Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(2); 2024 > Article
-
Review
Exploring histological predictive biomarkers for immune checkpoint inhibitor therapy response in non–small cell lung cancer -
Uiju Cho,1
 , Soyoung Im1
, Soyoung Im1 , Hyung Soon Park2
, Hyung Soon Park2
-
Journal of Pathology and Translational Medicine 2024;58(2):49-58.
DOI: https://doi.org/10.4132/jptm.2024.01.31
Published online: February 26, 2024
1Department of Pathology, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
2Division of Medical Oncology, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- Corresponding Author: Uiju Cho, MD, PhD, Department of Pathology, St. Vincent’s Hospital, 93 Jungbu-daero, Paldal-gu, Suwon 16247, Korea Tel: +82-31-249-7647, Fax: +82-31-244-6786, E-mail: 'hailtoya@catholic.ac.kr'
© 2024 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,304 Views
- 202 Download
Abstract
- Treatment challenges persist in advanced lung cancer despite the development of therapies beyond the traditional platinum-based chemotherapy. The early 2000s marked a shift to tyrosine kinase inhibitors targeting epidermal growth factor receptor, ushering in personalized genetic-based treatment. A further significant advance was the development of immune checkpoint inhibitors (ICIs), especially for non–small cell lung cancer. These target programmed death-ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4, which enhanced the immune response against tumor cells. However, not all patients respond, and immune-related toxicities arise. This review emphasizes identifying biomarkers for ICI response prediction. While PD-L1 is a widely used, validated biomarker, its predictive accuracy is imperfect. Investigating tumor-infiltrating lymphocytes, tertiary lymphoid structure, and emerging biomarkers such as high endothelial venule, Human leukocyte antigen class I, T-cell immunoreceptors with Ig and ITIM domains, and lymphocyte activation gene-3 counts is promising. Understanding and exploring additional predictive biomarkers for ICI response are crucial for enhancing patient stratification and overall care in lung cancer treatment.
- PD-L1 expression
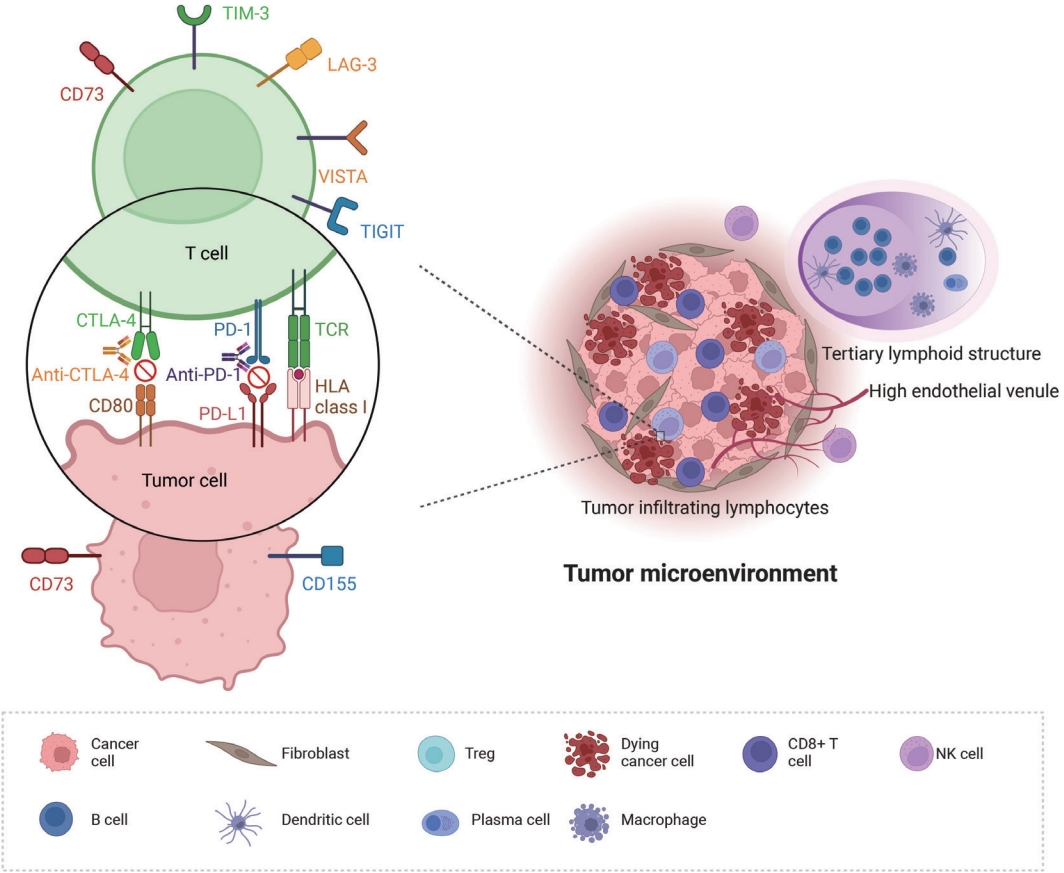
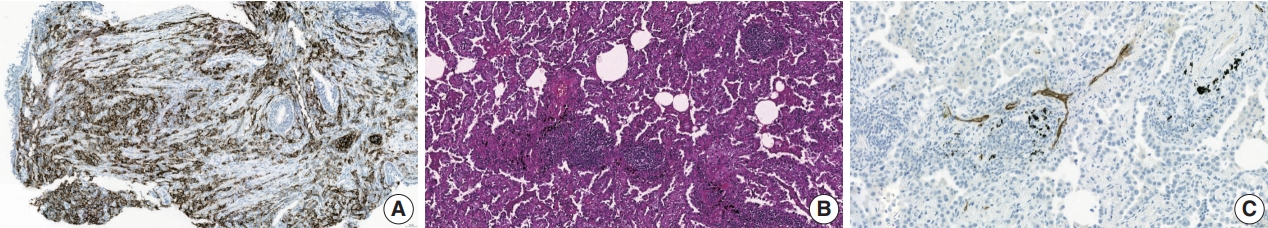
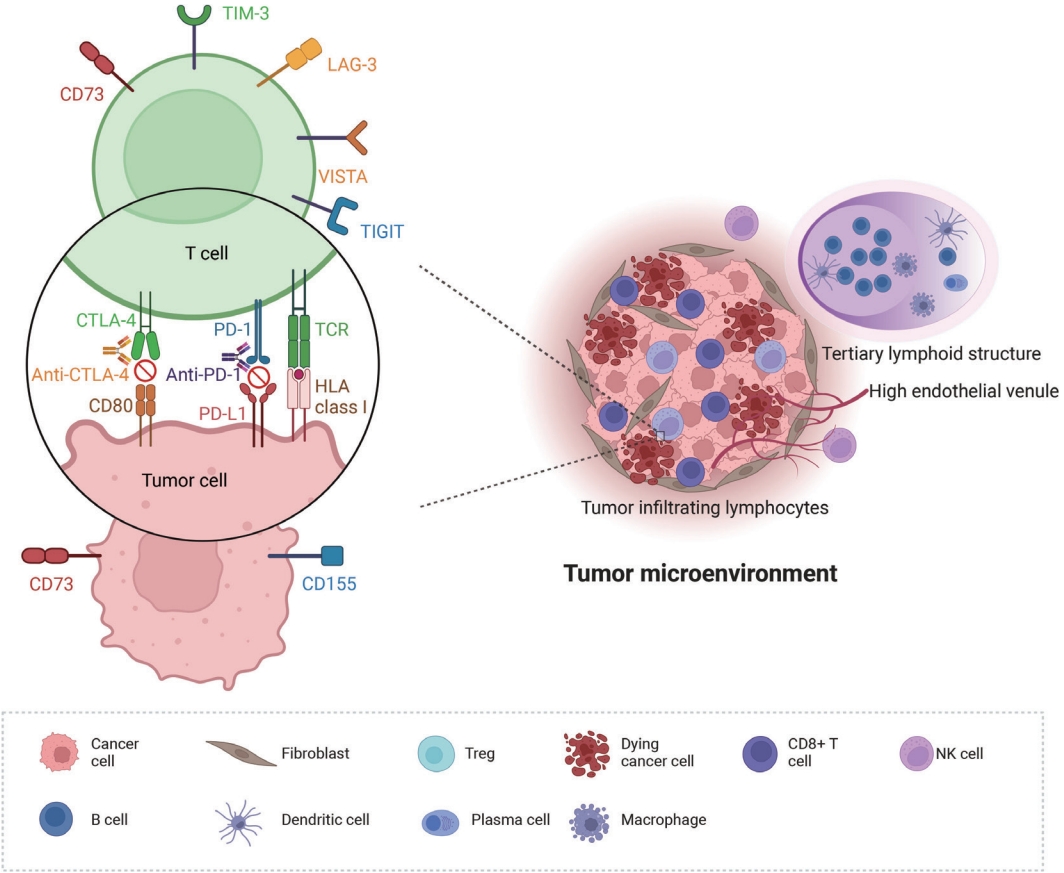
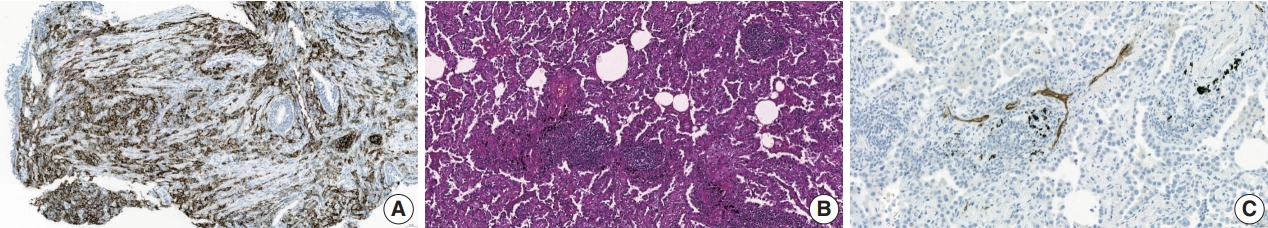
- PD-L1 expression is currently the only recommended biomarker in the National Comprehensive Cancer Network guidelines for determining the treatment approach to metastatic NSCLC, excluding genomic driver mutations [8]. It is also the sole companion diagnostic test for PD-L1 inhibitors (Fig. 2A) [12,14]. PD-L1 expression differs in different subtypes of NSCLC. In a literature review of 42 studies, PD-L1 expression was higher in squamous cell carcinoma than in adenocarcinoma, 41.05% vs. 34.72% at >1% cutoff [15]. Other subtypes of NSCLC have a limited number of case studies. However, sarcomatoid carcinoma, an aggressive, poorly differentiated subtype of NSCLC, demonstrated an impressively high rate of PD-L1 positivity. In a cohort of 41 patients, 78% of the patients showed positivity at PD-L1 SP142 ts, and a study by Domblides et al. [16] had 94.7% positivity at PD-L1 SP263 >5% [17]. Several clinical trials have demonstrated the predictive capability of PD-L1 expression in stage IV NSCLC [4]. These trial results both suggest that PD-L1 expression assists in patient selection and indicate its potential to predict the extent of a patient’s treatment response to PD-L1 inhibitors [12]. However, certain clinical trials have disputed the utility of programmed death-1 (PD-1)/PD-L1 expression in predicting treatment outcomes in patients receiving these inhibitors [18].
- PD-L1 expression does not provide absolute certainty in predicting response or resistance to treatment. Some studies of a PD-L1 <1% expression subgroup highlighted the need for novel and complementary methods to identify patients who respond to ICIs [18,19]. In the multicohort, open-label phase 1 CheckMate 012 trial, increasing PD-L1 expression level was associated with greater benefit, with an overall response rate of 50%, in which nivolumab was used as a first-line therapy for advanced NSCLC; however, clinical activity was also observed in a patient population with <1% PD-L1 expression [20]. The PD-L1 inhibitor pembrolizumab was administered to patients with a ≥50 PD-L1 tumor proportional score or combined positive score [21] (Table 1). Although PD-L1 is widely used as a biomarker in clinical practice, and PD-L1 positivity generally predicts therapeutic response, its predictive value is not absolute. Challenges associated with using PD-L1 as a biomarker include inter- and intra-tumor heterogeneity of PD-L1 expression, diversity in PDL1 assay methods, cutoff values, and interobserver bias [21,22]. To overcome one of the challenges, an artificial intelligence-powered PD-L1 analyzer was applied to PD-L1 scoring of NSCLC slides, and it was shown to improve pathologists’ consensus scoring and prediction of therapeutic response [23]. Such an effort would increase the reliability of PD-L1 interpretation in the future.
- Tumor-infiltrating lymphocytes
- Tumor-infiltrating lymphocytes (TILs) have undergone extensive study, revealing their crucial role in mediating the immune system’s anti-tumor activity. Additionally, tumor-immune system interactions have revealed insights into the causes of immunotherapy resistance [5]. Analyzing TIL nature at diagnosis both predicts immunotherapy outcomes and informs treatment strategies. Traditionally, cancer research centered on malignant cells, neglecting tumor microenvironment components. However, it has gradually been recognized that tumor cells possess antigenic properties, inducing an immune response of producing altered proteins that the host immune system perceives as harmful [24].
- Solid tumors encompass various cellular components in the tumor microenvironment, including the extracellular matrix, stromal, endothelial, and immune cells [25]. The density, location, and organization of immune cells collectively constitute the “immune context” [26]. The immune context at diagnosis correlates with clinical outcomes in various tumors, such as melanoma and colorectal, lung, and breast cancers [26]. The prognostic value of immune cells is based on their functional status and distinct phenotypes. Cytotoxic T cells, helper T cells, natural killer cells, and dendritic cells contribute to anti-tumor responses. In contrast, FOXP3-positive regulatory T cells (Tregs) and myeloid-derived suppressor cells have pro-tumorigenic effects and promote cancer growth and invasion [27]. Each immune cell type has different effects, so they carry distinct prognostic significance [26]. The prognostic importance of TIL in breast cancer has been extensively studied [28]. The association between TIL and breast cancer prognosis was first investigated by Aaltomaa et al. in the early 1990s [29]. Since then, several researchers have reported the prognostic and predictive value of TIL in breast cancer, and these results have been consistent across various randomized clinical trials [30]. Several studies have evaluated TIL as a continuous parameter in hematoxylin and eosin– stained tumor sections following the criteria proposed by Denkert et al. [31]. International collaborative efforts have sought to standardize TIL assessment and enhance reproducibility, and recommend scoring stromal TILs as a percentage of stromal areas between nests of carcinoma cells while excluding the areas occupied by the carcinoma cells from the total assessed surface area [32]. TIL assessment guidelines established for breast cancer are also being applied to NSCLC, melanoma, gastrointestinal tract carcinomas, and other solid tumors [33,33].
- In solid tumors, TILs primarily consist of CD4+ and CD8+ T cells [34]. CD8+ T cells act as cytotoxic T cells and can directly kill tumor cells. Increased CD8+ T-cell infiltration has been associated with improved patient survival [35,36]. CD4+ T cells function as helper T cells or Tregs that mediate diverse functions through cytokine secretion. Th cells recruit leukocytes, stimulate phagocytes and cytotoxic T cells to kill tumor cells, and promote B-cell antibody production [37]. In contrast, CD4+ FOXP3+ CD25high Treg cells, which express the transcription factor FOXP3, inhibit effector T cells by secreting cytokines such as transforming growth factor β and interleukin-10 or metabolites like adenosine, thereby conferring immunotolerance. Consistently, the presence of CD4+ FOXP3+ CD25high Tregs has been associated with worsened prognosis in breast cancer and is a marker of poor prognosis in NSCLC [38]. The proportion and type of TILs, along with their organizational level, could make them crucial biomarkers for improving candidate selection for ICI therapy. Although the distribution of CD markers (which reflect immune profiles in the tumor microenvironment) is a potential predictive biomarker of ICI effectiveness in NSCLC, comprehensive analyses or randomized clinical trials are yet to conclusively establish the utility of TIL as definitive predictive biomarkers [8,39,40]. Attempts have been made to create models that combine PD-L1 expression and TIL infiltration to classify the tumor microenvironment and discriminate tumors that respond best to PD-1 inhibitors [41]. Combining multiple biomarkers may be a rational approach for tailoring immunotherapeutic treatments and should be integrated into future clinical trials [42,43].
- Theoretically, TILs serve as the main activators of anti-tumor immunity, and if objectively measured in the entire tumor microenvironment, they could be a promising biomarker. However, quantifying TILs is labor-intensive and limited by spatial distribution in whole-slide images and interobserver heterogeneity [44]. Therefore, establishing clinically relevant TIL cutoff values is challenging. The current immunophenotyping concept is based on TIL status in the tumor microenvironment, dividing it into inflamed (intratumorally distributed TILs), immune-excluded (TILs excluded from the cancer stroma), and immune-desert (scant TILs in the tumor microenvironment) subtypes [45]. Although pathologists can easily confirm the abundance of TIL through microscopic examination, TIL quantification is challenging. Some cancer types lack lymphocytes, while others, such as lymphoepitheliomas, show tumor cells engulfed by lymphocytes. Although studies have suggested that immune phenotype predicts clinical outcomes of ICI therapy [11], there is a lack of standardized methodology for quantifying TILs. To address these issues, artificial intelligence–assisted methods have been introduced [46], and ongoing research studies are exploring TIL quantification using multiplex immunohistochemistry and spatial studies [47].
- Tertiary lymphoid structures
- Tertiary lymphoid structures (TLSs) are ectopic lymphoid formations that develop under prolonged inflammatory conditions, including cancer. Compared to B, T, and dendritic cells, TLSs exhibit varying levels of organization, ranging from locally concentrated immune cell aggregates to mature follicles with well-defined B-cell follicles and germinal centers (Fig. 2B) [48]. Immature TLSs display visible immune cell foci with segregated B and T cell zones but lack follicular dendritic cells and germinal centers, which are crucial sites for B-cell proliferation and affinity maturation [6]. TLS status demonstrates prognostic potential in various cancer types, including NSCLC, colon cancer, breast cancer, and melanoma [48,49]. Additionally, TLS status appears to have a predictive value in ICI therapy; it is strongly associated with improved survival and clinical outcomes in patients receiving ICI treatment for solid tumors such as sarcoma, melanoma, and renal cell carcinoma [50,51]. Recently, Helmink et al. [52] studied the association between renal cell carcinoma and TLS status via spatial transcriptomic analysis of formalinf-ixed paraffin-embedded samples and reported higher remission rates and longer progression-free survival in patients with TLSpositive tumors treated with ICI than in those with TLS-negative tumors.
- Some researchers argue that both the presence of immune cells and their organization into TLSs are crucial in response to immunotherapy [53]. However, the reason for the increase in TLS density in ICI responders during treatment remains unclear. Nonetheless, histologically evaluated CD20 density is higher at baseline in responding patients than in non-responding patients, with a further increase observed after ICI treatment [52]. Nevertheless, changes in TLSs due to ICI therapy require prospective validation in larger and more homogeneous patient cohorts for conclusive evidence, which could establish TLSs as an active and beneficial component of ICI treatment.
- The exact contribution of TLSs to anti-tumor responses has yet to be fully understood. B cells, a component of TILs, primarily reside within TLSs. The role of B cells in anti-tumor immunity is controversial, with studies suggesting that they contribute to humoral anti-tumor immune responses by generating antibodies against tumor-associated antigens and enhancing cellular immunity by secreting cytokines that increase the activation of antigen-presenting cells (APCs) [54,55]. Theoretically, intra-tumoral TLSs may lead to a complete B-cell response within the tumor, causing a direct anti-tumor effect by maintaining B-cell maturation and antibody production. Notably, B cells may alter T-cell activation and function, contributing to the enhanced therapeutic effects of ICI [54].
- These findings could have clinical applications in improving patient selection for ICI therapy, as TLSs can be easily detected in standard pathology laboratories. Prospective studies employing TLSs to select ICI candidates were reported in 2022. In one study, patients with advanced soft-tissue sarcoma known to have limited responses to ICIs were selected for anti–PD-1 therapy based on the presence of TLSs in tumor biopsy specimens. This TLS-positive cohort exhibited improved overall response rates and median progression-free survival compared with the previously unselected cohort. Following this, TLS status has been employed as an inclusion criterion in several ICI clinical trials; more results from similar clinical trials are expected in the near future (NCT04705818 and NCT03475953).
- It is noteworthy that previous studies have quantified TLS differently. Some lung cancer studies evaluated TLSs exclusively using the CD208+ dendritic cells present in them. In contrast, others used the follicular dendritic cell markers CD21 and CD23 or assessed TLSs based on the co-localization of CD3+ T cells and CD20+ B cells [48,56]. Although there is currently no consensus on a standardized TLS evaluation method, if clinical trial results with TLS inclusion criteria emerge, the methods used in such studies may be accepted as standardized approaches.
BIOMARKERS OF RESPONSE AND RESISTANCE TO IMMUNOTHERAPY IN NON–SMALL CELL LUNG CANCER
- High endothelial venule
- Tumor-associated high endothelial venules (TA-HEVs) originate from post-capillary venules and are characterized by an elevated expression of high endothelial venule-specific sulfated MECA-79 (PNAd) antigens (Fig. 2C). TA-HEVs play a crucial role in lymphocyte recirculation and TLS formation, and increased HEV values are associated with a favorable prognosis in gastric cancer [57,58]. Recent discoveries suggest that ICIs increase the network of TA-HEVs and enhance CD8+ T-cell infiltration [59]. Furthermore, anti-angiogenic therapy downregulates continuous angiogenic signaling, resulting in vasculature normalization and promoting TA-HEV formation [60,61]. Despite these significant developments, the characterization of TAHEVs in the context of ICI therapy for NSCLC remains poorly defined [62]. Studies have suggested an association between TAHEVs and the tumor microenvironment in solid tumors; however, further investigations are warranted to determine the usefulness of TA-HEVs in selecting patients for ICI therapy [63].
- HLA class I
- The major histocompatibility complex, the human leukocyte antigen (HLA), includes cell surface molecules responsible for presenting and recognizing self- and non-self peptides. It is encoded by a highly polymorphic gene complex. The HLA gene complex contains more than 200 loci on the short arm of chromosome 6. Population surveys have identified thousands of allelic variants of HLA molecules primarily influenced by the nature and composition of the peptide-binding groove [64], and these variants are associated with the risk of developing various diseases, including cancer [65].
- Human leukocyte antigen class I (HLA-I) promotes the clonal amplification and cellular activation of naïve TCD8 lymphocytes by presenting intracellular antigenic peptides. HLA-I exhibits polymorphisms in its antigenic peptide-binding region, allowing each variant to bind to a specific repertoire of peptide ligands [66]. The HLA genotype, which generates this diversity, has been linked to the prognosis of patients receiving ICIs, with certain supertypes associated with improved or lower survival [67]. Beta-2-microglobulin (B2M), a component of HLA-I, is required for antigen presentation by dendritic cells. B2M mutations can lead to resistance to ICI therapy, with B2M abnormalities associated with cancer progression in 29.4% of the cases [68]. B2M mutations or decreased expression have been linked with ICI outcomes in patients with head and neck squamous cell carcinoma and melanoma [69,70].
- The HLA class I antigen-derived peptide complex is crucial for presenting tumor antigens to naïve T cells. The activation of naïve T cells requires the interaction of HLA class I-derived peptide complexes with the T cell receptor and co-stimulatory ligands, such as the B7 family, on APCs [64]. The balance between co-stimulatory and co-inhibitory signaling, mediated by immune checkpoint molecules such as PD-1 and CTLA-4, tightly regulates T-cell activation. Some tumor cells evade the host immune system due to defects in their ability to present tumor antigens to naïve T cells via HLA class I molecules [70]. Research is ongoing to investigate the impact of HLA class I expression on the ICI response. In murine solid tumor models, HLA class I expression is reportedly a predictor of ICI response and an overall marker of immunogenicity [71,72]. In vivo studies using HLA class I and class II knockout mice treated with PD-1 antibodies showed strong anticancer effects [73]. Although several in vivo studies have suggested that HLA class I expression may be a predictive marker, clinical evidence remains limited. For instance, in patients with melanoma treated with ICI, post-treatment samples showed significantly lower HLA class I expression, particularly in the progressing metastases of non-responding patients [74]. Retrospective evaluation of patients with metastatic melanoma treated with ipilimumab or nivolumab revealed associations between HLA class I expression and tumor response, with different patterns observed for each treatment [67]. Further studies are underway to analyze whether the patient’s HLA class I subtype influences the ICI response, indicating its potential predictive value.
- Novel target immune checkpoint biomarkers
- PD-1/PD-L1 and CTLA-4 inhibitors are most widely used for ICI therapy of lung cancer; however, the development of drug resistance remains a challenge. Recently, novel immune checkpoint targets, such as T-cell immunoreceptors with Ig and ITIM domains (TIGIT), have shown promise in preclinical and early clinical studies, offering hope to overcome resistance to conventional ICIs [75]. TIGIT, which is expressed in activated natural killer and regulatory T cells, binds to CD155 (PVR) and CD112 (PVRL2 and nectin-2), ligands on tumor cells and antigen-presenting cells in the tumor microenvironment [75]. A recent randomized phase II clinical trial demonstrated that combining anti-TIGIT antibody tiragolumab and atezolizumab as firstline therapy for advanced PD-L1–positive NSCLC significantly increased objective response rate and progression-free survival compared to the control group. Based on these results, the FDA recently granted breakthrough therapy designation to tiragolumab [75]. TIGIT expression, particularly in CD3+ TIL and peritumoral lymphocyte infiltrates, indicates an “exhausted” T cell phenotype in the tumor microenvironment. Additionally, TIGIT expression positively correlates with PD-1 and PD-L1 density, indicating the synergy between these immune checkpoint axes in lung squamous cell carcinoma and melanoma [76,77]. In lung squamous cell carcinoma tissues analyzed using immunohistochemistry, 85.8% expressed CD155 (PVR) and 26.8% expressed PD-L1. High TIGIT density and high CD155/TIGIT expression correlated with advanced tumor, nodal, and metastasis (TNM) stage and worse overall survival when TIGIT-positive TIL were counted [76]. Although TIGIT expression has been studied in various solid tumors [75-77], data on TIGIT or TIGIT ligands as immunohistochemical biomarkers in NSCLC are limited. Currently, the immunohistochemical status of TIGIT is not a prerequisite for the use of TIGIT inhibitors, and PD-L1 positivity is considered sufficient. No data exist on the potential role of TIGIT as a predictive marker for anti-TIGIT regimens [75]. In most clinical trials, anti-TIGIT agents are administered in combination with anti–PD-1/PD-L1 or anti–CTLA-4 inhibitors; however, some trials on NSCLC have investigated anti-TIGIT monotherapy (NCT02964013, NCT04165070). Further studies are needed to determine the role of TIGIT expression as a predictive marker of response to anti-TIGIT therapy regimens, particularly in lung cancer, which has been the focus of most immunotherapy trials.
- Another novel ICI target is lymphocyte activation gene-3 (LAG-3), which is expressed in activated CD4+/CD8+ T cells, regulatory T cells, natural killer (NK) cells, B cells, and plasmacytoid dendritic cells. LAG-3 signaling plays a negative regulatory role in T helper 1 cell activation, proliferation, and cytokine secretion, allowing tumor cells to evade the host immune system [78]. Several LAG-3 inhibitors are under development, and some are undergoing phase II trials as first-line therapies for advanced NSCLC [79]. LAG-3 expression in TIL in NSCLC appears positively correlated with PD-1/PD-L1 expression [80]. However, similar to TIGIT, no prospective study has assessed the potential value of LAG-3 as a predictive NSCLC biomarker.
- Other novel ICI targets include T cell immunoglobulin and mucin-domain containing-3, NK group 2 member A (NKG2A), and CD73 [81]. CD73, acting as an immune checkpoint, generates adenosine, inhibiting immune activation via the A2A receptor [82]. CD73 is upregulated in various cancers, including lung cancer, and its overexpression in tumor tissues is associated with poor prognosis [83-85]. In preclinical studies, combination therapy with PD-1/PD-L1 and CD73 inhibitors has demonstrated synergistic anti-tumor effects [86]. CD73 expression in NSCLC positively correlates with a “hot” immune environment, including PD-L1 expression and the presence of TIL [85]. However, more data are necessary to comprehend the effects of CD73 on the tumor microenvironment [87,88]. Retrospective analyses of NSCLC patients treated with anti–PD-1/PD-L1 therapy indicate that high CD73 expression may predict a favorable response, particularly in EGFR-mutant patients [89]. Given the high EGFR mutation rate in the East Asian population, CD73 may be a crucial therapeutic target and predictive marker.
- Several other immune checkpoint targets, including V-domain immunoglobulin suppressor of T cell activation (VISTA), B7-H3 (CD276), indoleamine 2,3-dioxygenase 1, glucocorticoid-induced tumor necrosis factor receptor–related receptor, and CD47, are under investigation [81,90]. Ongoing clinical trials are evaluating these inhibitors alone and in combination with PD-1 and PD-L1 inhibitors [90]. As more ICIs are integrated into therapy, discovering predictive markers for optimal patient selection will become increasingly necessary.
EMERGING PREDICTIVE BIOMARKERS OF IMMUNE CHECKPOINT INHIBITOR THERAPY
- ICIs are increasingly utilized as standard treatments in clinical settings, particularly for stage III locally advanced NSCLC and extensive-stage small cell lung cancer, with an emphasis on metastatic NSCLC. Future indications for its use are expected to expand. However, additional research is required to identify biomarkers that are more reliable than PD-L1 expression and that are readily usable in daily practice. Discovering a cost-effective, easily accessible predictive biomarker would significantly enhance patient stratification and management, ultimately improving overall patient care. Furthermore, advancements in understanding the mechanisms of resistance to ICIs and strategies to overcome them, along with pathological methods for prediction, are crucial for further progress in this field.
CONCLUSION
-
Ethics Statement
Not applicable.
-
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
-
Code Availability
Not applicable.
-
Author Contributions
Conceptualization: UC, HSP. Data curation: UC, SI. Funding acquisition: UC. Investigation: UC. Methodology: UC, SI, HSP. Project administration: UC. Resources: UC, SI, HSP. Supervision: UC. Validation: UC, SI, HSP. Visualization: UC. Writing—original draft: UC. Writing—review & editing: UC, SI, HSP. Approval of the final manuscript: all authors.
-
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
-
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF- 2022R1I1A1A01071569).
Notes
Acknowledgments
- 1. Kim HC, Jung CY, Cho DG, et al. Clinical characteristics and prognostic factors of lung cancer in Korea: a pilot study of data from the Korean Nationwide Lung Cancer Registry. Tuberc Respir Dis (Seoul) 2019; 82: 118–25. ArticlePubMedPMCPDF
- 2. Vital Statistics Division, Statistics Korea; Shin HY, Kim J, et al. Cause-of-death statistics in 2018 in the Republic of Korea. J Korean Med Assoc 2020; 63: 286–97. ArticlePDF
- 3. Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung 2020; 198: 897–907. ArticlePubMedPMCPDF
- 4. Lee GW. Current advances in the treatment of lung cancer with immune checkpoint inhibitors. J Korean Med Assoc 2021; 64: 333–41. ArticlePDF
- 5. Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res 2015; 21: 976–84. ArticlePubMedPDF
- 6. Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI. Immunotherapy in lung cancer: current landscape and future directions. Front Immunol 2022; 13: 823618.ArticlePubMedPMC
- 7. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. ArticlePubMedPMCPDF
- 8. Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022; 20: 497–530. PubMed
- 9. Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol 2019; 16: 341–55. ArticlePubMedPDF
- 10. Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest 2018; 154: 1416–23. ArticlePubMedPMC
- 11. Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res 2020; 8: 34.ArticlePubMedPMCPDF
- 12. Uruga H, Mino-Kenudson M. Predictive biomarkers for response to immune checkpoint inhibitors in lung cancer: PD-L1 and beyond. Virchows Arch 2021; 478: 31–44. ArticlePubMedPDF
- 13. Pabst L, Lopes S, Bertrand B, et al. Prognostic and predictive biomarkers in the era of immunotherapy for lung cancer. Int J Mol Sci 2023; 24: 7577.ArticlePubMedPMC
- 14. Kim H, Chung JH. PD-L1 testing in non-small cell lung cancer: past, present, and future. J Pathol Transl Med 2019; 53: 199–206. ArticlePubMedPMCPDF
- 15. Ullah A, Pulliam S, Karki NR, et al. PD-L1 over-expression varies in different subtypes of lung cancer: will this affect future therapies? Clin Pract 2022; 12: 653–71. ArticlePubMedPMC
- 16. Domblides C, Leroy K, Monnet I, et al. Efficacy of immune checkpoint inhibitors in lung sarcomatoid carcinoma. J Thorac Oncol 2020; 15: 860–6. ArticlePubMed
- 17. Sharma J, Borczuk A, Liu H, et al. P2.01-056 Distinct PD-L1 expression in different components of pulmonary sarcomatoid carcinoma and its association with MET mutation. Topic: immune mechanisms in thoracic cancer and targeted therapy. J Thoracic Oncol 2017; 12(1 Suppl):S819–20.
- 18. Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer 2020; 20: 1185.ArticlePubMedPMCPDF
- 19. Li H, van der Merwe PA, Sivakumar S. Biomarkers of response to PD-1 pathway blockade. Br J Cancer 2022; 126: 1663–75. ArticlePubMedPMCPDF
- 20. Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 2980–7. ArticlePubMedPMC
- 21. Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 2018; 17: 129.ArticlePubMedPMCPDF
- 22. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017; 12: 208–22. ArticlePubMed
- 23. Choi S, Cho SI, Ma M, et al. Artificial intelligence-powered programmed death ligand 1 analyser reduces interobserver variation in tumour proportion score for non-small cell lung cancer with better prediction of immunotherapy response. Eur J Cancer 2022; 170: 17–26. ArticlePubMed
- 24. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–71. ArticlePubMedPMCPDF
- 25. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 2019; 51: 27–41. ArticlePubMedPMC
- 26. Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12: 298–306. ArticlePubMedPDF
- 27. Liu X, Hogg GD, DeNardo DG. Rethinking immune checkpoint blockade: ‘beyond the T cell’. J Immunother Cancer 2021; 9: e001460.ArticlePubMedPMC
- 28. El Bairi K, Haynes HR, Blackley E, et al. The tale of TILs in breast cancer: a report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2021; 7: 150.PubMedPMC
- 29. Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer 1992; 28A: 859–64. ArticlePubMed
- 30. Luen SJ, Savas P, Fox SB, Salgado R, Loi S. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology 2017; 49: 141–55. ArticlePubMed
- 31. Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28: 105–13. ArticlePubMed
- 32. Salgado R, Denkert C, Demaria S, et al. The evaluation of tumorinfiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015; 26: 259–71. PubMed
- 33. Fuchs TL, Sioson L, Sheen A, et al. Assessment of tumor-infiltrating lymphocytes using International TILs Working Group (ITWG) system is a strong predictor of overall survival in colorectal carcinoma: a study of 1034 patients. Am J Surg Pathol 2020; 44: 536–44. PubMed
- 34. Ahn SG, Jeong J, Hong S, Jung WH. Current issues and clinical evidence in tumor-infiltrating lymphocytes in breast cancer. J Pathol Transl Med 2015; 49: 355–63. ArticlePubMedPMCPDF
- 35. Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 2014; 25: 1536–43. ArticlePubMed
- 36. Raskov H, Orhan A, Christensen JP, Gogenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer 2021; 124: 359–67. ArticlePubMedPMCPDF
- 37. Speiser DE, Chijioke O, Schaeuble K, Munz C. CD4(+) T cells in cancer. Nat Cancer 2023; 4: 317–29. ArticlePubMedPDF
- 38. Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4(+) T cells in cancer immunotherapy: new insights into old paradigms. Cancer Gene Ther 2021; 28: 5–17. ArticlePubMedPMCPDF
- 39. Gataa I, Mezquita L, Rossoni C, et al. Tumour-infiltrating lymphocyte density is associated with favourable outcome in patients with advanced non-small cell lung cancer treated with immunotherapy. Eur J Cancer 2021; 145: 221–9. ArticlePubMed
- 40. Lopez de Rodas M, Nagineni V, Ravi A, et al. Role of tumor infiltrating lymphocytes and spatial immune heterogeneity in sensitivity to PD-1 axis blockers in non-small cell lung cancer. J Immunother Cancer 2022; 10: e004440.ArticlePubMedPMC
- 41. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2015; 75: 2139–45. ArticlePubMedPMCPDF
- 42. Jiang Z, Zhou Y, Huang J. A combination of biomarkers predict response to immune checkpoint blockade therapy in non-small cell lung cancer. Front Immunol 2021; 12: 813331.ArticlePubMedPMC
- 43. Deutsch JS, Lipson EJ, Danilova L, et al. Combinatorial biomarker for predicting outcomes to anti-PD-1 therapy in patients with metastatic clear cell renal cell carcinoma. Cell Rep Med 2023; 4: 100947.ArticlePubMedPMC
- 44. Swisher SK, Wu Y, Castaneda CA, et al. Interobserver agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast cancer using methodology proposed by the International TILs Working Group. Ann Surg Oncol 2016; 23: 2242–8. ArticlePubMedPDF
- 45. Chen DS, Mellman I. Elements of cancer immunity and the cancerimmune set point. Nature 2017; 541: 321–30. ArticlePubMedPDF
- 46. Park S, Ock CY, Kim H, et al. Artificial intelligence-powered spatial analysis of tumor-infiltrating lymphocytes as complementary biomarker for immune checkpoint inhibition in non-small-cell lung cancer. J Clin Oncol 2022; 40: 1916–28. ArticlePubMedPMC
- 47. Backman M, Strell C, Lindberg A, et al. Spatial immunophenotyping of the tumour microenvironment in non-small cell lung cancer. Eur J Cancer 2023; 185: 40–52. ArticlePubMed
- 48. Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019; 19: 307–25. ArticlePubMedPDF
- 49. Colbeck EJ, Ager A, Gallimore A, Jones GW. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front Immunol 2017; 8: 1830.ArticlePubMedPMC
- 50. Trub M, Zippelius A. Tertiary lymphoid structures as a predictive biomarker of response to cancer immunotherapies. Front Immunol 2021; 12: 674565.PubMedPMC
- 51. Zhang Q, Wu S. Tertiary lymphoid structures are critical for cancer prognosis and therapeutic response. Front Immunol 2022; 13: 1063711.ArticlePubMedPMC
- 52. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020; 577: 549–55. PubMedPMC
- 53. Sautes-Fridman C, Verneau J, Sun CM, et al. Tertiary lymphoid structures and B cells: clinical impact and therapeutic modulation in cancer. Semin Immunol 2020; 48: 101406.ArticlePubMed
- 54. Fridman WH, Petitprez F, Meylan M, et al. B cells and cancer: to B or not to B? J Exp Med 2021; 218: e20200851.ArticlePubMedPMCPDF
- 55. Silina K, Rulle U, Kalnina Z, Line A. Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: a novel anti-cancer treatment avenue? Cancer Immunol Immunother 2014; 63: 643–62. ArticlePubMedPMCPDF
- 56. Rakaee M, Kilvaer TK, Jamaly S, et al. Tertiary lymphoid structure score: a promising approach to refine the TNM staging in resected non-small cell lung cancer. Br J Cancer 2021; 124: 1680–9. ArticlePubMedPMCPDF
- 57. Blanchard L, Girard JP. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis 2021; 24: 719–53. ArticlePubMedPMCPDF
- 58. Hong SA, Hwang HW, Kim MK, et al. High endothelial venule with concomitant high CD8+ tumor-infiltrating lymphocytes is associated with a favorable prognosis in resected gastric cancer. J Clin Med 2020; 9: 2628.ArticlePubMedPMC
- 59. Fang J, Lu Y, Zheng J, et al. Exploring the crosstalk between endothelial cells, immune cells, and immune checkpoints in the tumor microenvironment: new insights and therapeutic implications. Cell Death Dis 2023; 14: 586.ArticlePubMedPMCPDF
- 60. Milutinovic S, Abe J, Godkin A, Stein JV, Gallimore A. The dual role of high endothelial venules in cancer progression versus immunity. Trends Cancer 2021; 7: 214–25. ArticlePubMedPMC
- 61. Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 2017; 9: eaak9679.ArticlePubMedPMC
- 62. Ye D, Jin Y, Weng Y, et al. High endothelial venules predict response to PD-1 inhibitors combined with anti-angiogenesis therapy in NSCLC. Sci Rep 2023; 13: 16468.ArticlePubMedPMCPDF
- 63. Hussain B, Kasinath V, Ashton-Rickardt GP, et al. High endothelial venules as potential gateways for therapeutics. Trends Immunol 2022; 43: 728–40. ArticlePubMedPMC
- 64. Hazini A, Fisher K, Seymour L. Deregulation of HLA-I in cancer and its central importance for immunotherapy. J Immunother Cancer 2021; 9: e002899.ArticlePubMedPMC
- 65. Pagliuca S, Gurnari C, Rubio MT, Visconte V, Lenz TL. Individual HLA heterogeneity and its implications for cellular immune evasion in cancer and beyond. Front Immunol 2022; 13: 944872.ArticlePubMedPMC
- 66. Wang C, Xiong C, Hsu YC, Wang X, Chen L. Human leukocyte antigen (HLA) and cancer immunotherapy: HLA-dependent and -independent adoptive immunotherapies. Ann Blood 2020; 5: 14.Article
- 67. Chowell D, Morris LG, Grigg CM, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2018; 359: 582–7. PubMed
- 68. Wang H, Liu B, Wei J. Beta2-microglobulin(B2M) in cancer immunotherapies: Biological function, resistance and remedy. Cancer Lett 2021; 517: 96–104. ArticlePubMed
- 69. Gavrielatou N, Vathiotis I, Aung TN, et al. Digital spatial profiling links beta-2-microglobulin expression with immune checkpoint blockade outcomes in head and neck squamous cell carcinoma. Cancer Res Commun 2023; 3: 558–63. ArticlePubMedPMCPDF
- 70. Sade-Feldman M, Jiao YJ, Chen JH, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun 2017; 8: 1136.ArticlePubMedPMCPDF
- 71. Lechner MG, Karimi SS, Barry-Holson K, et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother 2013; 36: 477–89. ArticlePubMedPMC
- 72. Sabbatino F, Liguori L, Polcaro G, et al. Role of human leukocyte antigen system as a predictive biomarker for checkpoint-based immunotherapy in cancer patients. Int J Mol Sci 2020; 21: 7295.ArticlePubMedPMC
- 73. Ashizawa T, Iizuka A, Nonomura C, et al. Antitumor effect of programmed death-1 (PD-1) blockade in humanized the NOG-MHC double knockout mouse. Clin Cancer Res 2017; 23: 149–58. ArticlePubMedPDF
- 74. Ladanyi A, Hegyi B, Balatoni T, et al. HLA class I downregulation in progressing metastases of melanoma patients treated with ipilimumab. Pathol Oncol Res 2022; 28: 1610297.PubMedPMC
- 75. Pescia C, Pini G, Olmeda E, Ferrero S, Lopez G. TIGIT in lung cancer: potential theranostic implications. Life (Basel) 2023; 13: 1050.ArticlePubMedPMC
- 76. Yang Z, Peng Y, Xu J, et al. PVR/TIGIT and PD-L1/PD-1 expression predicts survival and enlightens combined immunotherapy in lung squamous cell carcinoma. Transl Oncol 2022; 24: 101501.ArticlePubMedPMC
- 77. Kim SW, Kim YI, Mustafa B, et al. Distribution pattern of tumor infiltrating lymphocytes and tumor microenvironment composition as prognostic indicators in anorectal malignant melanoma. Mod Pathol 2021; 34: 141–60. ArticlePDF
- 78. Aggarwal V, Workman CJ, Vignali DA. LAG-3 as the third checkpoint inhibitor. Nat Immunol 2023; 24: 1415–22. ArticlePubMedPDF
- 79. Sauer N, Szlasa W, Jonderko L, et al. LAG-3 as a potent target for novel anticancer therapies of a wide range of tumors. Int J Mol Sci 2022; 23: 9958.ArticlePubMedPMC
- 80. He Y, Yu H, Rozeboom L, et al. LAG-3 protein expression in nonsmall cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol 2017; 12: 814–23. ArticlePubMed
- 81. Borgeaud M, Sandoval J, Obeid M, et al. Novel targets for immunecheckpoint inhibition in cancer. Cancer Treat Rev 2023; 120: 102614.ArticlePubMed
- 82. Inoue Y, Yoshimura K, Kurabe N, et al. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget 2017; 8: 8738–51. ArticlePubMedPMC
- 83. Loi S, Pommey S, Haibe-Kains B, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 2013; 110: 11091–6. ArticlePubMedPMC
- 84. Wu XR, He XS, Chen YF, et al. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol 2012; 106: 130–7. ArticlePubMedPDF
- 85. Rocha P, Salazar R, Zhang J, et al. CD73 expression defines immune, molecular, and clinicopathological subgroups of lung adenocarcinoma. Cancer Immunol Immunother 2021; 70: 1965–76. ArticlePubMedPMCPDF
- 86. Bendell JC, LoRusso P, Overman MJ, et al. Safety and efficacy of the anti-CD73 monoclonal antibody (mAb) oleclumab ± durvalumab in patients (pts) with advanced colorectal cancer (CRC), pancreatic ductal adenocarcinoma (PDAC), or EGFR-mutant non-small cell lung cancer (EGFRm NSCLC). J Clin Oncol 2021; 39: 9047.Article
- 87. Jin D, Fan J, Wang L, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res 2010; 70: 2245–55. ArticlePubMedPMCPDF
- 88. Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 2006; 103: 13132–7. ArticlePubMedPMC
- 89. Ishii H, Azuma K, Kawahara A, et al. Predictive value of CD73 expression for the efficacy of immune checkpoint inhibitors in NSCLC. Thorac Cancer 2020; 11: 950–5. ArticlePubMedPMCPDF
- 90. Lee JB, Ha SJ, Kim HR. Clinical insights into novel immune checkpoint inhibitors. Front Pharmacol 2021; 12: 681320.ArticlePubMedPMC
References
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission