and bone marrow involvement. Our case exhibited clinical features of the ‘Asian variant’ with fever, hepatosplenomegaly, and nephrotic range proteinuria.
- Renal involvement of IVLBL is infrequent. Forty-three cases, including the present case, have been reported in English literature (Table 1). There were 19 males and 21 females, ranging from 35 to 85 years of age (mean age, 60.5 years). The clinical signs of renal involvement were proteinuria (90.0%), some with nephrotic range (21.0%), renal failure (60.5%), and enlargement of the kidney upon radiologic evaluation (34.3%). Fifty percent of the cases also exhibited extrarenal involvement. Bone marrow involvement was identified in three out of 16 cases. Hemophagocytosis was observed in four cases, including the present case. All four cases occurred in Asians.
- Histologically, malignant lymphoid cells demonstrate generally large, vesicular nuclei, one or more nucleoli, and scanty cytoplasm [1]. These cells usually express pan B cell markers including CD20, CD79a, and bcl-2, bcl-6, MUM-1, and variable expressions of CD5 and CD10 [7]. Although glomerular hypercellularity can be seen in hemophagocytic lymphohistiocytosis, cellular atypia and immunohistochemistry could differentiate malignant B lymphocytes from macrophages. Neoplastic cells lack surface molecules including CD29, CD54, and CD49d, facilitating the transvascular migration of the tumor cells. The aberrant expression of CXCR3 has also been described [8].
- Of the 43 cases of intrarenal IVLBL with histologic descriptions, 35 cases exhibited the glomerular infiltration of tumor cells with or without peritubular and interstitial involvement. In four cases, the tumor cells were localized in peritubular capillaries without glomerular involvement. Our case showed glomerular and peritubular involvement. The microvascular infiltration and obliteration by tumor cells may be responsible for proteinuria in our case through podocyte and endothelial injuries [9].
- IVLBL generally shows aggressive clinical courses, often worsened by the delay of diagnosis due to its nonspecific clinical presentation [10]. In terms of prognosis, at 6 months after diagnosis, 15 patients were alive and seven patients had died. Recently, rituximab-based chemotherapy has significantly improved clinical outcomes with a 3-year survival rate of 60%–80%. In addition, IVLBL only limited to the skin has been reported to exhibit better prognoses [11]. Our case is under rituximab-based chemotherapy for 3 months without further clinical deterioration.
- In summary, we described a case of the renal involvement of IVLBL. Although the renal involvement of IVLBL is rare, this entity should be included as a differential diagnosis if unexplained hemophagocytic syndrome coupled with proteinuria persists.
DISCUSSION
Supplementary Materials
Ethics Statement
This work was approved by a faculty research grant of Yonsei University College of Medicine (4-2020-0150). The patient provided written informed consent for the publication of associated data and accompanying images.
Author contributions
Conceptualization: HJJ. Data curation: MSK. Formal analysis: HJJ. Funding acquisition: HJJ. Investigation: MSK. Methodology: MSK, WIY, HRC. Project administration: HJJ. Resources: HJJ, WIY. Supervision: HJJ. Validation: HJJ, WIY, HRC. Visualization: HJJ, WIY, HRC. Writing—original draft: MSK. Writing—review & editing: HJJ, WIY, HRC. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
N/A, not available; G, glomerulus; F, female; M, male; I, interstitium; NR, not remarkable; P, peritubular capillaries; HTLV-1, Human T-cell leukemia virus type 1; DCMP, dilated cardiomyopathy; AF, Atrial fibrillation; HTN, hypertension; DM, diabetes mellitus; CHO, hypercholesterolemia.
aThe reference list is included in the Supplementary Material.
- 1. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood 2007; 109: 478-85. ArticlePubMedPDF
- 2. Desclaux A, Lazaro E, Pinaquy JB, Yacoub M, Viallard JF. Renal intravascular large B-cell lymphoma: a case report and review of the literature. Intern Med 2017; 56: 827-33. ArticlePubMedPMC
- 3. Zuckerman D, Seliem R, Hochberg E. Intravascular lymphoma: the oncologist’s “great imitator”. Oncologist 2006; 11: 496-502. ArticlePubMed
- 4. Hasegawa J, Hoshino J, Suwabe T, et al. Characteristics of intravascular large B-cell lymphoma limited to the glomerular capillaries: a case report. Case Rep Nephrol Dial 2015; 5: 173-9. ArticlePubMedPMC
- 5. Ponzoni M, Ferreri AJ. Intravascular lymphoma: a neoplasm of 'homeless' lymphocytes? Hematol Oncol 2006; 24: 105-12. ArticlePubMed
- 6. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol 2007; 25: 3168-73. ArticlePubMed
- 7. Brunet V, Marouan S, Routy JP, et al. Retrospective study of intravascular large B-cell lymphoma cases diagnosed in Quebec: a retrospective study of 29 case reports. Medicine (Baltimore) 2017; 96: e5985.ArticlePubMedPMC
- 8. Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med 2012; 136: 333-8. ArticlePubMedPDF
- 9. Sekulic M, Martin S, Lal A, Weins A. Intravascular large B-cell lymphoma of the kidney. Kidney Int Rep 2018; 3: 1501-5. ArticlePubMedPMC
- 10. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol 2004; 127: 173-83. ArticlePubMed
- 11. Ferreri AJ, Dognini GP, Bairey O, et al. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma. Br J Haematol 2008; 143: 253-7. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- EBV-Positive Intravascular Large B-Cell Lymphoma of the Small Intestine: A Case Report and Literature Review
Chenglong Pan, Xiaoling Ma, Yanfei Yao, Chunyan Wang
International Journal of Surgical Pathology.2024; 32(3): 586. CrossRef - Intravascular large B‐cell lymphoma in renal cell carcinoma incidentally detected by robot‐assisted partial nephrectomy
Michio Noda, Yutaka Enomoto, Yukari Shirasugi, Sumiyo Ando, Yukimasa Matsuzawa, Haruki Kume
IJU Case Reports.2022; 5(3): 191. CrossRef - Case Report: Intravascular Large B-Cell Lymphoma: A Clinicopathologic Study of Four Cases With Review of Additional 331 Cases in the Literature
Yingying Han, Qingjiao Li, Dan Wang, Lushan Peng, Tao Huang, Chunlin Ou, Keda Yang, Junpu Wang
Frontiers in Oncology.2022;[Epub] CrossRef - Renal Involvement of CD20-Negative Intravascular Large B Cell Lymphoma with Neurological Manifestations
Faten Aqeel, Serena M. Bagnasco, Duvuru Geetha, Yoshihide Fujigaki
Case Reports in Nephrology.2022; 2022: 1. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
-
- Fibrin-associated large B-cell lymphoma arising in an endovascular graft: first case report in Korea
- Intravascular NK/T-cell lymphoma: a case report and literature review
- Metastatic choroidal melanoma in the breast: a case report and review of the literature
- Comment on “Breast implant-associated anaplastic large cell lymphoma: the first South Korean case”
- Breast implant–associated anaplastic large cell lymphoma: the first South Korean case



Fig. 1.
Fig. 2.
Fig. 3.
| No. | Referencea | Sex | Age (yr) | Renal disease | Extrarenal involvement | Proteinuria | Fever | Bone marrow finding | Other disease | Radiologic evaluation | Lymphoma cell location | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Jothy et al. | N/A | N/A | Renal failure | N/A | + | N/A | N/A | N/A | N/A | G | N/A |
| 2 | Jothy et al. | N/A | N/A | Renal failure | N/A | + | N/A | N/A | N/A | N/A | G | N/A |
| 3 | D’Agati | F | 62 | Nephrotic syndrome | + | + | + | NR | - | Bilateral enlarged kidney | G/I | Died 1 mo after the biopsy |
| 4 | Axelsen et al. | F | 60 | Nephrotic syndrome | – | + | + | N/A | N/A | N/A | G | Alive (8 mo |
| 5 | Nishikawa et al. | M | 52 | Renal failure | – | + | + | N/A | N/A | N/A | G | Alive (6 mo) |
| 6 | Agar et al. | F | 70 | Renal failure | – | + | N/A | N/A | N/A | N/A | G/P | N/A |
| 7 | Wood et al. | M | 61 | Renal failure | + | + | + | N/A | N/A | NR | G | Died 1 mo after the biopsy |
| 8 | Cheng et al. | F | 35 | N/A | + | N/A | + | Hemophagocytosis | Anemia | Bilateral enlarged kidney | G | N/A |
| 9 | Sepandj et al. | F | 75 | Renal failure | + | + | + | Erythroid hyperplasia and monocytosis | Anemia and monocytosis | Bilateral enlarged kidney | G/P | Alive (3 mo) |
| 10 | Charasse et al. | M | 71 | N/A | N/A | + | N/A | N/A | N/A | N/A | G | N/A |
| 11 | Katoh et al. | F | 64 | Renal failure | + | + | + | N/A | N/A | NR | G | N/A |
| 12 | Katoh et al. | M | 65 | Renal failure | + | + | + | N/A | N/A | Bilateral enlarged kidney | G/P/I | Post-mortem diagnosis |
| 13 | Katoh et al. | M | 82 | Renal failure | + | - | + | N/A | N/A | NR | G | Post-mortem diagnosis |
| 14 | Shaknovich et al. | F | 85 | Renal failure | – | + | - | N/A | Depression and hypertension | NR | G/I | Alive (3 mo) |
| 15 | Jourdan et al. | F | 49 | N/A | – | + | N/A | N/A | N/A | N/A | G | N/A |
| 16 | Törnroth et al. | M | 69 | Renal failure | – | + | + | N/A | Urosepsis | Diffusely hyperechoic | G/P | Died 1 mo after the biopsy |
| 17 | Törnroth et al. | M | 63 | Renal failure | + | + | - | N/A | Deep vein thrombosis | NR | G | Died 21 months after the biopsy |
| 18 | Fozza et al. | F | 53 | Nephrotic syndrome | + | + | + | Hypercellularity and increased reticulin fiber | Pancytopenia | NR | G | Died 6 mo after the biopsy |
| 19 | Kakumitsu et al. | M | 58 | Nephrotic syndrome | + | + | + | Hemophagocytosis | HTLV-1 positive | NR | G/I | Alive (1.5 mo) |
| 20 | Ozolek et al. | M | 72 | NR | – | + | - | N/A | DCMP, AF | NR | G | Alive (3 mo) |
| 21 | Cossu et al. | F | 56 | Nephrotic syndrome | + | + | - | N/A | N/A | NR | G/I | Died 6 mo after the biopsy |
| 22 | Kusaba et al. | M | 48 | Renal failure | – | + | - | N/A | N/A | NR | G | Alive (24 mo) |
| 23 | Chroboczek et al. | M | 56 | NR | + | + | + | N/A | N/A | N/A | N/A | Alive (8 mo) |
| 24 | Dauchy et al. | M | 67 | Renal failure | + | + | + | N/A | N/A | NR | G | Died 9 mo after the biopsy |
| 25 | Sawa et al. | F | 35 | Renal failure | – | + | + | Hypercellular marrow | NR | Bilateral enlarged kidney | P | Alive (6 mo) |
| 26 | Balkema et al. | F | 41 | N/A | + | N/A | + | NR | NR | Bilateral enlarged kidney | N/A | N/A |
| 27 | Yoo et al. | F | 74 | Renal failure | – | + | + | NR | Anemia | NR | G/I | N/A |
| 28 | Niitsu et al. | M | 52 | Renal failure | – | - | - | NR | NR | Bilateral enlarged kidney | G/I | Alive (26 mo) |
| 29 | Kameoka et al. | F | 40 | N/A | – | + | - | N/A | N/A | NR | G | Alive (24 mo) |
| 30 | Yago et al. | M | 76 | Renal failure | + | + | + | N/A | N/A | Bilateral enlarged kidney | N/A | N/A |
| 31 | Deisch et al. | M | 47 | Renal failure | + | + | + | Rare atypical large cells | NR | NR | G/P | Alive (6 mo) |
| 32 | Bai et al. | M | 41 | Nephrotic syndrome | + | + | + | Immature cells of unknown origin | NR | Bilateral enlarged kidney | I/P | Alive (9 mo) |
| 33 | Kado et al. | F | 72 | Renal failure | – | N/A | + | N/A | N/A | Bilateral enlarged kidney | p | Alive (4 mo) |
| 34 | Iwagami et al. | F | 78 | Nephrotic syndrome | + | + | + | N/A | N/A | NR | G/P | Died 3 mo after the biopsy |
| 35 | Zhu et al. | M | 55 | NR | – | + | - | Hypercellular marrow | Anemia | NR | G | N/A |
| 36 | Kamalanathan et al. | F | 77 | Renal failure | – | + | - | N/A | HTN, sicca syndrome | NR | G | Alive (48 mo) |
| 37 | Hasegawa et al. | F | 65 | Renal failure | – | + | - | N/A | HTN | NR | G | Alive (109 mo) |
| 38 | Vankalakunti et al. | M | 45 | Renal failure | – | + | N/A | Hemophagocytosis | N/A | NR | P | N/A |
| 39 | Desclaux et al. | F | 52 | NR | – | – | + | NR | NR | Bilateral enlarged kidney | G/P/I | Alive (30 mo) |
| 40 | Kimura et al. | M | 67 | NR | + | – | + | Presence of malignant lymphoid cells | Anemia and thrombocytopenia | Bilateral enlarged kidney | P | N/A |
| 41 | Sekulic et al. | M | 59 | Nephrolithiasis | – | + | + | Hypercellular marrow without abnormal lymphoid cells | DM, HTN | NR | G/P | Died less than 1 mo after the biopsy |
| 42 | Pothen et al. | F | 64 | Nephrotic syndrome | + | + | + | N/A | HTN, CHO | NR | G | Alive (24 mo) |
| 43 | Current case | F | 66 | NR | – | + | + | Normocellular marrow without abnormal lymphoid cells | Hemophagocytosis | NR | G/P/I | Alive (3mo) |
N/A, not available; G, glomerulus; F, female; M, male; I, interstitium; NR, not remarkable; P, peritubular capillaries; HTLV-1, Human T-cell leukemia virus type 1; DCMP, dilated cardiomyopathy; AF, Atrial fibrillation; HTN, hypertension; DM, diabetes mellitus; CHO, hypercholesterolemia. The reference list is included in the

 E-submission
E-submission