Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(1); 2016 > Article
-
Original Article
Eosinophils in Colorectal Neoplasms Associated with Expression of CCL11 and CCL24 - Hyuck Cho, Sung-Jig Lim,1, Kyu Yeoun Won1, Go Eun Bae2, Gou Young Kim1, Ji Won Min, Byeong-joo Noh3
-
Journal of Pathology and Translational Medicine 2015;50(1):45-51.
DOI: https://doi.org/10.4132/jptm.2015.10.16
Published online: December 14, 2015
Department of Pathology, Kyung Hee University Medical Center, Graduate School of Medicine, Kyung Hee University, Seoul, Korea
1Department of Pathology, Kyung Hee University Hospital of Gangdong, Seoul, Korea
2Department of Pathogy, Kyung Hee University Hospital of Gangdong, Graduate School of Medicine, Kyung Hee University, Seoul, Korea
3Department of Pathology, Kyung Hee University Medical Center, Seoul, Korea
- Corresponding Author: Sung-Jig Lim, MD Department of Pathology, Kyunghee University Hospital at Gangdong, 892 Dongnam-ro, Gangdong-gu, Seoul 05278, Korea Tel: +82-2-440-7550 Fax: +82-2-440-7564 E-mail: 'sungjig@khu.ac.kr'
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background:
- A decrease in the number of tissue eosinophils is known to reflect the malignancy potential of neoplastic lesions and even prognosis. Increased levels of the chemokines CCL11 and CCL24 in serum and tissue are also known to have diagnostic value as serum tumor markers or prognostic factors. The aim of this study was to evaluate the correlation between the degree of tissue eosinophilia and the expression of these chemokines in the glandular and stromal cells of colorectal neoplastic lesions ranging from benign to malignant tumors.
-
Methods:
- We counted the number of infiltrating eosinophils in neoplastic lesion tissue and we evaluated the expression of CCL11 and CCL24 in glandular cells and stromal cells by immunohistochemical staining.
-
Results:
- The results showed that the number of eosinophils decreased significantly and the expression of CCL11 and CCL24 in glandular cells decreased with tumor progression, whereas the stromal expression of CCL11 and CCL24 appeared to increase.
-
Conclusions:
- The discrepancy in CCL11 and CCL24 expression between glandular cells and stromal cells might shed light on how colorectal cancer evades the immune system, which would enable further development of immunotherapies that target these chemokines. Further research on eosinophil biology and the expression pattern of chemokines in tumor cells is needed.
- Patients and tissue samples
- Among the patients who underwent colonoscopic biopsy at Kyung Hee Medical Center and were diagnosed with colorectal tubular adenoma with any degree of dysplasia or colorectal cancer, a list of 50 patients was generated and their clinicopathological data were retrospectively collected. Patients were categorized into separate groups based on whether they had tubular adenoma with low-grade dysplasia, tubular adenoma with high-grade dysplasia, or adenocarcinoma. For adenocarcinoma cases, patients who had undergone resection were selected. Specimens with severe squeezing or cautery artifacts and specimens from patients with a history of other malignancies were excluded. For colorectal cancers, selection was performed among those who had undergone surgical resection and whose surgical specimens were retrieved from Kyung Hee Medical Center. Every diagnosis was reviewed and confirmed by pathologists. Each paraffin block from the specimen was cut into 4–5-µm thick sections and stained with hematoxylin and eosin for direct counting of eosinophils under microscopy. This study was approved by the Institutional Review Board (IRB) of Kyung Hee University (IRB 2015-08-039).
- Evaluation of tissue eosinophilia
- Tissue eosinophils were counted directly on hematoxylin and eosin-stained slides of each specimen using an Olympus BX-53 microscope (Olympus, Tokyo, Japan). Eosinophils in the mucosa and submucosa were counted in three “hotspots” near the neoplastic lesion under a high-power field (×400). Controversial results were resolved by consensus of more than two pathologists using a multiview microscope.
- Immunohistochemical staining
- Each specimen was prepared into 4–5-µm thick paraffin-embedded sections for immunohistochemical staining. Monoclonal mouse antibodies against CCL11 (LS-C139009, LifeSpan Biosciences, Seattle, WA, USA) and monoclonal mouse antibodies against CCL24 (LS-C104346, LifeSpan Biosciences) were used for immunohistochemical staining of the specimens. Unstained slides from each specimen were processed for 20 minutes in a pressure cooker for antigen retrieval. Immunohistochemical staining of all slides was performed using BOND-MAX (Leica Biosystems, Nusslock, Germany) with a dilution ratio of 1:200 for CCL11 and 1:800 for CCL24. Staining of CCL11 and CCL24 in glandular cells of neoplastic lesions was evaluated according to the number of glandular cells showing positive staining and the intensity of the staining over the slide. The number of positively stained glandular cells ranged between 1 and 60, and individual counts were scored as 0 (0–15 cells), 1 (16–30 cells), 2 (31–45 cells), or 3 (≥46 cells). Staining intensity was scored as 1 (faint), 2 (intermediate-strong), and 3 (strong granular). The sum of the immunohistochemical stain scores ranged from 1 to 6, and each summed score was then divided into categories of low (score 1–2), intermediate (score 3–4), and high (score 5–6). We also conducted immunohistochemical staining of CCL11 and CCL24 in stromal cells. Each specimen contained a different amount of stroma because most were endoscopically biopsied samples. Thus, expression in the stromal cells needed to be measured as a proportion rather than an absolute count. Positively stained stromal cells were counted under a microscope and scored as low, intermediate, or high according to the cutoff values of 5%, 15%, and >30% for the percentage of cells showing positive staining relative to the total number of stromal cells in the high-power field.
- Statistical analyses
- SPSS ver. 20.0 (IBM Co., Armonk, NY, USA) was used for the statistical analyses. Correlation between the number of eosinophils and tumor progression was analyzed with a one-way ANOVA. Correlation between the expression of CCL11 and CCL24 in glandular and stromal cells according to each different tumor progression group was analyzed via chi-square tests.
MATERIALS AND METHODS
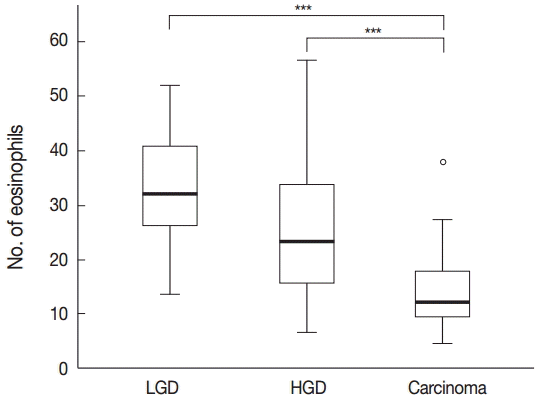
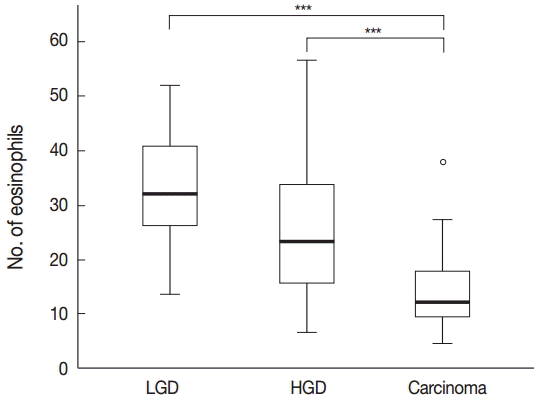
- Tissue eosinophilia in the neoplastic lesions was strikingly different according to progression of the lesion. Tubular adenoma with low-grade dysplasia included a stunning number of infiltrating eosinophils, whereas less eosinophils were present in cases of adenoma with high-grade dysplasia, and only a few eosinophils were counted in adenocarcinoma cases. These infiltrating eosinophils were mostly found adjacent to neoplastic lesions, close to neoplastic glands in tumor stroma.
- One-way ANOVA analysis was performed to evaluate the differences, and the results confirmed that the number of eosinophils differed significantly according to the malignant potential of the lesion (p<.001) (Fig. 1).
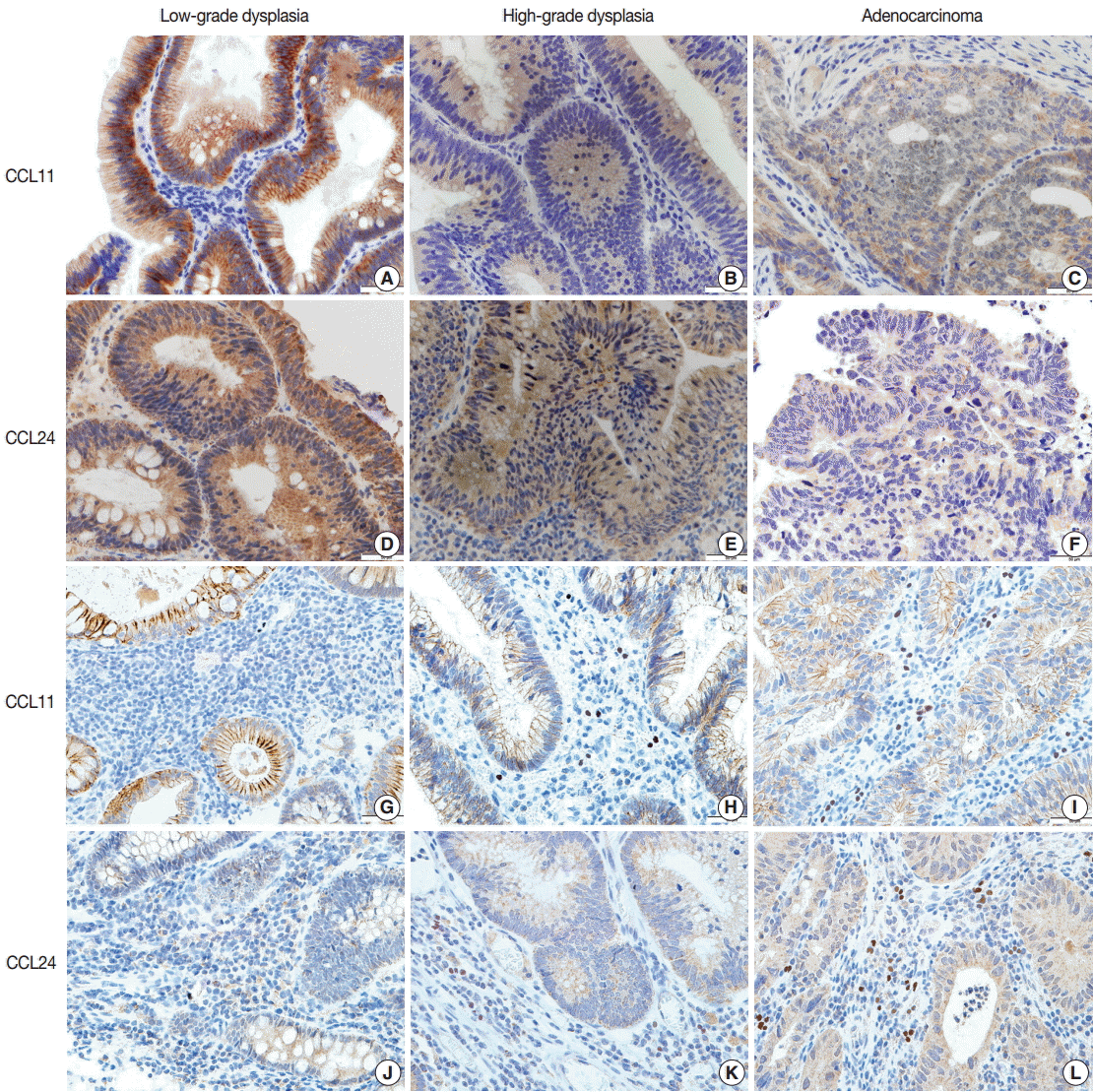
- Immunohistochemical staining of CCL11 in the glandular cells of neoplastic lesions appeared was strong and granular in cases of low-grade dysplasia (Fig. 2A). A mix of strong and faint staining was observed in high-grade dysplasia cases (Fig. 2B). In contrast, most of the staining in adenocarcinoma cases was weak and faint (Fig. 2C).
- Expression of CCL24 in glandular cells of neoplastic lesions was similar to CCL11 expression. CCL24 staining revealed a mostly strong and granular pattern of expression in low-grade dysplasia cases (Fig. 2D), a mix of strong and weak staining in high-grade dysplasia cases, and an intermediate pattern in cases that are between low-grade dysplasia and adenocarcinoma (Fig. 2E). In contrast, a weak and faint pattern of staining was dominant in adenocarcinoma cases (Fig. 2F). Analysis of immunohistochemical staining showed significant differences between the groups (p<.001 in CCL11 and p<.001 in CCL24) (Table 1).
- CCL24 staining in the stromal cells of patients with low-grade dysplasia was scarce and weak (Fig. 1G). Slightly more staining was observed in high-grade dysplasia cases (Fig. 2H), whereas stromal cells with CCL11 reactivity were frequently seen in adenocarcinoma cases (Fig. 2I). CCL11 expression appeared to increase with increased progression of the neoplastic lesion (p<.001) (Table 1).
- CCL24 expression in stromal cells had a similar pattern to that of CCL11. CCL24-expressing stromal cells were scant and faintly stained in low-grade dysplasia cases (Fig. 2J), whereas a small number of positive stromal cells were observed in high-grade dysplasia cases (Fig. 2K). CCL24-positive stromal cells were frequently identified in adenocarcinoma cases (Fig. 2L). StromalCCL24 expression also increased significantly with tumor progression (p<.001) (Table 1).
- Comparisons of tumoral and stromal expression of CCL11 and CCL24 with the degree of eosinophilia revealed that tumoral expression of CCL11 and CCL24 decreased and stromal expression of CCL11 and CCL24 increased while the number of tissue-infiltrating eosinophils decreased (both comparisons, p<.001) (Table 2).
RESULTS
- Our study showed that the number of tissue infiltrating eosinophils in colorectal neoplasms decreased significantly in colorectal adenocarcinoma cases compared to tubular adenoma cases with low-grade dysplasia and tubular adenoma cases with high-grade dysplasia, which is consistent with the results of previous studies [17]. Colorectal cancer is well known as a non-immunogenic tumor that induces an impaired immune response to the tumor itself, thus evading the host immune response to cancer [29-31]. It is also known that an increase in tissue-infiltrating eosinophils in colorectal cancer is associated with a better prognosis. Therefore, decreased tissue eosinophilia may be an immuneevading strategy of colon cancer. Because eosinophils develop and migrate to tissues in response to chemokine signaling, chemokine expression in colorectal cancer should reveal the eosinophil-infiltration potential of colorectal cancer in individual cases.
- In colorectal cancer patients, both the serum level and concentration of CCL11 in tumor tissue are elevated [24,29,30]. Since CCL11 enhances tissue recruitment of eosinophils, an increased concentration of CCL11 should attract more eosinophils and cause more severe eosinophilia in tissues where the concentration of CCL11 is high. Indeed, a study on eosinophilia in colorectal neoplastic lesions showed that tissue extracted from a tumor with a greater number of eosinophils was also highly chemotactic for eosinophils, which was thought to reflect chemokine concentrations in the tissue [19,23]. Another study involving immunohistochemical staining of colorectal cancer tissue revealed that expression of CCL11 was elevated in stromal cells, such as fibroblasts or lymphocytes [24]. The results of this study are consistent with our data showing that expression of CCL11 and CCL24 is increased in the stromal cells of adenocarcinomas compared to those that are dysplastic. The CCL11/CCL24-secreting stromal cells in our study were mostly mononuclear inflammatory cells. Increased expression of CCL11 and CCL24 in the stromal cells of tumors might explain the elevated serum chemokine levels and elevated tissue concentration of chemokines. However, previous studies on eosinophilia in colorectal neoplastic lesions reported decreased numbers of eosinophils in tissues with adenocarcinoma or high-grade dysplasia [17,19], which is consistent with our results. Thus the question remains: why is decreased eosinophilia observed in association with colorectal malignant neoplastic lesions even when the concentration of chemokines for eosinophils is elevated?
- Our results showed that CCL11 and CCL24 expression was lower in glandular cells of adenocarcinomas compared to the expression levels in stromal cells. These findings might provide insight into the discrepancy between tissue eosinophilia and tissue chemokine concentrations in colorectal neoplasms. As we previously mentioned, the population of tissue-infiltrating eosinophils is lower in colorectal adenocarcinomas, as is the expression of CCL11 and CCL24 in neoplastic glandular cells of adenocarcinomas. However, one study found increased tissue concentration of CCL11 associated with colorectal adenocarcinoma [23]. We postulate that increased expression of CCL11 and CCL24 in the stromal cells of tumors might explain increased tissue CCL11 concentration. If lower expression of CCL11 and CCL24 in neoplastic glandular cells is responsible for decreased eosinophilia in adenocarcinomas, modulation of chemokine expression could contribute to the immune-evasion mechanisms of colorectal adenocarcinomas by inhibiting recruitment of eosinophils, which function as effector cells for the neoplasm.
- Further studies on other chemokines involved in eosinophil physiology and studies on the status of eosinophils recruited to tumor tissues are needed for a more detailed understanding of the nature of peritumoral eosinophilia and its significance for the immunologic characteristics of colorectal cancer. CCL24 has previously been a target of anti-cancer immune therapies [23]. If reduced expression of chemokines contributes to immune evasion by colorectal cancer, modulation of chemokine expression in cancer cells could be a possible target for anticancer therapies as well as a prognostic factor for colorectal cancer [31,32]. As some studies on leukemia and CCL24 have suggested, specific kinds of chemokines might affect the migration of specific types of eosinophils in colorectal cancer [33].
- In conclusion, we found a significant correlation between eosinophil numbers and immunohistochemical staining of CCL11 and CCL24 chemokines in the glandular cells of colorectal neoplasms. Lower expression of CCL11 and CCL24 was observed in tumor glandular cells, while greater expression was observed in tumor stromal cells. This differential expression of chemokines might help explain the decreased eosinophilia observed in colorectal cancer despite an apparent increased concentration of CCL11, and could provide insight into immune evasion mechanisms of colorectal cancer. Considering that eosinophils are antitumoral effector immune cells, cancer appears to induce a decrease in eosinophilia through decreased expression of chemokines in glandular cells, which is consistent with the decreased expression of CCL11 and CCL24 shown in our study, and thus, achieves immune evasion. Further study on eosinophil-associated chemokines and the nature of the eosinophils recruited by colorectal cancer cells might enhance our understanding of the immunologic characteristics and roles of eosinophils in colorectal cancer.
DISCUSSION
-
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Notes
- 1. Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol 2006; 24: 147–74. ArticlePubMed
- 2. Adkinson NF, Middleton E Jr. Middleton’s allergy: principles and practice. Philadelphia: Mosby/Elsevier, 2009.
- 3. Legrand F, Driss V, Delbeke M, et al. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol 2010; 185: 7443–51. ArticlePubMed
- 4. Capron M, Legrand F. Functions of eosinophil granulocytes: from anti-parasite immunity to anti-tumoral potential. Bull Acad Natl Med 2009; 193: 339–46. PubMed
- 5. Pretlow TP, Keith EF, Cryar AK, et al. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res 1983; 43: 2997–3000. PubMed
- 6. Teo PZ, Utz PJ, Mollick JA. Using the allergic immune system to target cancer: activity of IgE antibodies specific for human CD20 and MUC1. Cancer Immunol Immunother 2012; 61: 2295–309. ArticlePubMed
- 7. Woerly G, Roger N, Loiseau S, Dombrowicz D, Capron A, Capron M. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J Exp Med 1999; 190: 487–95. ArticlePubMedPMC
- 8. Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology 2002; 41: 152–7. ArticlePubMed
- 9. Fujii M, Yamashita T, Ishiguro R, Tashiro M, Kameyama K. Significance of epidermal growth factor receptor and tumor associated tissue eosinophilia in the prognosis of patients with nasopharyngeal carcinoma. Auris Nasus Larynx 2002; 29: 175–81. ArticlePubMed
- 10. Isaacson NH, Rapoport P. Eosinophilia in malignant tumors: its significance. Ann Intern Med 1946; 25: 893–902. ArticlePubMed
- 11. Goldsmith MM, Belchis DA, Cresson DH, Merritt WD 3rd, Askin FB. The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg 1992; 106: 27–33. ArticlePubMedPDF
- 12. Thompson AC, Bradley PJ, Griffin NR. Tumor-associated tissue eosinophilia and long-term prognosis for carcinoma of the larynx. Am J Surg 1994; 168: 469–71. ArticlePubMed
- 13. Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brünner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol 1999; 189: 487–95. ArticlePubMed
- 14. Fernández-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer 2000; 88: 1544–8. ArticlePubMed
- 15. Fisher ER, Paik SM, Rockette H, Jones J, Caplan R, Fisher B. Prognostic significance of eosinophils and mast cells in rectal cancer: findings from the National Surgical Adjuvant Breast and Bowel Project (protocol R-01). Hum Pathol 1989; 20: 159–63. ArticlePubMed
- 16. Oliveira DT, Biassi TP, Faustino SE, Carvalho AL, Landman G, Kowalski LP. Eosinophils may predict occult lymph node metastasis in early oral cancer. Clin Oral Investig 2012; 16: 1523–8. ArticlePubMed
- 17. Kiziltaş S, Sezgin Ramadan S, Topuzoğlu A, Küllü S. Does the severity of tissue eosinophilia of colonic neoplasms reflect their malignancy potential? Turk J Gastroenterol 2008; 19: 239–44. PubMed
- 18. Polydorides AD, Mukherjee B, Gruber SB, McKenna BJ, Appelman HD, Greenson JK. Adenoma-infiltrating lymphocytes (AILs) are a potential marker of hereditary nonpolyposis colorectal cancer. Am J Surg Pathol 2008; 32: 1661–6. ArticlePubMedPMC
- 19. Moezzi J, Gopalswamy N, Haas RJ Jr, Markert RJ, Suryaprasad S, Bhutani MS. Stromal eosinophilia in colonic epithelial neoplasms. Am J Gastroenterol 2000; 95: 520–3. ArticlePubMed
- 20. Provost V, Larose MC, Langlois A, Rola-Pleszczynski M, Flamand N, Laviolette M. CCL26/eotaxin-3 is more effective to induce the migration of eosinophils of asthmatics than CCL11/eotaxin-1 and CCL24/eotaxin-2. J Leukoc Biol 2013; 94: 213–22. ArticlePubMed
- 21. Schaefer D, Meyer JE, Pods R, et al. Endothelial and epithelial expression of eotaxin-2 (CCL24) in nasal polyps. Int Arch Allergy Immunol 2006; 140: 205–14. ArticlePubMed
- 22. Menzies-Gow A, Ying S, Sabroe I, et al. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J Immunol 2002; 169: 2712–8. ArticlePubMed
- 23. Cheadle EJ, Riyad K, Subar D, et al. Eotaxin-2 and colorectal cancer: a potential target for immune therapy. Clin Cancer Res 2007; 13: 5719–28. ArticlePubMed
- 24. Erreni M, Bianchi P, Laghi L, et al. Expression of chemokines and chemokine receptors in human colon cancer. Methods Enzymol 2009; 460: 105–21. ArticlePubMed
- 25. Cui G, Shi Y, Cui J, Tang F, Florholmen J. Immune microenvironmental shift along human colorectal adenoma-carcinoma sequence: is it relevant to tumor development, biomarkers and biotherapeutic targets? Scand J Gastroenterol 2012; 47: 367–77. ArticlePubMed
- 26. Wågsäter D, Löfgren S, Hugander A, Dienus O, Dimberg J. Analysis of single nucleotide polymorphism in the promoter and protein expression of the chemokine eotaxin-1 in colorectal cancer patients. World J Surg Oncol 2007; 5: 84.ArticlePubMedPMC
- 27. Watanabe H, Miki C, Okugawa Y, Toiyama Y, Inoue Y, Kusunoki M. Decreased expression of monocyte chemoattractant protein-1 predicts poor prognosis following curative resection of colorectal cancer. Dis Colon Rectum 2008; 51: 1800–5. ArticlePubMed
- 28. Agarwal M, He C, Siddiqui J, Wei JT, Macoska JA. CCL11 (eotaxin-1): a new diagnostic serum marker for prostate cancer. Prostate 2013; 73: 573–81. ArticlePubMedPMC
- 29. Monson JR, Ramsden C, Guillou PJ. Decreased interleukin-2 production in patients with gastrointestinal cancer. Br J Surg 1986; 73: 483–6. ArticlePubMed
- 30. King J, Caplehorn JR, Ross WB, Morris DL. High serum carcinoembryonic antigen concentration in patients with colorectal liver metastases is associated with poor cell-mediated immunity, which is predictive of survival. Br J Surg 1997; 84: 1382–5. ArticlePubMed
- 31. Nielsen HJ, Moesgaard F, Hammer JH. Effect of ranitidine and low-dose interleukin-2 in vitro on NK-cell activity in peripheral blood from patients with liver metastases from colorectal cancer. Eur J Surg Oncol 1995; 21: 526–30. ArticlePubMed
- 32. Bovo G, Brivio F, Brenna A, et al. Pre-operative interleukin-2 immunotherapy induces eosinophilic infiltration in colorectal neoplastic stroma. Pathologica 1995; 87: 135–8. PubMed
- 33. Yoshida N, Aizu-Yokota E, Sonoda Y, Moriwaki Y, Kishi K, Kasahara T. Production and regulation of eotaxin-2/CCL24 in a differentiated human leukemic cell line, HT93. Biol Pharm Bull 2007; 30: 1826–32. ArticlePubMed
References
Figure & Data
References
Citations

- Genetic fusion of CCL11 to antigens enhances antigenicity in nucleic acid vaccines and eradicates tumor mass through optimizing T-cell response
Hailong Qi, Zhongjie Sun, Tianle Gao, Yanling Yao, Yu Wang, Weiwei Li, Xudong Wang, Xiaofang Wang, Defang Liu, Jian-Dong Jiang
Molecular Cancer.2024;[Epub] CrossRef - Eosinophilia in cancer and its regulation by sex hormones
Sandeep Artham, Ching-Yi Chang, Donald P. McDonnell
Trends in Endocrinology & Metabolism.2023; 34(1): 5. CrossRef - A novel inflammation-related signature for predicting prognosis and characterizing the tumor microenvironment in colorectal cancer
Jinna Li, Jiapeng Yang, Rui Xing, Ying Wang
Aging.2023; 15(7): 2554. CrossRef - Potential Mechanisms of Melatonin in Osteosarcoma and Bone-Related
Neoplasms: Updated Review
Parisa Maleki Dana, Fatemeh Sadoughi, Russel J. Reiter, Bahman Yousefi, Zatollah Asemi
Mini-Reviews in Medicinal Chemistry.2023; 23(3): 290. CrossRef - Epitope Mapping of Anti-Mouse CCR3 Monoclonal Antibodies (C3Mab-6 and C3Mab-7)
Nami Tateyama, Teizo Asano, Tomohiro Tanaka, Yu Isoda, Yuki Okada, Hiyori Kobayashi, Guanjie Li, Ren Nanamiya, Takeo Yoshikawa, Mika K. Kaneko, Hiroyuki Suzuki, Yukinari Kato
Monoclonal Antibodies in Immunodiagnosis and Immunotherapy.2023; 42(2): 68. CrossRef - Improved colorectal cancer screening by adding noninvasive serum-based biomarkers
Ayman M. Farouk, Mona K. ElDeeb, Mona H. Kandil, Noha A. ElBanna, Mohamed M. Shamseya, Amel S. Elsedafy, Nevine L. Micheal, Mohamed A. Selimah
The Egyptian Journal of Surgery.2023; 42(1): 1. CrossRef - Eosinophils in the tumor microenvironment: implications for cancer immunotherapy
Sasan Ghaffari, Nima Rezaei
Journal of Translational Medicine.2023;[Epub] CrossRef - A Novel Gene Signature Associated with Protein Post-translational Modification to Predict Clinical Outcomes and Therapeutic Responses of Colorectal Cancer
Jun Liu, Peng Zhu
Molecular Biotechnology.2023;[Epub] CrossRef - Assessing serum cytokine profiles in inflammatory breast cancer patients using Luminex® technology
Maryem Bessaad, Azza Habel, Mariem Hadj Ahmed, Weili Xu, Mouna Stayoussef, Hanen Bouaziz, Monia Hachiche, Amel Mezlini, Anis Larbi, Besma Yaacoubi-Loueslati
Cytokine.2023; 172: 156409. CrossRef - Systemic Evaluation of the Effect of Diabetes Mellitus on Breast Cancer in a Mouse Model
Nana Wei, Jinmiao Lu, Zhibing Lin, Xiaoyu Wang, Mengmeng Cai, Shengyao Jiang, Xiaoyu Chen, Shilan Zhu, Dong Zhang, Li Cui
Frontiers in Oncology.2022;[Epub] CrossRef - Extracellular DNA Traps: Origin, Function and Implications for Anti-Cancer Therapies
Medina Mamtimin, Akif Pinarci, Chao Han, Attila Braun, Hans-Joachim Anders, Thomas Gudermann, Elmina Mammadova-Bach
Frontiers in Oncology.2022;[Epub] CrossRef - Immune-Related Biomarkers Associated with Lung Metastasis from the Colorectal Cancer Microenvironment
Wang Da, Wu Yinhang, Zhuang Jing, Xu Jiamin, Gao Xinyi, Song Yongmao, Pan Yuefen
Journal of Interferon & Cytokine Research.2022; 42(5): 220. CrossRef - Cancer-Associated Fibroblasts Promote Tumor Aggressiveness in Head and Neck Cancer through Chemokine Ligand 11 and C-C Motif Chemokine Receptor 3 Signaling Circuit
Wen-Yen Huang, Yaoh-Shiang Lin, Yu-Chun Lin, Shin Nieh, Yi-Ming Chang, Tsai-Yu Lee, Su-Feng Chen, Kuender D. Yang
Cancers.2022; 14(13): 3141. CrossRef - Eosinophils Decrease Pulmonary Metastatic Mammary Tumor Growth
Rachel A. Cederberg, Sarah Elizabeth Franks, Brennan J. Wadsworth, Alvina So, Lisa R. Decotret, Michael G. Hall, Rocky Shi, Michael R. Hughes, Kelly M. McNagny, Kevin L. Bennewith
Frontiers in Oncology.2022;[Epub] CrossRef - An Immune-Related Prognostic Risk Model in Colon Cancer by Bioinformatics Analysis
Qing Lai, Haifei Feng, Shuli Yang
Evidence-Based Complementary and Alternative Medicine.2022; 2022: 1. CrossRef - Epitope Mapping of Anti-Mouse CCR3 Monoclonal Antibodies Using Flow Cytometry
Nami Tateyama, Teizo Asano, Hiroyuki Suzuki, Guanjie Li, Takeo Yoshikawa, Tomohiro Tanaka, Mika K. Kaneko, Yukinari Kato
Antibodies.2022; 11(4): 75. CrossRef - Granulocytes and Cells of Granulocyte Origin—The Relevant Players in Colorectal Cancer
Izabela Siemińska, Ewa Poljańska, Jarek Baran
International Journal of Molecular Sciences.2021; 22(7): 3801. CrossRef - CCL24 Protects Renal Function by Controlling Inflammation in Podocytes
Youdi Wang, Xue Wu, Mengya Geng, Jiamin Ding, Kangjia Lv, Hui Du, Jiahui Ding, Wenjun Pei, Xin Hu, Jing Gu, Lizhuo Wang, Yao Zhang, Jialin Gao, Roberta Rizzo
Disease Markers.2021; 2021: 1. CrossRef - Primary tumors from mucosal barrier organs drive unique eosinophil infiltration patterns and clinical associations
Sharon Grisaru-Tal, Michal Itan, Daniel G Grass, Javier Torres-Roca, Steven A Eschrich, Yaara Gordon, Avishay Dolitzky, Inbal Hazut, Shmuel Avlas, Elizabeth A Jacobsen, Tomer Ziv-Baran, Ariel Munitz
OncoImmunology.2021;[Epub] CrossRef - Discovery of core gene families associated with liver metastasis in colorectal cancer and regulatory roles in tumor cell immune infiltration
Wei-Qing Liu, Wen-Liang Li, Shu-Min Ma, Lei Liang, Zhi-Yong Kou, Jun Yang
Translational Oncology.2021; 14(3): 101011. CrossRef - Metastasis-Entrained Eosinophils Enhance Lymphocyte-Mediated Antitumor Immunity
Sharon Grisaru-Tal, Shai Dulberg, Lir Beck, Chunyan Zhang, Michal Itan, Soroor Hediyeh-zadeh, Julie Caldwell, Perri Rozenberg, Avishay Dolitzky, Shmuel Avlas, Inbal Hazut, Yaara Gordon, Ophir Shani, Shlomo Tsuriel, Motti Gerlic, Neta Erez, Nicolas Jacquel
Cancer Research.2021; 81(21): 5555. CrossRef - Tumor Immunology and Tumor Evolution: Intertwined Histories
Jérôme Galon, Daniela Bruni
Immunity.2020; 52(1): 55. CrossRef - A new dawn for eosinophils in the tumour microenvironment
Sharon Grisaru-Tal, Michal Itan, Amy D. Klion, Ariel Munitz
Nature Reviews Cancer.2020; 20(10): 594. CrossRef - Eotaxins and Their Receptor in Colorectal Cancer—A Literature Review
Monika Zajkowska, Barbara Mroczko
Cancers.2020; 12(6): 1383. CrossRef - Eosinophilic peritonitis with colon cancer: a case report
Ryo Ataka, Hirokazu Tanaka, Shintaro Yagi, Kei Yamane, Kenji Yoshino, Tomoyuki Miyauchi, Tomoaki Yoh, Keiichi Arafuka, Shinichi Fujita, Akihiko Hamada, Bunji Endo, Shinji Uemoto
BMC Gastroenterology.2020;[Epub] CrossRef - Hypoxia Alters the Expression of CC Chemokines and CC Chemokine Receptors in a Tumor–A Literature Review
Jan Korbecki, Klaudyna Kojder, Katarzyna Barczak, Donata Simińska, Izabela Gutowska, Dariusz Chlubek, Irena Baranowska-Bosiacka
International Journal of Molecular Sciences.2020; 21(16): 5647. CrossRef - CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4
Jan Korbecki, Klaudyna Kojder, Donata Simińska, Romuald Bohatyrewicz, Izabela Gutowska, Dariusz Chlubek, Irena Baranowska-Bosiacka
International Journal of Molecular Sciences.2020; 21(21): 8412. CrossRef - Opposing roles of eosinophils in cancer
Sonja C. S. Simon, Jochen Utikal, Viktor Umansky
Cancer Immunology, Immunotherapy.2019; 68(5): 823. CrossRef - Prognostic significance of inflammatory cell response in patients with colorectal cancer
Katarzyna Jakubowska, Mariusz Koda, Wojciech Kisielewski, Luiza Kańczuga‑Koda, Waldemar Famulski
Oncology Letters.2019;[Epub] CrossRef - Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer
Hadar Reichman, Michal Itan, Perri Rozenberg, Tal Yarmolovski, Eli Brazowski, Chen Varol, Nathan Gluck, Shiran Shapira, Nadir Arber, Udi Qimron, Danielle Karo-Atar, James J. Lee, Ariel Munitz
Cancer Immunology Research.2019; 7(3): 388. CrossRef - Developmental endothelial locus‐1 (Del‐1) antagonizes Interleukin‐17‐mediated allergic asthma
Shu Yan, Li Chen, Qi Zhao, Ya‐Nan Liu, Rui Hou, Jing Yu, Hong Zhang
Immunology & Cell Biology.2018; 96(5): 526. CrossRef - Expression of Galectins-1 and Galectin-3 in Stomach and Colorectal Cancer with Tissue Eosinophilia
Yu. V. Kolobovnikova, A. I. Dmitrieva, K. I. Yankovich, O. A. Vasil’eva, I. L. Purlik, V. S. Poletika, V. V. Novitskii, O. I. Urazova
Bulletin of Experimental Biology and Medicine.2018; 165(2): 256. CrossRef - The expression of CCL11/eotaxin, CCR3 receptor and eosinophil peroxidase in tumor tissue in gastric and colon cancers
Yu. V. Kolobovnikova, K. I. Yankovich, E. V. Romanova, A. I. Dmitrieva, V. V. Novitskiy, O. I. Urazova
Bulletin of Siberian Medicine.2018; 17(3): 80. CrossRef - Friend or foe?
Tommaso Colangelo, Giovanna Polcaro, Livio Muccillo, Giovanna D'Agostino, Valeria Rosato, Pamela Ziccardi, Angelo Lupo, Gianluigi Mazzoccoli, Lina Sabatino, Vittorio Colantuoni
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2017; 1867(1): 1. CrossRef - CCL24 contributes to HCC malignancy via RhoB- VEGFA-VEGFR2 angiogenesis pathway and indicates poor prognosis
Lei Jin, Wei-Ren Liu, Meng-Xin Tian, Xi-Fei Jiang, Han Wang, Pei-Yun Zhou, Zhen-Bin Ding, Yuan-Fei Peng, Zhi Dai, Shuang-Jian Qiu, Jian Zhou, Jia Fan, Ying-Hong Shi
Oncotarget.2017; 8(3): 5135. CrossRef - Inflammatory cytokines are associated with response and prognosis in patients with esophageal cancer
Susanne Blank, Henrik Nienhüser, Lena Dreikhausen, Leila Sisic, Ulrike Heger, Katja Ott, Thomas Schmidt
Oncotarget.2017; 8(29): 47518. CrossRef - The formation mechanisms of tumor-associated tissue eosinophilia in gastric cancer and colon cancer
В.В. Новицкий, К.И. Янкович, Ю.В. Колобовникова, А.И. Дмитриева, О.И. Уразова, И.Л. Пурлик, Л.А. Кудяков, С.П. Чумакова
ZHurnal «Patologicheskaia fiziologiia i eksperimental`naia terapiia».2017; (4(61)): 74. CrossRef - LncRNAs expression in adjuvant-induced arthritis rats reveals the potential role of LncRNAs contributing to rheumatoid arthritis pathogenesis
Hui Jiang, Xiu-Juan Qin, Wei-Ping Li, Rong Ma, Ting Wang, Zhu-Qing Li
Gene.2016; 593(1): 131. CrossRef - Emerging Roles for Eosinophils in the Tumor Microenvironment
Hadar Reichman, Danielle Karo-Atar, Ariel Munitz
Trends in Cancer.2016; 2(11): 664. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission