Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(1); 2025 > Article
-
Original Article
PLUNC downregulates the expression of PD-L1 by inhibiting the interaction of DDX17/β-catenin in nasopharyngeal carcinoma -
Ranran Feng1,2,*
 , Yilin Guo1,3,*
, Yilin Guo1,3,* , Meilin Chen4,*
, Meilin Chen4,* , Ziying Tian1,5
, Ziying Tian1,5 , Yijun Liu1,5
, Yijun Liu1,5 , Su Jiang1,5
, Su Jiang1,5 , Jieyu Zhou1,5
, Jieyu Zhou1,5 , Qingluan Liu1,5
, Qingluan Liu1,5 , Xiayu Li6
, Xiayu Li6 , Wei Xiong7,8
, Wei Xiong7,8 , Lei Shi9
, Lei Shi9 , Songqing Fan9
, Songqing Fan9 , Guiyuan Li7,8
, Guiyuan Li7,8 , Wenling Zhang,1,5
, Wenling Zhang,1,5
-
Journal of Pathology and Translational Medicine 2025;59(1):68-83.
DOI: https://doi.org/10.4132/jptm.2024.11.27
Published online: January 15, 2025
1Department of Laboratory Medicine, The Third Xiangya Hospital, Central South University, Changsha, China
2Department of Laboratory Medicine, Reproductive and Genetic Hospital of CITIC-Xiangya, Changsha, China
3Department of Blood Transfusion, Children's Hospital Affiliated to Zhengzhou University, Henan Children's Hospital, Zhengzhou Children's Hospital, Zhengzhou, China
4The First Affiliated Hospital of Sun Yatsen University, Guangzhou, China
5Department of Medical Laboratory Science , Xiangya School of Medicine, Central South University, Changsha, China
6Hunan Key Laboratory of Nonresolving Inflammation and Cancer, Disease Genome Research Center, The Third Xiangya Hospital, Central South University, Changsha, China
7NHC Key Laboratory of Carcinogenesis, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
8The Key Laboratory of Carcinogenesis and Cancer Invasion of the Chinese Ministry of Education, Cancer Research Institute and School of Basic Medicine Sciences, Central South University, Changsha, China
9Department of Pathology, Second Xiangya Hospital, Central South University, Changsha, China
- Corresponding Author: Wenling Zhang, MD Department of Laboratory Medicine, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Yuelu District Changsha, Hunan 410013, China Tel, Fax: +86-0731-82650348, E-mail: zhangwenling73@126.com
- *Ranran Feng, Yilin Guo, and Meilin Chen contributed equally to this work.
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Nasopharyngeal carcinoma (NPC) is characterized by high programmed death-ligand 1 (PD-L1) expression and abundant infiltration of non-malignant lymphocytes, which renders patients potentially suitable candidates for immune checkpoint blockade therapies. Palate, lung, and nasal epithelium clone (PLUNC) inhibit the growth of NPC cells and enhance cellular apoptosis and differentiation. Currently, the relationship between PLUNC (as a tumor-suppressor) and PD-L1 in NPC is unclear.
-
Methods
- We collected clinical samples of NPC to verify the relationship between PLUNC and PD-L1. PLUNC plasmid was transfected into NPC cells, and the variation of PD-L1 was verified by western blot and immunofluorescence. In NPC cells, we verified the relationship of PD-L1, activating transcription factor 3 (ATF3), and β-catenin by western blot and immunofluorescence. Later, we further verified that PLUNC regulates PD-L1 through β-catenin. Finally, the effect of PLUNC on β-catenin was verified by co-immunoprecipitation (Co-IP).
-
Results
- We found that PLUNC expression was lower in NPC tissues than in paracancer tissues. PD-L1 expression was opposite to that of PLUNC. Western blot and immunofluorescence showed that β-catenin could upregulate ATF3 and PD-L1, while PLUNC could downregulate ATF3/PD-L1 by inhibiting the expression of β-catenin. PLUNC inhibits the entry of β-catenin into the nucleus. Co-IP experiments demonstrated that PLUNC inhibited the interaction of DEAD-box helicase 17 (DDX17) and β-catenin.
-
Conclusions
- PLUNC downregulates the expression of PD-L1 by inhibiting the interaction of DDX17/β-catenin in NPC.
- The 2020 Global Cancer Statistics report showed that there were more than 133,000 new cases of nasopharyngeal cancer (NPC) worldwide [1]. NPC occurs mainly in East and South Asia. NPC is sensitive to radiotherapy, so radiotherapy is the preferred treatment. Radiotherapy plus adjuvant chemotherapy is the standard treatment for advanced NPC. However, 20%–30% of NPC patients will experience recurrence, with most recurrences occurring within the first 2 years after treatment [2]. Because of its unique immune environment, NPC is considered a highly immune-inflamed tumor. Massive lymphocytic infiltrations, high programmed death-ligand 1 (PD-L1) expression, and several key immune molecules that regulate the activation of T-cells (CD40, CD70, CD80, and CD86) are often observed in Epstein-Barr virus (EBV)–induced NPC [3]. Since NPC is characterized by high PD-L1 expression and significant non-malignant lymphocyte infiltration, patients may be suitable for immune checkpoint-blocking therapies [4]. Fortunately, anti–programmed death-1 (PD-1)/PD-L1 treatment has made certain progress in metastatic or recurrent metastatic NPC as a promising method to prolong the survival rate of patients [5]. Therefore, understanding the regulatory mechanism of PD-L1 is of great significance for the immunotherapy of NPC.
- Palatal, lung, and nasal epithelial clone (PLUNC) protein is encoded by a 300-KB gene segment on chromosome 20, which is specifically expressed in the upper airway and nasopharyngeal regions and is involved in host defense [6,7]. PLUNC contributes to the overall surface tension of airway epithelial secretions, and this activity may interfere with the formation of biofilms in airway pathogens [8,9]. In innate immune defense, the PLUNC protein is involved in antimicrobial activity and can directly kill Escherichia coli and Acinetobacter haemolyticus through cell wall permeability [10]. In patients with chronic sinusitis, reduced levels of PLUNC expression are associated with repeated sinus surgery and the amount of bacterial colonization [11]. In acute pulmonary exacerbations (AEs), PLUNC expression decreases sharply as inflammation increases. In stable patients, lower PLUNC expression correlates with an increased AE risk. Thus, the downregulation of PLUNC could predict AE at the early stages [12]. However, in gastric hepatoid adenocarcinoma, PLUNC expression may be an important factor that can indicate the tumor's malignant potential, as positive cases show vascular invasion and lymph node metastasis [13].
- A previous study suggested that PLUNC was downregulated in NPC and related to the poor prognosis of NPC patients [14]. PLUNC can inhibit the tumor inflammatory microenvironment by regulating the Toll-like receptor 9/nuclear factor κB signaling pathway, thereby reducing the inflammatory response of EBV-induced NPC cells [15]. PLUNC may be a tumor-suppressor gene in NPC; in previous research, it inhibited the growth of NPC cells and promoted cellular apoptosis and differentiation [16,17]. Our results revealed that PD-L1 is highly expressed in nasopharyngeal carcinoma. However, the relationship between PLUNC and PD-L1 in NPC is unclear.
- Wnt/β-catenin signaling plays a crucial role in cancer. Abnormal Wnt/β-catenin signaling had been found to be closely related to the occurrence, development, and malignant transformation of cancer [18]. β-catenin is highly expressed in NPC tissues, and its positive expression negatively correlates with the survival rate of NPC patients [19]. It had been reported that β-catenin promotes the proliferation and metastasis of NPC cells [20,21]. However, it is still unclear whether β-catenin regulates PD-L1 in NPC.
- In our study, we found a negative correlation between PLUNC and PD-L1 expression in NPC, and PLUNC suppressed PD-L1 expression by downregulating β-catenin. Mechanically, PLUNC downregulated PD-L1 expression by inhibiting DEAD-box helicase 17 (DDX17)/β-catenin interaction. In summary, our research will provide new ideas for immune checkpoint blockade therapy in NPC.
INTRODUCTION
- Bioinformatic analysis
- We conducted gene expression differential analysis using a t test based on data from head and neck cancer cases in the GEPIA public database(http://gepia.cancer-pku.cn/detail.php. In addition, the differences in EBV infection between high- and low-expression groups of the PLUNC gene in the GSE102349 dataset from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/GEO) were compared using the t test, and the correlation between EBV infection and PLUNC gene expression was analyzed using Pearson’s correlation analysis.
- Cell culture
- NPC cell lines (5-8F, HNE2, S18, and HONE1) and the 293T cell line come from the Cancer Research Institute of Central South University. 5-8F, HNE2, S18, and HONE1 were used for the study of protein expression, localization, and signal transduction in nasopharyngeal carcinoma cells. 293T cells are the most commonly used cells in the study of protein interactions in articles. These cells were maintained at 37°C and 5% CO2 in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (Biological Industries, Kibbutz Beit-Haemek, Israel), 100 U/mL of penicillin, and 100 μg/mL of streptomycin (GE Healthcare, Waukesha, WI, USA).
- Cell transfection
- Transfection plasmids (including PLUNC overexpression), PLUNC control vectors, PLUNC/β-catenin/activating transcription factor 3 (ATF3) small hairpin RNAs (shRNAs), and their negative controls were purchased from Shanghai Genechem (Shanghai, China). DDX17/β-catenin/ATF3 lentiviral expression vectors were constructed by inserting expanded DDX17/β-catenin/ATF3 cDNA fragments into a lentiviral shuttle vector. The cells were seeded into six-well plates, and, upon reaching 80% confluence, recombinant plasmids were transfected using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer's protocol. The indicated cells infected with the recombinant lentiviral vectors were selected with puromycin or G418 for 2 weeks.
- Co-immunoprecipitation assay
- Cell lysis ice immunoprecipitation lysis buffer was supplemented with protease and phosphatase inhibitors for 15 minutes. Add immunoprecipitation lysis buffer containing protease inhibitors and phosphatase inhibitors to the cells and lyse on ice for 15 minutes. After cell lysis, centrifuge at 16,000 ×g for 10 minutes at 4°C and incubate the supernatant with antibodies. Following incubation, the cells were added into pre-washed agarose beads, coupled with protein G or protein A, and incubated at 4°C for 3 hours. The immune complex was purified on the magnet by extensive washing of the lysis buffer. The chemiluminescence signals were collected by a BIO-RAD Gel imaging analysis system scanner (Bio-Rad Laboratories, Hercules, CA, USA).
- Western blot analysis
- Cells were lysed with RIPA lysis buffer, and the protein concentration was measured using a bicinchoninic acid protein assay kit (KeyGen Biotech, Nanjing, China). Protein samples (30 μg/well) were loaded onto 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes. After blocking with skim milk, membranes were incubated in primary antibodies at 4°C overnight and then in horseradish peroxidase–conjugated secondary antibodies for 1 hour at 37°C. Primary antibodies used for experiments included anti-PLUNC (ab131163, Abcam, Cambridge, UK), glyceraldehyde 3-phosphate dehydrogenase (Abcam), anti–β-catenin, T-cell factor 4 (TCF4) (Cell Signaling Technology, Danvers, MA, USA), DDX17 (sc398168, Santa Cruz Biotechnology, Dallas, TX, USA), PD-L1 (28076-1-AP, Proteintech, Rosemont, IL, USA), ATF3 (ab254268, Abcam), glycogen synthase kinase 3β (GSK-3β; 67329-1-Ig, Proteintech), and p-GSK-3β (67558-1-Ig, Proteintech). Protein bands were imaged and captured using ECL reagent (Biosharp, Hefei City, China).
- Immunofluorescence staining
- Cells were seeded on coverslips, fixed with 4% paraformaldehyde, and then permeabilized with 0.2% Triton X-100. After blocking with 5% bovine serum albumin, the cells were incubated with a primary antibody (anti-ATF3 [Abcam]; anti-Plunc, anti–β-catenin, and anti–PD-L1 [Proteintech]; and anti-DDX17 [Santa Cruz Biotechnology]) overnight at 4°C, which was followed by incubation with an Alexa Fluor 488– or Alexa Fluor 594–conjugated secondary antibody (Abcam). DAPI was used to stain cell nuclei. Immunofluorescence images were captured with a fluorescence microscope (Olympus Corp., Tokyo, Japan).
- Clinical NPC samples and immunohistochemistry
- NPC samples and paired paracancer tissues were collected from 30 NPC patients at the Second Xiangya Hospital of Central South University (Changsha, China). The study was approved by the Joint Ethics Committee of the Central South University Health Authority, and informed consent was obtained from each participant.
- For immunohistochemistry, tissue sections of formalin-fixed and paraffin-embedded NPC tissues were incubated with anti-PLUNC (10413-1-AP, Proteintech), β-catenin (51067-2-AP, Proteintech), PD-L1 (28076-1-AP, Proteintech), DDX17 (sc398168, Santa Cruz Biotechnology) or control IgG1 (1 μg/mL). After washing with phosphate buffered saline, the slides were reacted with the Prolink-2 Plus horseradish peroxidase rabbit polymer detection kit (Golden Bridge International, Bothell, WA, USA), and the images were scanned using Aperio ScanScope CS software (Aperio Technologies, Vista, CA, USA). Two independent pathologists evaluated and scored the slides based on the intensity and extent of staining (double-blinded). A staining index (values, 0–9) was obtained from the intensity of the positive staining (negative = 0 points, weak = 1 points, moderate = 2 points, strong = 3 points), and the proportion of positive cells of interest (<25% = 1 point, 25%–50% = 2 points, ≥50% = 3 points) was calculated. The final results were then designated and rescored as follows: 0 points = negative, 1–3 points = weak positive (1 point), 4–6 points = moderately positive (2 points), or 7–9 points = strongly positive (3 points). All sections were scored independently by two pathologists blinded to the clinicopathological features and the clinical course.
- Statistical analysis
- Data were analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Graphed data were presented as mean ± standard error of the mean values, and statistical significance was determined using Student’s t test. Pearson’s chi-squared or Fisher's exact test was used to analyze clinicopathological parameters. Multivariable Cox proportional hazards modeling was performed to identify independent risk factors for the prognosis of NPC. p ≤ 0.05 was considered statistically significant.
MATERIALS AND METHODS
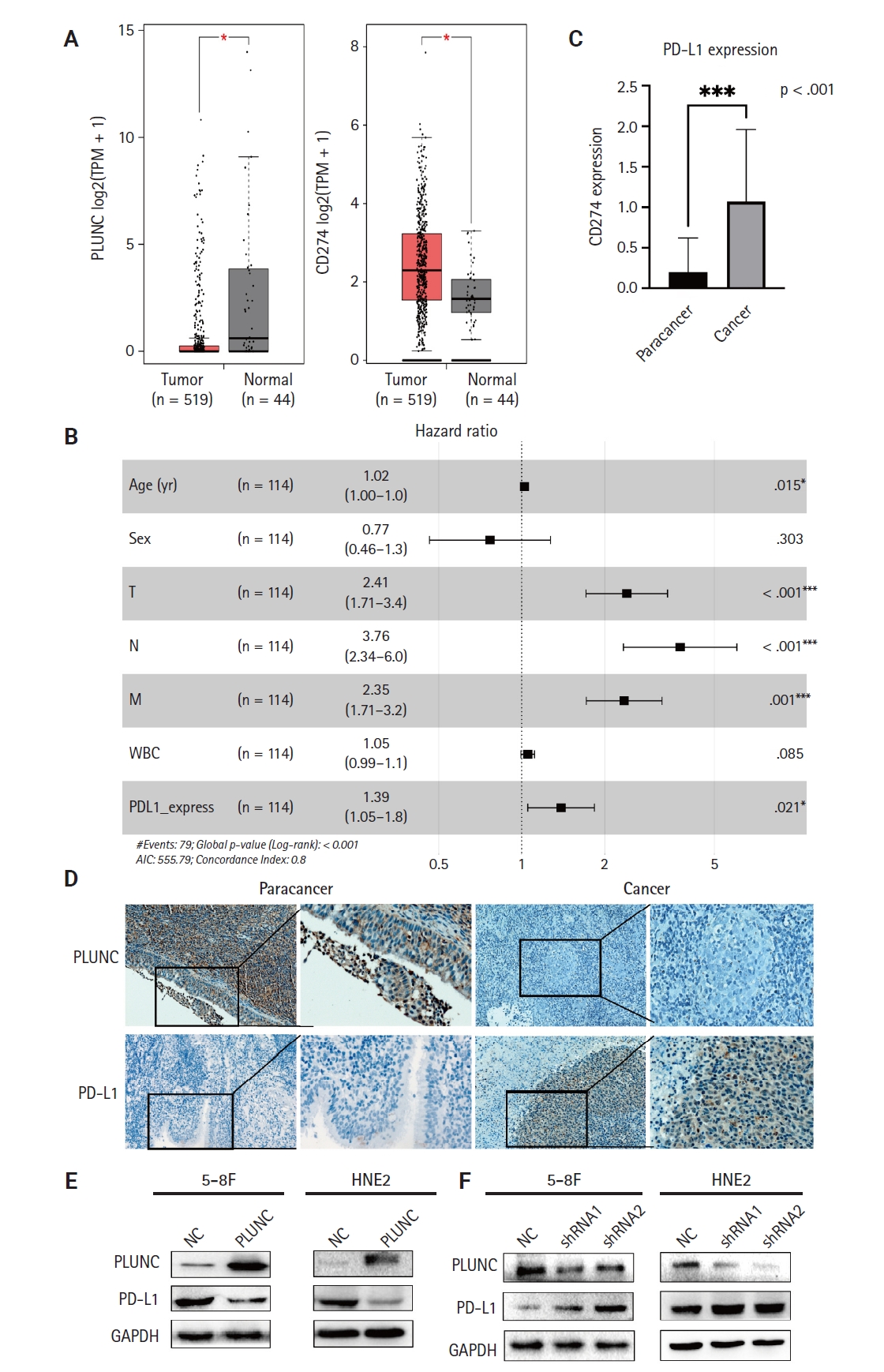
- PLUNC negatively correlates with PD-L1 expression in NPC
- In the GEPIA online database (http://gepia.cancer-pku.cn/detail.php), the expression of the PLUNC gene (BPIFA1) was low in head and neck squamous cell carcinoma, while that of the PD-L1 gene (CD274) was high (Fig. 1A). The X-axis represents the tumor group and the normal group, while the Y-axis uses log2 (TPM+1) values to represent gene-expression levels. Based on the information of 114 NPC patients represented in tissue microarrays (Superbiotek, Shanghai, China) (for relevant clinical information, see Supplementary Table S1), we conducted univariate logistic regression analysis according to the immunohistochemical staining score of PD-L1 (0–3 points; 0 points = negative, >0 points = positive) and found that PD-L1 is a risk factor affecting the prognosis of nasopharyngeal carcinoma patients (Fig. 1B, C). Considering the close correlation between NPC occurrence and EBV infection, we analyzed the effect of EBV on PLUNC gene expression. The results showed no difference in the degree of EBV infection in NPC with high and low expression of PLUNC (Supplementary Fig. S1A). Moreover, there was no correlation between EBV infection and PLUNC gene-expression level (R = −0.16, p = 0.083) (Supplementary Fig. S1B). To investigate the correlation between PLUNC and PD-L1, we collected paraffin sections from 30 NPC patients in the Second Xiangya Hospital of Central South University for immunohistochemical staining. The results showed that the expression of PLUNC was low in NPC and high in paracancer tissues, while the expression of PD-L1 was opposite to that of PLUNC (Fig. 1D). The statistical analysis of PD-L1 scores in tumor tissues and adjacent tissues of 30 NPC patients is presented in Supplementary Fig. S1C, indicating that the expression of PD-L1 was significantly greater in tumor tissues than in paracancer tissues. The negative and positive control results of immunohistochemical staining are shown in Supplementary Fig. S1D. Next, we validated the relationship between PLUNC and PD-L1 in NPC cells. In the HNE2 and 5-8F cell lines, we overexpressed PLUNC and found that PD-L1 was downregulated (Fig. 1E). However, when PLUNC was knocked down, the expression of PD-L1 was upregulated (Fig. 1F). Therefore, we considered a negative correlation to exist between the expression of PLUNC and PD-L1 in NPC.
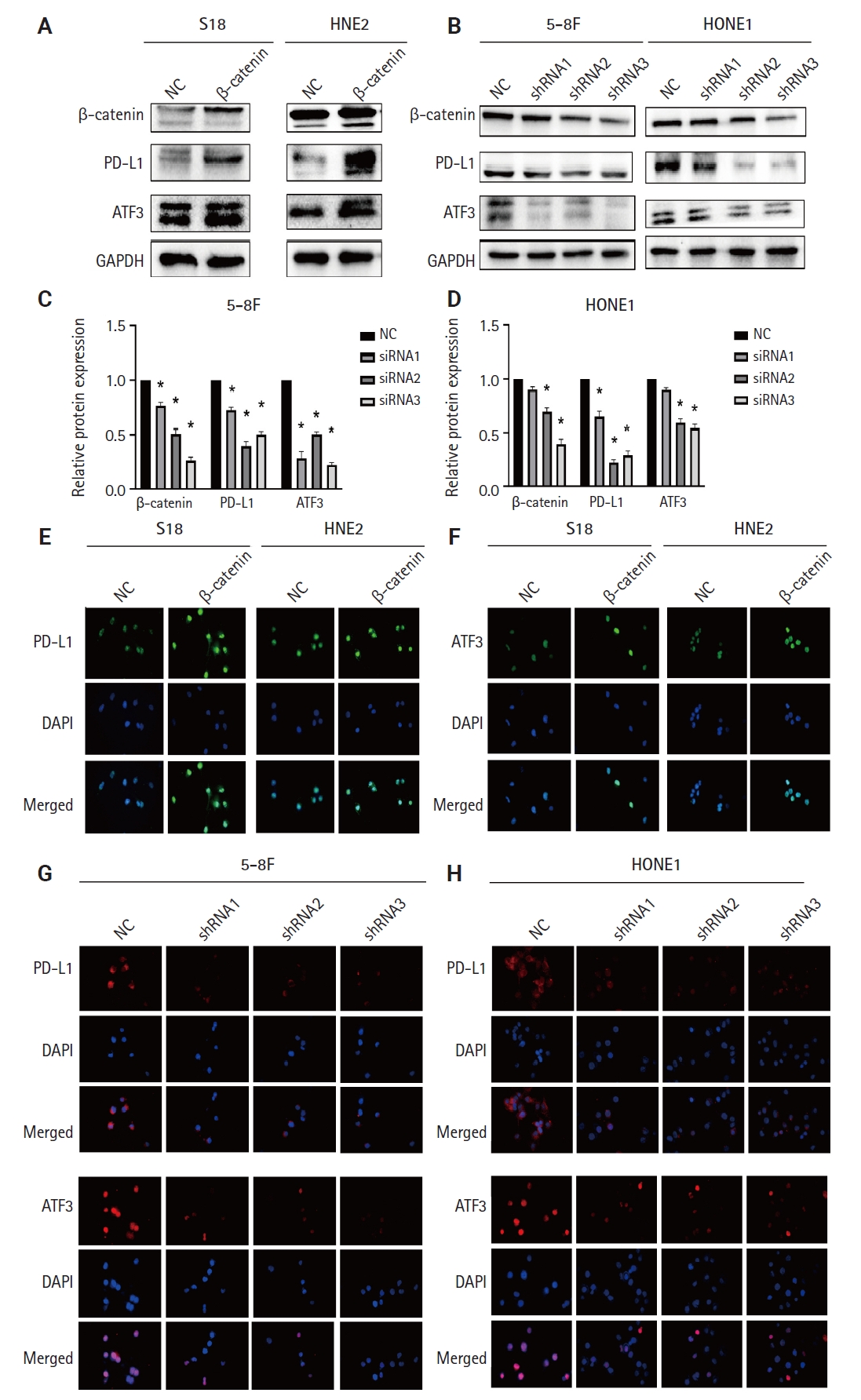
- β-catenin upregulates ATF3/PD-L1 expression
- ATF3 is a transcription factor; as a member of the ATF/CREB family, it can regulate the expression of PD-L1 in melanoma cells [22]. We validated the regulation of PD-L1 by ATF3 in NPC cells. When we overexpressed ATF3 in 5-8F and S18 cells, the expression of PD-L1 was upregulated (Supplementary Fig. S2A, B). Conversely, when we knocked down ATF3, the expression of PD-L1 was downregulated (Supplementary Fig. S2C, D). The high expression of β-catenin is a risk factor for poor prognosis in NPC patients [23]. β-catenin is an upstream molecule of PD-L1 in glioblastoma [24]. However, whether β-catenin regulates PD-L1 in NPC remains unclear, so we validated the relationship between β-catenin and PD-L1 in NPC cells. When we overexpressed β-catenin in S18 cells and HNE2 cells, the expressions of ATF3 and PD-L1 were upregulated (Fig. 2A). Conversely, when we knocked down β-catenin in 5-8F and HONE1 cells, the expression of both PD-L1 and ATF3 was downregulated (Fig. 2B; statistical results are shown in Fig. 2C, D). Meanwhile, we validated the mRNA- and protein-expression levels of β-catenin after transfection with shRNA (Supplementary Fig. S3A, B). In addition, we also confirmed the expression of both ATF3 and PD-L1 through immunofluorescence experiments (Fig. 2E-H). Therefore, we confirmed that β-catenin upregulated the expression of PD-L1 and ATF3 in NPC cells.
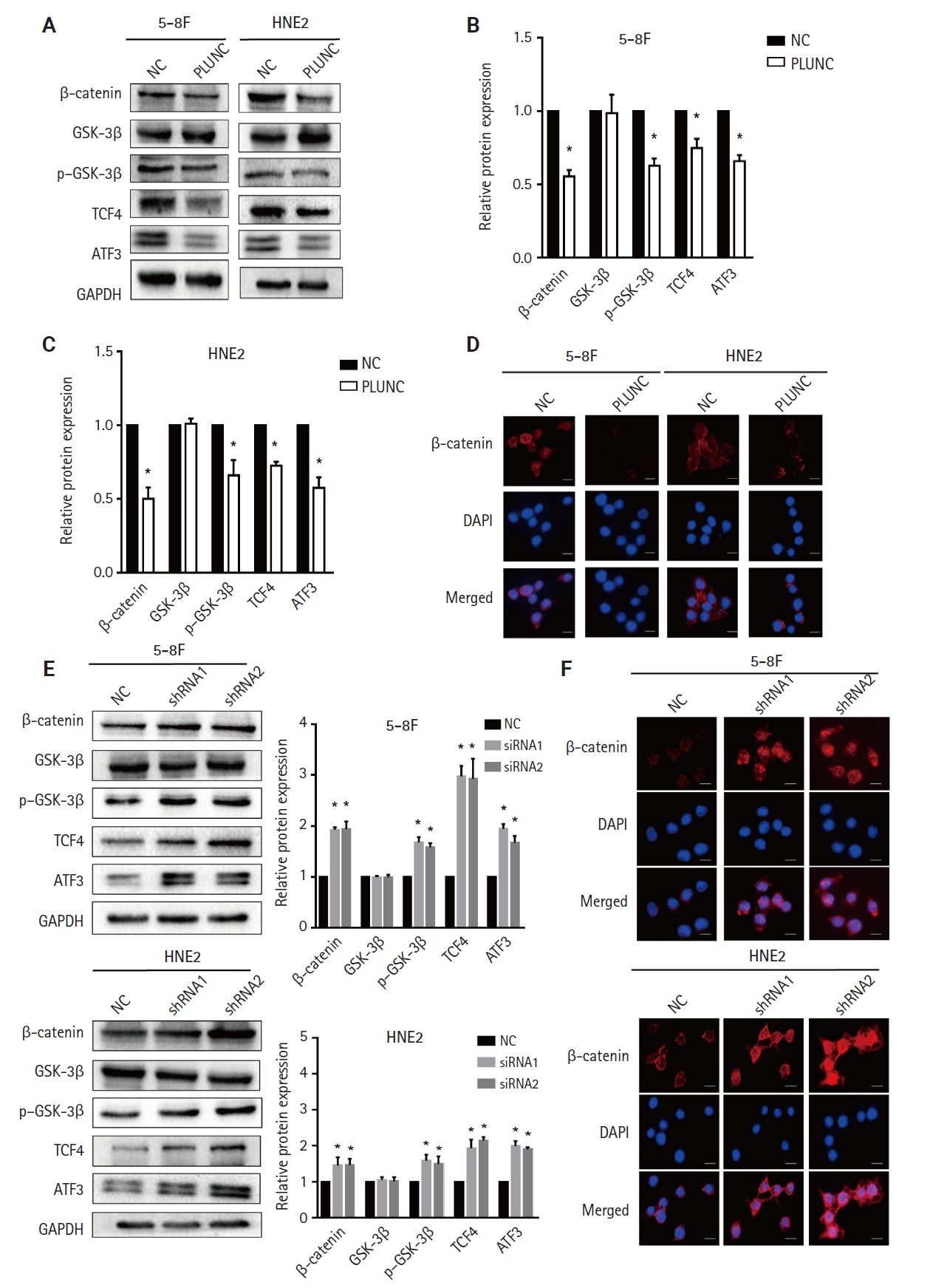
- PLUNC suppresses the β-catenin pathway
- Since β-catenin could upregulate PD-L1 expression and PLUNC could downregulate PD-L1 expression, could PLUNC inhibit the β-catenin pathway? After overexpressing PLUNC in 5-8F and HNE2 cells (Supplementary Fig. S3C), we found that GSK-3β (which promotes β-catenin ubiquitination degradation) [25] was upregulated. Conversely, phosphorylated GSK-3β (its inactivated form) was upregulated. The expression of TCF4, which could interact with β-catenin to regulate downstream gene transcription [26], was also inhibited (Fig. 3A). The relative changes in β-catenin, GSK-3β, p–GSK-3β, TCF4, and ATF3 are shown in Fig. 3B and C. We also found that overexpression of PLUNC resulted in the downregulation of β-catenin expression by immunofluorescence experiments (Fig. 3D). On the contrary, knocking down PLUNC in HNE2 and 5-8F cells (Supplementary Fig. S3D) weakened the inhibition of the β-catenin pathway (Fig. 3E, F). Therefore, we considered that PLUNC could inhibit the β-catenin pathway.
- PLUNC downregulates the expression of ATF3/PD-L1 through inhibiting the β-catenin pathway
- After overexpressing PLUNC in 5-8F and HNE2 cells, we observed that the expression of ATF3/PD-L1 decreased (Fig. 4A–D). Conversely, when we knocked down PLUNC in 5-8F and HNE2 cells, we observed that the expression of both ATF3 and PD-L1 increased (Fig. 4E–H). After treating cells using XAV-939 as an inhibitor of the Wnt/β-catenin pathway, the expression of β-catenin, p-GSK3β, ATF3, and PD-L1 decreased. However, when PLUNC was knocked down in 5-8F and HNE2 cells, the expressions of β-catenin, p-GSK3β, ATF3, and PD-L1 increased. When PLUNC-shRNA was combined with XAV-939, the expressions of β-catenin, p-GSK3β, ATF3, and PD-L1 were weakened (Fig. 4I, J). Thus, we believed that PLUNC downregulated the expression of ATF3/PD-L1 through inhibiting the β-catenin pathway.
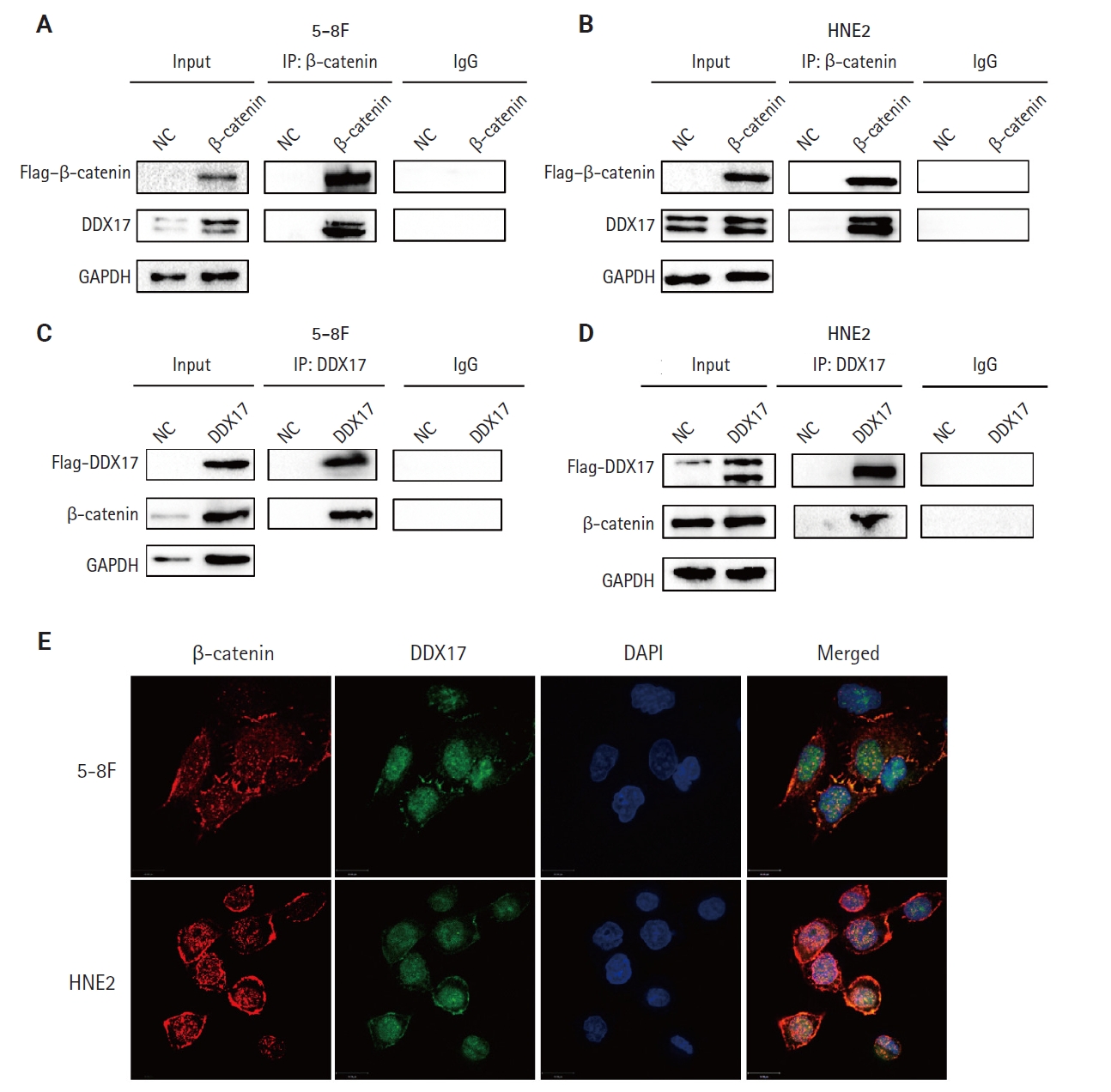
- DDX17 interacts with β-catenin in NPC cells
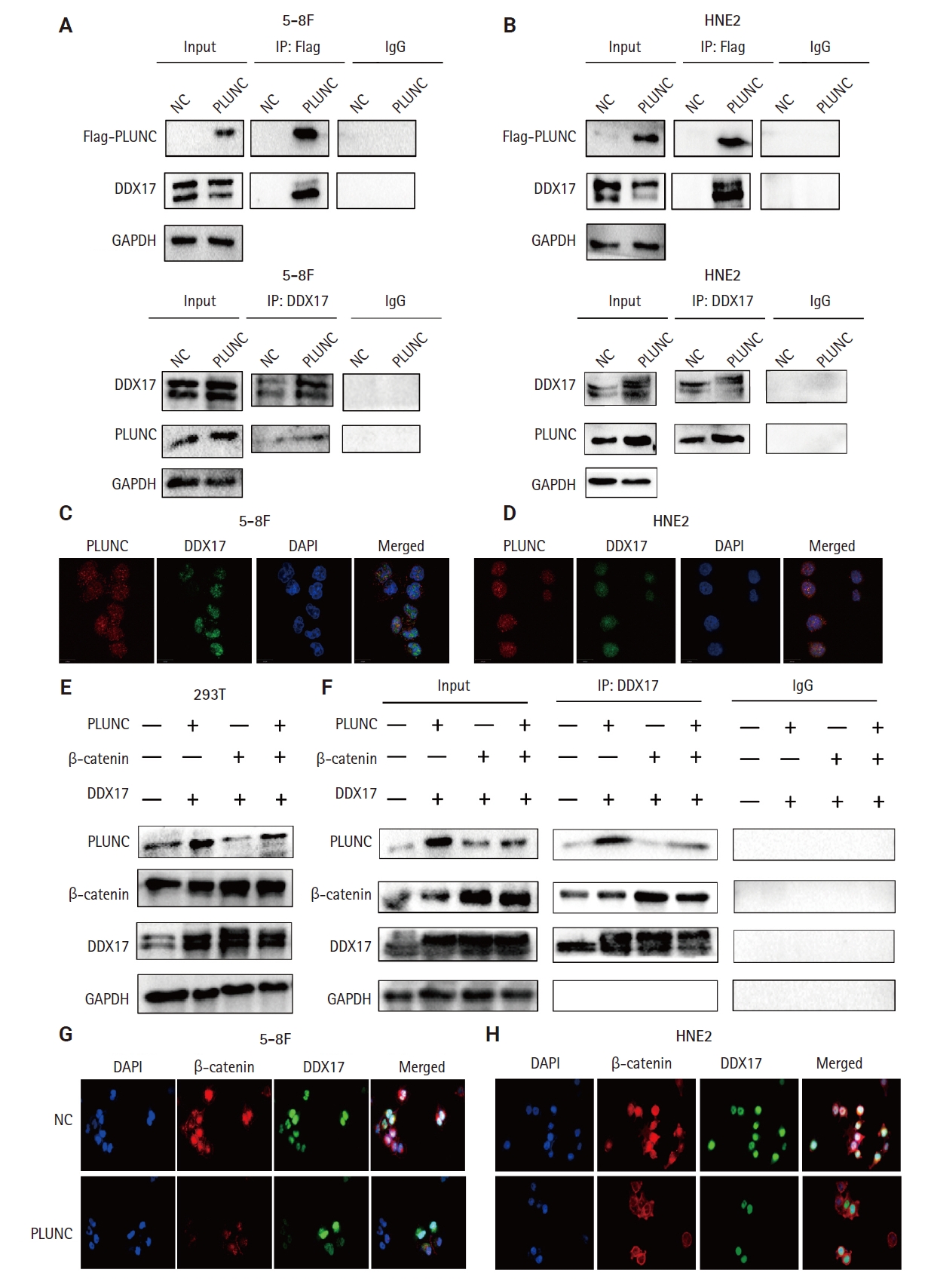
- However, the mechanism by which PLUNC downregulated β-catenin remains unclear. In 5-8F and HNE2 cells, PLUNC cannot bind directly to β-catenin, so we speculated that PLUNC may regulate β-catenin through another mechanism. The RNA helicase DDX17 is a member of a large family of highly conserved proteins that are involved in gene-expression regulation [27]. DDX17 acts as a co-activator or co-inhibitor of transcription factors of cell differentiation [28]. We explored the association of DDX17 with β-catenin in NPC cells. Just as seen in Fig. 5A–D, we confirmed DDX17 interactions with β-catenin by co-immunoprecipitation (Co-IP). Then, we demonstrated their co-localization by immunofluorescence experiments (Fig. 5E). Therefore, we further speculate whether PLUNC is related to the combination of DDX17 and β-catenin.
- PLUNC inhibits the interaction of DDX17 and β-catenin
- In NPC cells, we confirmed the interactions between PLUNC and DDX17 by Co-IP (Fig. 6A, B). In addition, the co-localization of PLUNC and DDX17 was confirmed by immunofluorescence (Fig.6C, D). Therefore, we speculate that PLUNC and β-catenin compete to combine with DDX17. We co-transfected PLUNC, β-catenin, and DDX17 plasmids in 293T cells and successfully validated their expression (Fig. 6E). Then, we used the DDX17 antibody to co-precipitate PLUNC and β-catenin. We found that, following transfection with PLUNC, the interaction of DDX17 and β-catenin decreased, while, after transfection with β-catenin, the interaction of PLUNC and DDX17 decreased (Fig. 6F). In addition, through immunofluorescence experiments, we found that, after transfection with PLUNC, the co-localization of β-catenin and DDX17 decreased (Fig. 6G, H). Therefore, the results confirmed that PLUNC inhibits the interaction of DDX17 and β-catenin.
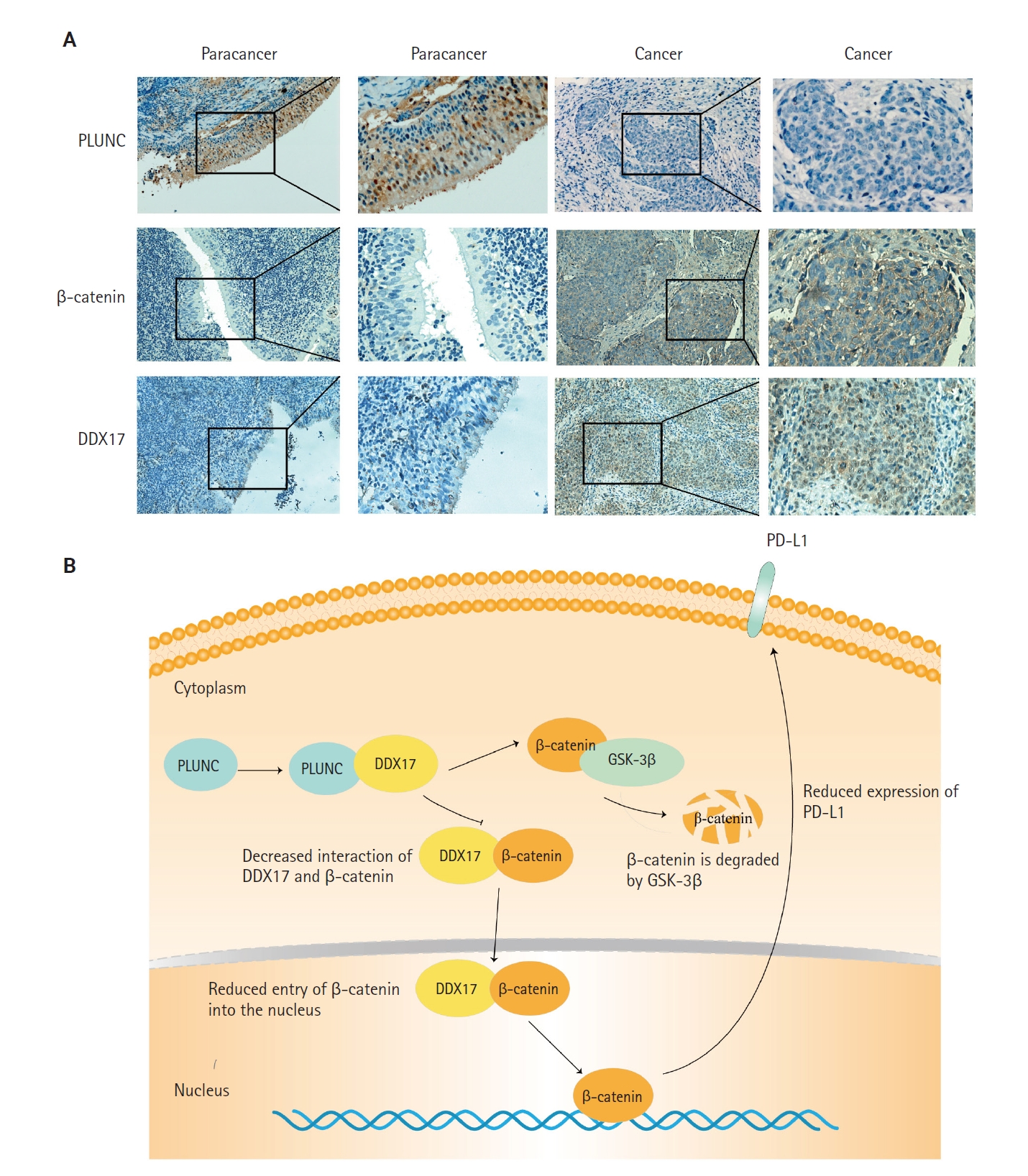
- PLUNC negatively correlates with DDX17 and β-catenin expression in NPC
- In NPC tissue sections, we confirmed a negative correlation between PLUNC and the expressions of DDX17 and β-catenin. In NPC tissue, the expression of PLUNC was low, while those of DDX17 and β-catenin were high. However, in paracancer tissues, their expressions are exactly opposite (Fig. 7A). In addition, we present representative images with immunohistochemical scores in Supplementary Fig. S4.
- Finally, we concluded that the interaction of PLUNC and DDX17 inhibited the interaction of β-catenin and DDX17, thereby promoting the degradation of β-catenin by GSK-3β. However, the reduction of β-catenin's entry into the nucleus inhibited the expression of PD-L1, ultimately leading to a reduced expression of PLUNC (Fig. 7B).
RESULTS
- EBV is closely related to the occurrence and development of NPC. It had been reported that EBV could upregulate the expression of PD-L1 in NPC cells [29-31]. Therefore, immunotherapy targeting PD-1/PD-L1 has become a new strategy for the treatment of NPC [32,33]. Our results indicated that high expression of PD-L1 is a risk factor for poor prognosis in patients with NPC. Therefore, inhibiting the expression of PD-L1 will benefit patients with NPC.
- PLUNC was originally identified in the trachea and bronchi [34]. It was originally called PLUNC, and its gene product was later renamed short PLUNC 1 (SPLUNC1), also known as SPURT, LUNX, NASG, or BPIFA1. The secreted protein encoded by SPLUNC1 is abundant in human tracheobronchial secretions and saliva [35], nasal lavage [36], apical secretions of cultured human tracheobronchial epithelial cells [37,38], and specific granules of human neutrophils [39]. Previous research results from our group show that the poor prognosis of NPC patients closely relates to the low expression of PLUNC, which may represent a candidate molecular marker for early diagnosis of NPC [14]. In recent years, immunotherapy has become a promising treatment for NPC and has made great progress [40]. Upregulation of the PD-1/PD-L1 pathway is one of the possible mechanisms of immune-evasion in EBV-associated NPC [41]. PD-1/PD-L1 immune checkpoint inhibitors were approved in China in 2021 for the treatment of relapsed or metastatic NPC. In our study, PLUNC could downregulate the expression of PD-L1 in NPC.
- Under physiological conditions, Wnt/β-catenin signaling is turned off. In the absence of Wnt signaling, a protein complex formed by AXIN, adenomatous polyposis coli (APC), serine/threonine kinase GSK-3β, CK1, and E3 ubiquitin ligase β-trcp degrades β-catenin [42]. Abnormal activation of Wnt/β-catenin signaling is common in tumors. When Wnt signaling is activated, GSK-3β is deactivated after phosphorylation, which leads to disassembly of the Axin/GSK3β/APC complex, thereby inhibiting the phosphorylation and degradation of β-catenin, and dephosphorylated β-catenin could be transported to the nucleus to regulate target genes [43]. Studies have shown that nuclear β-catenin can bind to the CD274 promoter region, promoting PD-L1 transcription and upregulating PD-L1 expression in nasopharyngeal carcinoma cells [44]. We also confirmed the expression of β-catenin in the nucleus and cytoplasm (Supplementary Fig. S3E, F). The results indicated that PLUNC could inhibit the expression of β-catenin in the nucleus and cytoplasm. However, the entry of β-catenin into the nucleus is reduced, leading to a decrease in the transcription of PD-L1 and ultimately downregulating the expression of PD-L1.
- DDX17 (DEAD Asp-Glu-Ala-Asp) box helicase 17, also known as p72, is a typical member of the DEAD box family and is of interest for its role as an RNA helicase [45]. Among the DEAD-box RNA-binding proteins, DDX17 is a well-characterized cofactor of the Drosha/DGCR8 microprocessor, which mediates the recognition and processing of primary miRNAs (pri-miRNAs) to become mature miRNAs [46-48]. In addition, more and more studies are showing that DDX17 acts as a transcription factor-related protein that is associated with the function of transcription factors such as NFAT5, histone deacetylase 1, and SOX [49-52]. The abnormal expression of DDX17 could promote progression of various tumors [28,53,54]. Furthermore, DDX17 exerts its powerful oncogenic effects by acting as a co-activator or co-repressor of transcription factors, such as estrogen receptor α [55]. DDX17 could interact with β-catenin, while PLUNC could inhibit the β-catenin pathway. Therefore, we doubted whether PLUNC could regulate β-catenin through DDX17. Our experimental results also showed that PLUNC can directly interact with DDX17 and inhibited the interaction of DDX17 with β-catenin. After PLUNC knockdown, the expressions of both PD-L1 and β-catenin were increased.
- ATF3 is a transcription factor that plays vital roles in modulating metabolism, immunity, and oncogenes that has sparked intense attention [56]. ATF3 could participate in the immune-regulation of the body and affect the occurrence, development, and treatment of tumors. Our results showed that β-catenin could upregulate ATF3 and PD-L1, while PLUNC can inhibit the Wnt/β-catenin pathway, thereby inhibiting the expressions of ATF3 and PD-L1. However, our experimental results showed that ATF3 was indeed regulated by β-catenin, while PD-L1 was indeed regulated by ATF3, but whether β-catenin directly regulates PD-L1 expression or relies on ATF3 to regulate PD-L1 still requires further experimental verification.
- In conclusion, this study confirmed that PLUNC could downregulate the expression of PD-L1 in NPC by inhibiting the interaction of DDX17/β-catenin. This will provide new ideas for the immunotherapy of NPC.
DISCUSSION
Supplementary Information
Ethics Statement
All procedures performed in the current study were approved by Xiangya Hospital of Central South University ethics committee (reference number: 202207389 Date:2022.07.05) in accordance with the 1964 Helsinki declaration and its later amendments. All specimens in this study had obtained informed consent from the patients.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: RF, YG, MC, WZ. Formal analysis: JZ. Funding acquisition: WZ. Investigation: YG, MC, YL, QL, ZT, SJ, LS, SF. Supervision: XL, WX, GL, WZ. Writing—original draft: RF. Writing—review & editing: RF, WZ. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This work was funded by the National Natural Science Foundation of China (Grant No. 81672688) and the Hunan Province Natural Science Foundation (Grant No. 2020JJ4780).
Acknowledgments
We thank The Third Xiangya Hospital of Central South University and The Cancer Research Institute of Central South University for its technical support.







- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209-49. ArticlePubMedPDF
- 2. Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst 2016; 108: djv291.ArticlePubMed
- 3. Xu JY, Wei XL, Wang YQ, Wang FH. Current status and advances of immunotherapy in nasopharyngeal carcinoma. Ther Adv Med Oncol 2022; 14: 17588359221096214.ArticlePubMedPMCPDF
- 4. Chen YP, Chan AT, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet 2019; 394: 64-80. ArticlePubMed
- 5. Adkins DR, Haddad RI. Clinical trial data of anti-PD-1/PD-L1 therapy for recurrent or metastatic nasopharyngeal Carcinoma: a review. Cancer Treat Rev 2022; 109: 102428.ArticlePubMed
- 6. Bingle CD, Craven CJ. PLUNC: a novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Hum Mol Genet 2002; 11: 937-43. ArticlePubMed
- 7. Geetha C, Venkatesh SG, Bingle L, Bingle CD, Gorr SU. Design and validation of anti-inflammatory peptides from human parotid secretory protein. J Dent Res 2005; 84: 149-53. ArticlePubMedPDF
- 8. Bartlett JA, Gakhar L, Penterman J, et al. PLUNC: a multifunctional surfactant of the airways. Biochem Soc Trans 2011; 39: 1012-6. ArticlePubMedPMCPDF
- 9. Ben-Meir E, Perrem L, Shaw M, Ratjen F, Grasemann H. SPLUNC1 as a biomarker of pulmonary exacerbations in children with cystic fibrosis. J Cyst Fibros 2024; 23: 288-92. ArticlePubMed
- 10. Liu H, Zhang X, Wu J, French SW, He Z. New insights on the palate, lung, and nasal epithelium clone (PLUNC) proteins: based on molecular and functional analysis of its homolog of YH1/SPLUNC1. Exp Mol Pathol 2016; 100: 363-9. ArticlePubMed
- 11. Tsou YA, Peng MT, Wu YF, et al. Decreased PLUNC expression in nasal polyps is associated with multibacterial colonization in chronic rhinosinusitis patients. Eur Arch Otorhinolaryngol 2014; 271: 299-304. ArticlePubMedPDF
- 12. Khanal S, Webster M, Niu N, et al. SPLUNC1: a novel marker of cystic fibrosis exacerbations. Eur Respir J 2021; 58: 2000507.ArticlePubMedPMC
- 13. Osada M, Aishima S, Hirahashi M, et al. Combination of hepatocellular markers is useful for prognostication in gastric hepatoid adenocarcinoma. Hum Pathol 2014; 45: 1243-50. ArticlePubMed
- 14. Zhang W, Zeng Z, Wei F, et al. SPLUNC1 is associated with nasopharyngeal carcinoma prognosis and plays an important role in all-trans-retinoic acid-induced growth inhibition and differentiation in nasopharyngeal cancer cells. FEBS J 2014; 281: 4815-29. ArticlePubMed
- 15. Ou C, Sun Z, Zhang H, et al. SPLUNC1 reduces the inflammatory response of nasopharyngeal carcinoma cells infected with the EB virus by inhibiting the TLR9/NF-kappaB pathway. Oncol Rep 2015; 33: 2779-88. ArticlePubMed
- 16. Liu H, Tang L, Gong S, et al. USP7 inhibits the progression of nasopharyngeal carcinoma via promoting SPLUNC1-mediated M1 macrophage polarization through TRIM24. Cell Death Dis 2023; 14: 852.ArticlePubMedPMCPDF
- 17. Tang L, Peng L, Liu H, et al. SPLUNC1 regulates LPS-induced progression of nasopharyngeal carcinoma and proliferation of myeloid-derived suppressor cells. Med Oncol 2022; 39: 214.ArticlePubMedPDF
- 18. Yu F, Yu C, Li F, et al. Wnt/beta-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther 2021; 6: 307.ArticlePubMedPMC
- 19. Pang Q, Hu W, Zhang X, Pang M. Wnt/beta-catenin signaling pathway-related proteins (DKK-3, beta-catenin, and c-MYC) are involved in prognosis of nasopharyngeal carcinoma. Cancer Biother Radiopharm 2019; 34: 436-43. ArticlePubMed
- 20. Hu Z, Meng J, Cai H, et al. KIF3A inhibits nasopharyngeal carcinoma proliferation, migration and invasion by interacting with beta-catenin to suppress its nuclear accumulation. Am J Cancer Res 2022; 12: 5226-40. ArticlePubMedPMC
- 21. Ou X, Zhang Y, Xu Y, et al. PICK1 inhibits the malignancy of nasopharyngeal carcinoma and serves as a novel prognostic marker. Cell Death Dis 2024; 15: 294.ArticlePubMedPMCPDF
- 22. Liu N, Yan M, Tao Q, et al. Inhibition of TCA cycle improves the anti-PD-1 immunotherapy efficacy in melanoma cells via ATF3-mediated PD-L1 expression and glycolysis. J Immunother Cancer 2023; 11: e007146. ArticlePubMedPMC
- 23. Jin PY, Zheng ZH, Lu HJ, et al. Roles of beta-catenin, TCF-4, and survivin in nasopharyngeal carcinoma: correlation with clinicopathological features and prognostic significance. Cancer Cell Int 2019; 19: 48.ArticlePubMedPMC
- 24. Du L, Lee JH, Jiang H, et al. Beta-catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J Exp Med 2020; 217: e20191115. ArticlePubMedPMC
- 25. Xue W, Yang L, Chen C, Ashrafizadeh M, Tian Y, Sun R. Wnt/beta-catenin-driven EMT regulation in human cancers. Cell Mol Life Sci 2024; 81: 79.ArticlePubMedPMC
- 26. Delgado-Bellido D, Zamudio-Martinez E, Fernandez-Cortes M, et al. VE-cadherin modulates beta-catenin/TCF-4 to enhance vasculogenic mimicry. Cell Death Dis 2023; 14: 135.ArticlePubMedPMC
- 27. Yang S, Winstone L, Mondal S, Wu Y. Helicases in R-loop formation and resolution. J Biol Chem 2023; 299: 105307.ArticlePubMedPMC
- 28. Zhao G, Wang Q, Zhang Y, et al. DDX17 induces epithelial-mesenchymal transition and metastasis through the miR-149-3p/CYBRD1 pathway in colorectal cancer. Cell Death Dis 2023; 14: 1.ArticlePubMedPMCPDF
- 29. Wang J, Ge J, Wang Y, et al. EBV miRNAs BART11 and BART17-3p promote immune escape through the enhancer-mediated transcription of PD-L1. Nat Commun 2022; 13: 866.ArticlePubMedPMCPDF
- 30. Ge J, Wang J, Xiong F, et al. Epstein-Barr virus-encoded circular RNA CircBART2.2 promotes immune escape of nasopharyngeal carcinoma by regulating PD-L1. Cancer Res 2021; 81: 5074-88. ArticlePubMedPMCPDF
- 31. Kase K, Kondo S, Wakisaka N, et al. Epstein-Barr virus LMP1 induces soluble PD-L1 in nasopharyngeal carcinoma. Microorganisms 2021; 9: 603.ArticlePubMedPMC
- 32. Wang FH, Wei XL, Feng J, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02). J Clin Oncol 2021; 39: 704-12. ArticlePubMedPMC
- 33. Mai HQ, Chen QY, Chen D, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med 2021; 27: 1536-43. ArticlePubMedPDF
- 34. Weston WM, LeClair EE, Trzyna W, et al. Differential display identification of plunc, a novel gene expressed in embryonic palate, nasal epithelium, and adult lung. J Biol Chem 1999; 274: 13698-703. ArticlePubMed
- 35. Vitorino R, Lobo MJ, Ferrer-Correira AJ, et al. Identification of human whole saliva protein components using proteomics. Proteomics 2004; 4: 1109-15. ArticlePubMed
- 36. Ghafouri B, Kihlstrom E, Stahlbom B, Tagesson C, Lindahl M. PLUNC (palate, lung and nasal epithelial clone) proteins in human nasal lavage fluid. Biochem Soc Trans 2003; 31: 810-4. ArticlePubMedPDF
- 37. Di YP, Harper R, Zhao Y, Pahlavan N, Finkbeiner W, Wu R. Molecular cloning and characterization of spurt, a human novel gene that is retinoic acid-inducible and encodes a secretory protein specific in upper respiratory tracts. J Biol Chem 2003; 278: 1165-73. ArticlePubMed
- 38. Campos MA, Abreu AR, Nlend MC, Cobas MA, Conner GE, Whitney PL. Purification and characterization of PLUNC from human tracheobronchial secretions. Am J Respir Cell Mol Biol 2004; 30: 184-92. ArticlePubMed
- 39. Bartlett JA, Hicks BJ, Schlomann JM, Ramachandran S, Nauseef WM, McCray PB Jr. PLUNC is a secreted product of neutrophil granules. J Leukoc Biol 2008; 83: 1201-6. ArticlePubMedPDF
- 40. Huang H, Yao Y, Deng X, et al. Immunotherapy for nasopharyngeal carcinoma: current status and prospects (review). Int J Oncol 2023; 63: 97.ArticlePubMedPMC
- 41. Johnson D, Ma BB. Targeting the PD-1/ PD-L1 interaction in nasopharyngeal carcinoma. Oral Oncol 2021; 113: 105127.ArticlePubMed
- 42. Liu J, Xiao Q, Xiao J, et al. Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther 2022; 7: 3.ArticlePubMedPMC
- 43. Wang D, Wu S, He J, et al. FAT4 overexpression promotes antitumor immunity by regulating the beta-catenin/STT3/PD-L1 axis in cervical cancer. J Exp Clin Cancer Res 2023; 42: 222.ArticlePubMedPMC
- 44. Wang H, Luo K, Zhan Y, Peng S, Fan S, Wang W. Role of beta-catenin in PD-L1 expression of nasopharyngeal carcinoma. Heliyon 2023; 9: e18130. ArticlePubMedPMC
- 45. Moy RH, Cole BS, Yasunaga A, et al. Stem-loop recognition by DDX17 facilitates miRNA processing and antiviral defense. Cell 2014; 158: 764-77. ArticlePubMedPMC
- 46. Terrone S, Valat J, Fontrodona N, et al. RNA helicase-dependent gene looping impacts messenger RNA processing. Nucleic Acids Res 2022; 50: 9226-46. ArticlePubMedPMCPDF
- 47. Suthapot P, Xiao T, Felsenfeld G, Hongeng S, Wongtrakoongate P. The RNA helicases DDX5 and DDX17 facilitate neural differentiation of human pluripotent stem cells NTERA2. Life Sci 2022; 291: 120298.ArticlePubMed
- 48. Zhou HZ, Li F, Cheng ST, et al. DDX17-regulated alternative splicing that produced an oncogenic isoform of PXN-AS1 to promote HCC metastasis. Hepatology 2022; 75: 847-65. ArticlePubMedPDF
- 49. Li K, Mo C, Gong D, et al. DDX17 nucleocytoplasmic shuttling promotes acquired gefitinib resistance in non-small cell lung cancer cells via activation of beta-catenin. Cancer Lett 2017; 400: 194-202. ArticlePubMed
- 50. Germann S, Gratadou L, Zonta E, et al. Dual role of the ddx5/ddx17 RNA helicases in the control of the pro-migratory NFAT5 transcription factor. Oncogene 2012; 31: 4536-49. ArticlePubMedPDF
- 51. Wilson BJ, Bates GJ, Nicol SM, Gregory DJ, Perkins ND, Fuller-Pace FV. The p68 and p72 DEAD box RNA helicases interact with HDAC1 and repress transcription in a promoter-specific manner. BMC Mol Biol 2004; 5: 11.ArticlePubMedPMCPDF
- 52. Alqahtani H, Gopal K, Gupta N, et al. DDX17 (P72), a Sox2 binding partner, promotes stem-like features conferred by Sox2 in a small cell population in estrogen receptor-positive breast cancer. Cell Signal 2016; 28: 42-50. ArticlePubMed
- 53. Shin S, Rossow KL, Grande JP, Janknecht R. Involvement of RNA helicases p68 and p72 in colon cancer. Cancer Res 2007; 67: 7572-8. ArticlePubMedPDF
- 54. Liu X, Li L, Geng C, et al. DDX17 promotes the growth and metastasis of lung adenocarcinoma. Cell Death Discov 2022; 8: 425.ArticlePubMedPMCPDF
- 55. Wortham NC, Ahamed E, Nicol SM, et al. The DEAD-box protein p72 regulates ERalpha-/oestrogen-dependent transcription and cell growth, and is associated with improved survival in ERalpha-positive breast cancer. Oncogene 2009; 28: 4053-64. ArticlePubMedPMCPDF
- 56. Ku HC, Cheng CF. Master regulator activating transcription factor 3 (ATF3) in metabolic homeostasis and cancer. Front Endocrinol (Lausanne) 2020; 11: 556.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- The Potential Role of SP-G and PLUNC in Tumor Pathogenesis and Wound Healing in the Human Larynx
Aurelius Scheer, Lars Bräuer, Markus Eckstein, Heinrich Iro, Friedrich Paulsen, Fabian Garreis, Martin Schicht, Antoniu-Oreste Gostian
Biomedicines.2025; 13(5): 1240. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure









 E-submission
E-submission