Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(1); 2024 > Article
-
Newsletter
What’s new in genitourinary pathology 2023: WHO 5th edition updates for urinary tract, prostate, testis, and penis -
Bonnie Choy, MD1
 , Maria Tretiakova, MD, PhD2
, Maria Tretiakova, MD, PhD2 , Debra L. Zynger, MS, MD,3
, Debra L. Zynger, MS, MD,3
-
Journal of Pathology and Translational Medicine 2024;58(1):45-48.
DOI: https://doi.org/10.4132/jptm.2023.12.11
Published online: December 27, 2023
1Department of Pathology Northwestern University Feinberg School of Medicine, Chicago, IL, USA
2Department of Laboratory Medicine and Pathology, University of Washington, Seattle, WA, USA
3Department of Pathology, The Ohio State University Wexner Medical Center, Columbus, OH, USA
- Corresponding Author: Debra L. Zynger, MS, MD Department of Pathology, The Ohio State University Wexner Medical Center, Columbus, OH, USA E-mail: debra.zynger@osumc.edu
- This article has been published jointly, with consent, in both Journal of Pathology and Translational Medicine and PathologyOutlines.com.
• Received: August 15, 2023 • Accepted: December 11, 2023
© 2024 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- The 5th edition WHO Classification of Urinary and Male Genital Tumours (2022) introduced many significant changes relevant to urologic daily practice, mainly to renal tumors which was covered in the What’s New newsletter in September 2022. In this newsletter, we summarize the notable changes to bladder, prostate, testis, and penis based on the 5th edition of the WHO.
- Inverted urothelial papilloma
- • This is reserved for almost exclusively inverted lesions and continues to be in a separate section.
- • Use of the descriptor “inverted” in other papillary tumors with inverted histology (non-invasive papillary urothelial carcinoma and papillary urothelial neoplasm of low malignant potential) is discussed within each respective section in the WHO.
- Non-invasive papillary urothelial carcinoma, low-grade
- • Papillary urothelial hyperplasia and urothelial proliferation with undetermined malignant potential (tented architecture with short, non-branching papillae covered by mildly atypical urothelium) are no longer regarded as distinct entities but are considered early low-grade non-invasive papillary carcinoma.
- Non-invasive papillary urothelial carcinoma, high-grade
- • To address grade heterogeneity, there are now new proposed criteria for reporting papillary tumors.
- – A tumor with ≥ 5% high-grade component is diagnosed as “high-grade”.
- – A tumor with < 5% high-grade component is diagnosed as “low-grade with < 5% high-grade component”.
- Urothelial carcinoma in situ
- • Urothelial dysplasia is no longer discussed in its own section but continues to be used as a diagnostic term for lesions that fall short of carcinoma in situ, despite the lack of reproducible criteria.
- Invasive urothelial carcinoma
- • Presence of TERT promoter mutations can help to: 1) distinguish urothelial carcinoma from a non-neoplastic proliferative process and 2) establish urothelial origin.
- • Advanced urothelial carcinoma with FGFR3 alterations may be eligible for treatment with anti-FGFR agents, while mutations in ERCC2 and other DNA damage repair genes may determine those likely to benefit from cisplatin-based chemotherapy.
- • Predictors of response to immune checkpoint inhibitors include PDL1 expression in tumor and host immune cells, tumor mutation burden, and microsatellite instability/mismatch repair defect status.
- • Terminology is standardized to characterize genetic alterations as “variants,” distinct morphologies as “histologic patterns,” and unique morphologies with prognostic significance as tumor “subtypes.”
- • Muscle-invasive bladder carcinoma is divided into 6 molecular subgroups with differing prognosis: luminal-papillary (24%), luminal non-specified (8%), luminal-unstable (15%), stroma-rich (15%), basal-squamous (35%), and neuroendocrine-like (3%).
URINARY TRACT
- Prostatic intraepithelial neoplasia
- • Low-grade prostatic intraepithelial neoplasia has no increased risk of cancer detection and it is not possible to reliably differentiate from benign glandular hyperplasia; thus it was removed as a reportable entity.
- Intraductal carcinoma of the prostate (IDC-P)
- • The cribriform growth pattern of high-grade prostatic intraepithelial neoplasia (HGPIN) is no longer regarded as a distinct entity but rather a spectrum of intraductal proliferation that falls between HGPIN and IDC-P; it is increasingly being referred to as atypical intraductal proliferation (AIP), although there is no consensus on the best terminology.
- • IDC-P may represent two distinct entities: 1) the majority are late events associated with invasive high-grade carcinoma spreading into non-neoplastic prostatic ducts and acini and 2) a small subset which arises from a HGPIN precursor and may represent carcinoma in situ.
- • The presence of IDC-P should be reported but whether to incorporate areas of IDC-P into Gleason grading remains controversial, with diverging recommendations by leading societies.
- Prostatic acinar adenocarcinoma
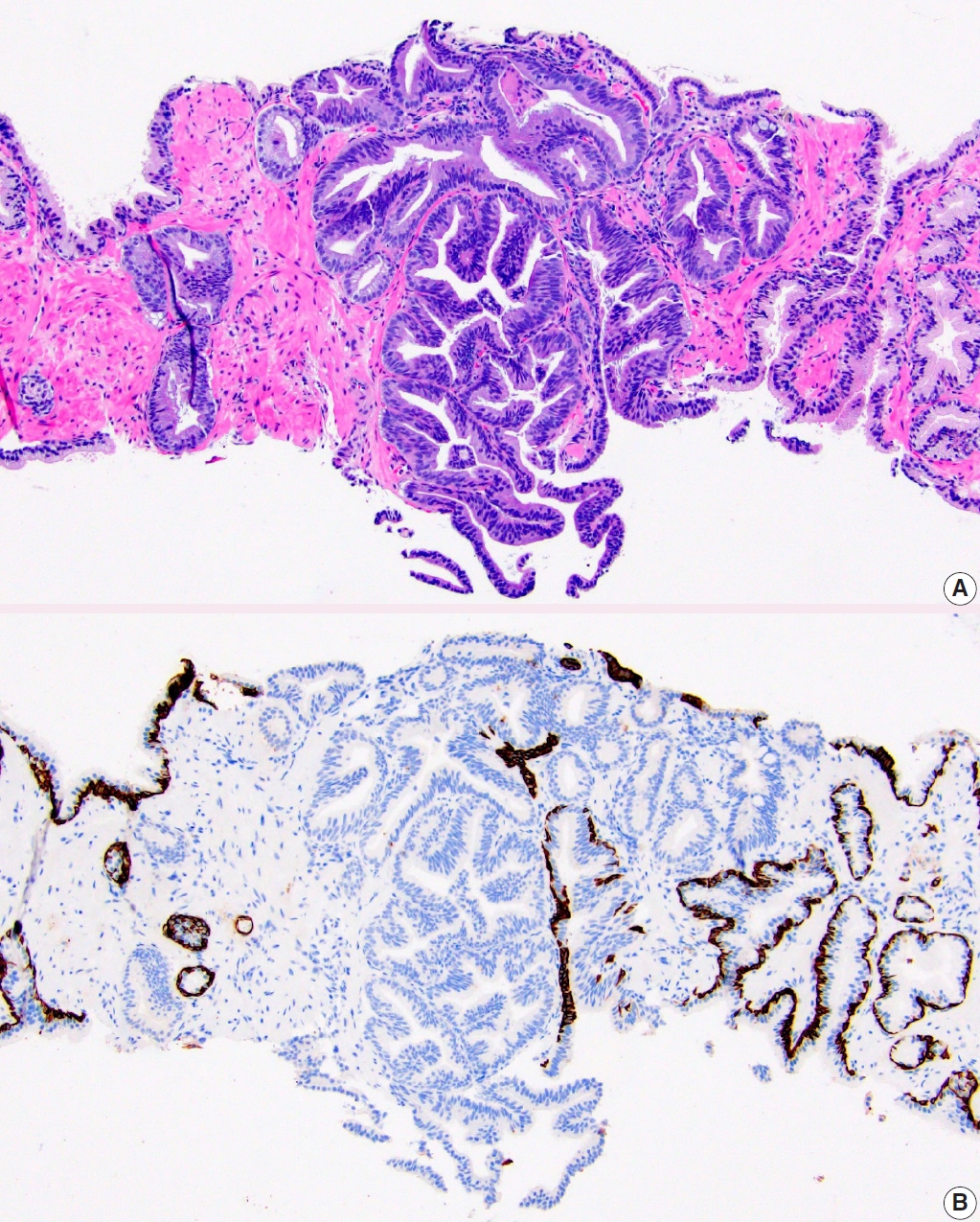
- • PIN-like carcinoma is now discussed as a subtype of acinar adenocarcinoma (Fig. 1). It generally has favorable behavior and has the recent distinctive molecular finding of frequent activating mutations in the RAF/RAS pathway.
- • Prostate cancers harboring homologous recombination repair defects may respond to poly (ADP-ribose) polymerase (PARP) inhibition, while those with mismatch repair defects may respond to immune checkpoint inhibitors.
- Adenoid cystic (basal cell) carcinoma of the prostate
- • Adenoid cystic carcinoma of the prostate is now the preferred diagnostic term, renamed from basal cell carcinoma, to avoid confusion with the skin tumor.
PROSTATE
- Noninvasive germ cell neoplasia
- • Gonadoblastoma has been moved from the category called “tumor containing both germ cell and sex cord-stromal elements” into the category “noninvasive germ cell neoplasia”.
- Germ cell tumors derived from germ cell neoplasia in situ
- • Teratoma is regarded as containing a somatic-type malignancy if the component that resembles a neoplasm has overgrowth of at least a 5 mm focus (consistent with the previous definition of 1 field at 4x magnification).
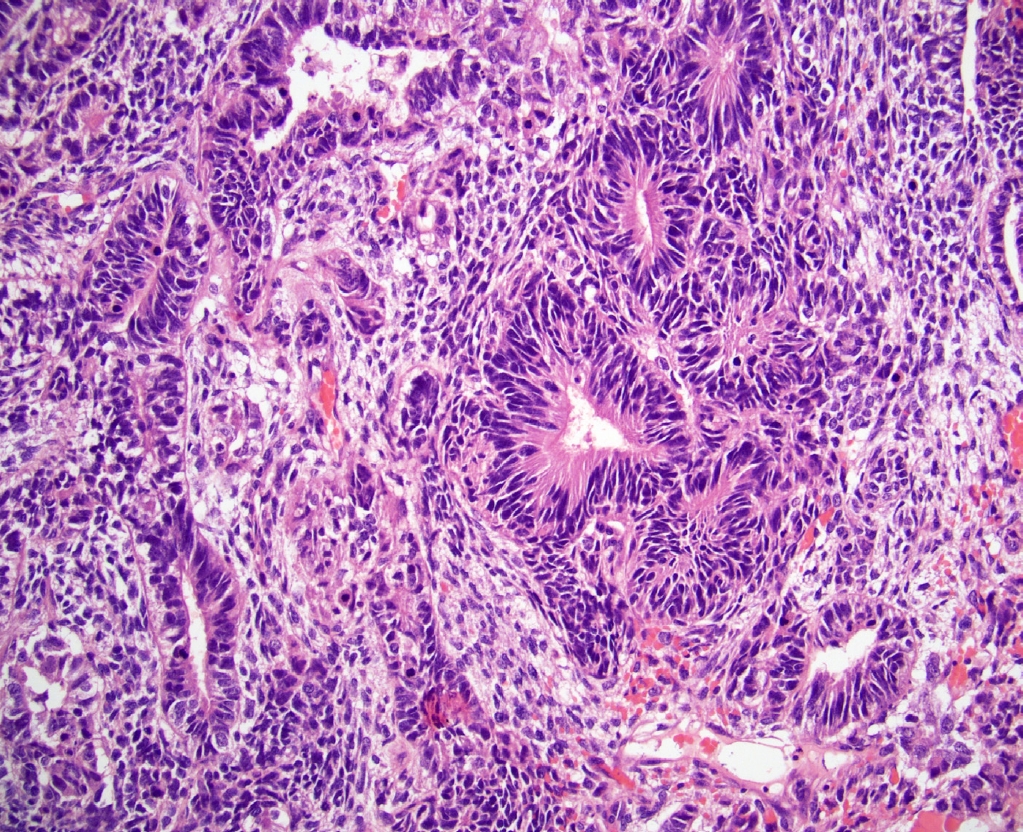
- • Somatic malignancy composed of immature neuroectoderm was formerly referred to as primitive neuroectodermal tumor (PNET) but has been renamed to teratoma with embryonic-type neuroectodermal tumor (Fig. 2). This helps avoid confusion with the unrelated tumor, Ewing sarcoma.
- Germ cell tumors unrelated to germ cell neoplasia in situ
- • Well-differentiated neuroendocrine tumor is renamed testicular neuroendocrine tumor, prepubertal-type.
- Sex cord stromal tumors
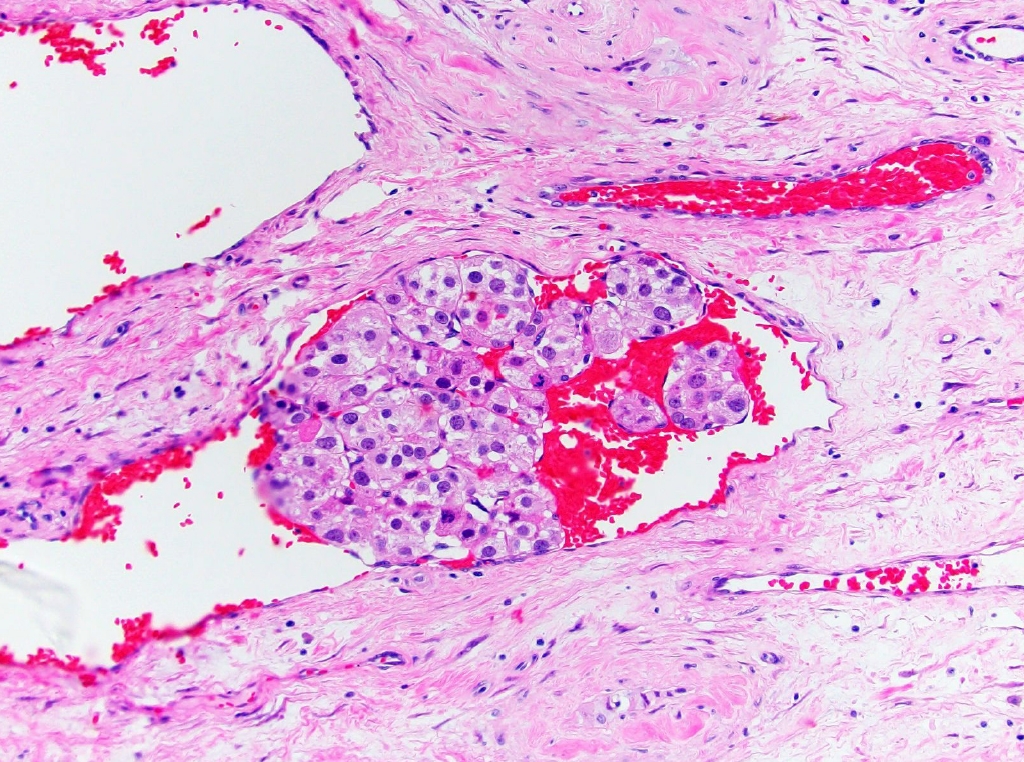
- • The recently proposed Leydig cell tumor Scaled Score (LeSS), in which Leydig cell tumor is classified as high or low risk based on size, mitoses, necrosis, infiltrative pattern, and vascular invasion, is mentioned in the WHO but is noted to require further validation (Fig. 3).
- • Leydig cell tumor can be FH deficient and this may merit FH testing, particularly in high risk tumors in which mutations may occur more frequently.
- • Sertoliform cystadenoma of the rete testis has been moved from being classified as an adenoma within the tumors of the collecting duct and rete testis, to being a part of Sertoli cell tumor.
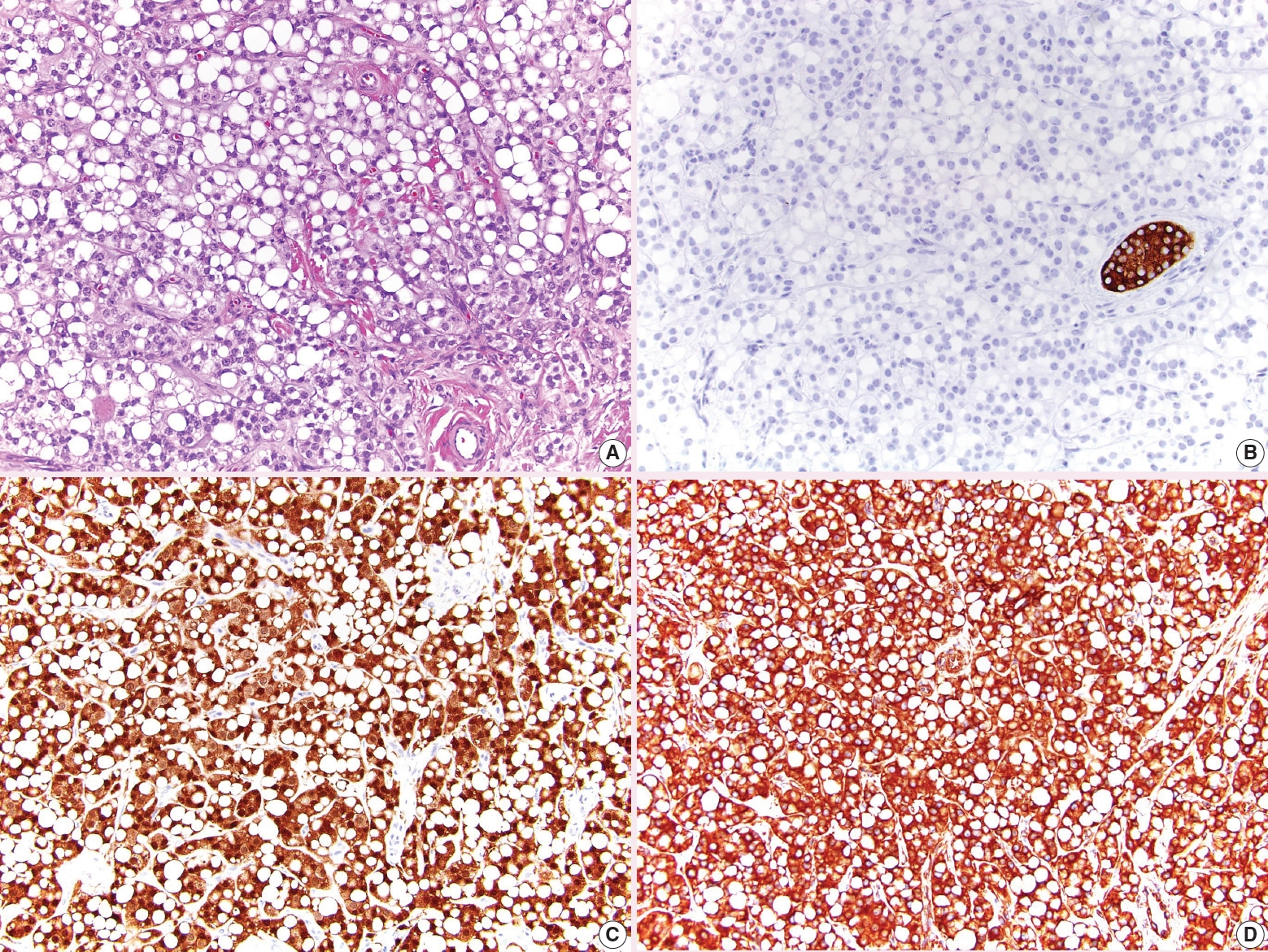
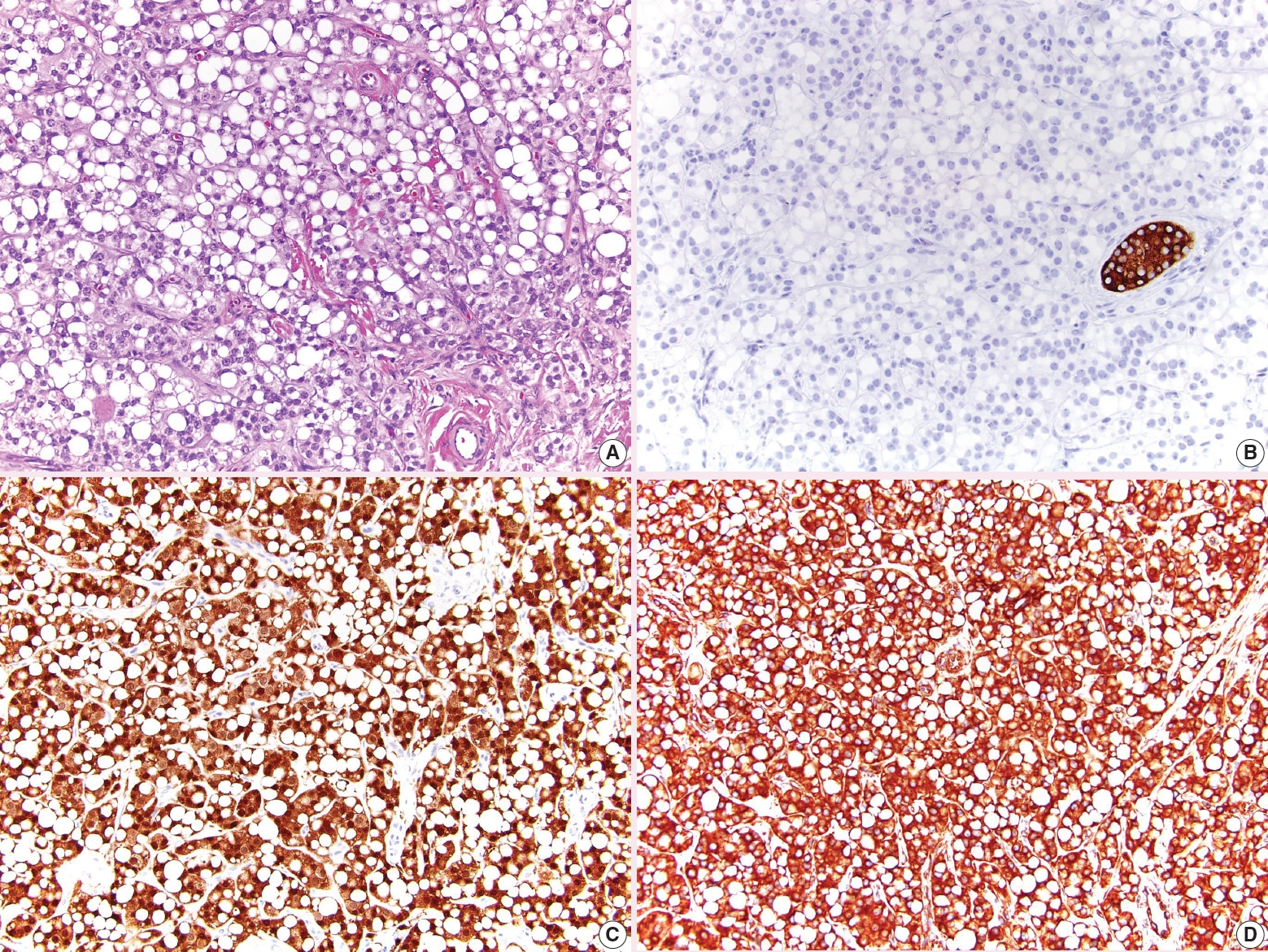
- • The signet ring pattern of Sertoli cell tumor is now listed as a separate tumor called signet ring stromal tumor (Fig. 4). It is composed of signet ring cells which do not contain mucin, is positive for vimentin and β-catenin, and is negative for inhibin. These tumors appear to have indolent behavior.
- • Myoid gonadal stromal tumor has been changed from a provisional entity to a confirmed entity. This tumor features densely packed, bland spindle cells positive for SMA and S100. Reported tumors have indolent behavior.
- Paratesticular mesothelial tumors
- • Well-differentiated papillary mesothelioma is renamed to well-differentiated papillary mesothelial tumor.
TESTIS
- • The terminology for squamous cell carcinoma groupings has been changed from non-HPV-related to HPV-independent and from HPV-related to HPV-associated.
- • Papillary basaloid carcinoma, warty basaloid carcinoma, pseudohyperplastic carcinoma, pseudoglandular carcinoma, carcinoma cuniculatum, and mixed squamous cell carcinoma have been removed as distinct subtypes.
- • Pseudohyperplastic and pseudoglandular patterns are now a part of squamous cell carcinoma, usual type.
- • Carcinoma cuniculatum pattern is now a part of verrucous carcinoma.
PENIS
- Dr. Choy has been an author for PathologyOutlines since 2020 and a part of the editorial board since 2021 as the Prostate and Cytopathology subspecialty section editor. She is currently an Assistant Professor and Associate Program Director of the Pathology Residency and Cytopathology Fellowship at Northwestern University where she practices GU Pathology and Cytopathology.
- Dr. Tretiakova has been an author for PathologyOutlines since 2015, part of the PathologyOutlines editorial board since 2019, and Deputy Editor in Chief for GU Pathology since 2021. She is currently a Professor and Director of the GU Fellowship and Immunohistochemistry Laboratories at the University of Washington where she primarily practices GU Pathology.
- Dr. Zynger has been an author for PathologyOutlines since 2013, a part of the editorial board since 2013, and is the subspecialty section editor for Adrenal, Penis, and Testis. She is currently a Professor and Director of Urologic Pathology at The Ohio State University where she primarily practices GU Pathology.
Meet the Authors
Fig. 1.PIN-like prostatic adenocarcinoma. A. It is comprised of large discrete glands with flat or tufted pseudostratified epithelium. B. Immunostaining with HMWCK highlights lack of basal cells in PIN-like carcinoma focus.
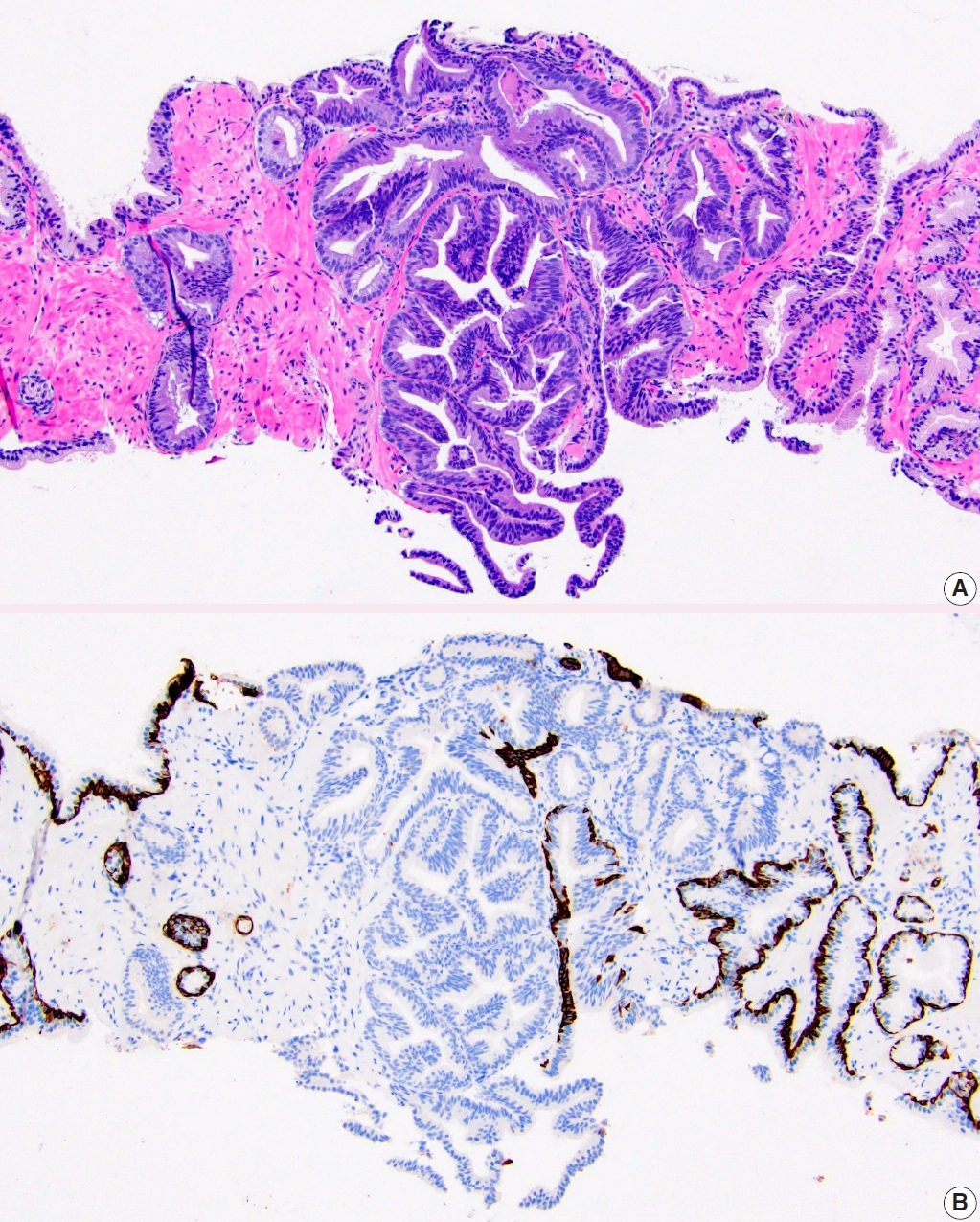

Figure & Data
References
Citations
Citations to this article as recorded by 

- Predicting variant histology in bladder cancer: the role of multiparametric MRI and vesical imaging-reporting and data system (VI-RADS)
Serdar Aslan, Merve Nur Tasdemir, Ertugrul Cakir, Ural Oguz, Birgul Tok
Abdominal Radiology.2025; 50(10): 4700. CrossRef - Pictorial review of multiparametric MRI in bladder urothelial carcinoma with variant histology: pearls and pitfalls
Yuki Arita, Sungmin Woo, Lisa Ruby, Thomas C. Kwee, Keisuke Shigeta, Ryo Ueda, Sunny Nalavenkata, Hiromi Edo, Kosuke Miyai, Jeeban Das, Pamela I. Causa Andrieu, Hebert Alberto Vargas
Abdominal Radiology.2024; 49(8): 2797. CrossRef - Oncological outcomes and prognostic implications of T1 histo-anatomic substaging in the management of high-Grade non-muscle invasive bladder cancer: results from a large single centre series
Marco Finati, Antonio Fanelli, Francesco Cinelli, Nicola Schiavone, Ugo Giovanni Falagario, Anna Ricapito, Nicola d’Altilia, Richard Naspro, Angelo Porreca, Felice Crocetto, Biagio Barone, Ciro Imbimbo, Carlo Bettocchi, Francesca Sanguedolce, Luigi Cormio
World Journal of Urology.2024;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
-
- What’s new in hematopathology 2025: myeloid neoplasms in the WHO 5th edition and ICC
- What’s new in neuropathology 2024: CNS WHO 5th edition updates
- What’s new in adrenal gland pathology: WHO 5th edition for adrenal cortex
- What’s new in thyroid pathology 2024: updates from the new WHO classification and Bethesda system
- What’s new in dermatopathology 2023: WHO 5th edition updates
What’s new in genitourinary pathology 2023: WHO 5th edition updates for urinary tract, prostate, testis, and penis




Fig. 1. PIN-like prostatic adenocarcinoma. A. It is comprised of large discrete glands with flat or tufted pseudostratified epithelium. B. Immunostaining with HMWCK highlights lack of basal cells in PIN-like carcinoma focus.
Fig. 2. Teratoma with embryonic-type neuroectodermal tumor. The area with exclusive somatic malignancy spanned over 3 cm.
Fig. 3. Leydig cell tumor with lymphovascular invasion, categorized as high risk by LeSS.
Fig. 4. Signet ring stromal tumor. A. The tumor has large cytoplasmic vacuoles. B. Inhibin is negative. C. β-catenin has diffuse nuclear and cytoplasmic expression. D. Vimentin is diffusely positive.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
What’s new in genitourinary pathology 2023: WHO 5th edition updates for urinary tract, prostate, testis, and penis

 E-submission
E-submission