Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(2); 2012 > Article
-
Original Article
Clinicopathologic Features of IgA-Dominant Postinfectious Glomerulonephritis - Tai Yeon Koo, Gheun-Ho Kim, Moon Hyang Park1
-
Korean Journal of Pathology 2012;46(2):105-114.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.2.105
Published online: April 25, 2012
Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
1Department of Pathology, Hanyang University College of Medicine, Seoul, Korea.
- Corresponding Author: Moon Hyang Park, M.D. Department of Pathology, Hanyang University College of Medicine, 222 Wangsimni-ro, Seongdong-gu, Seoul 133-791, Korea. Tel: +82-2-2290-8249, Fax: +82-2-2296-7502, 'parkmh@hanyang.ac.kr'
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- IgA-dominant acute postinfectious glomerulonephritis (APIGN) is a recently recognized morphologic variant of APIGN, but its clinicopathologic features were not clearly characterized. We will present demographic, clinical and renal biopsy findings from seven patients with IgA-dominant APIGN with a literature review.
-
Methods
- All renal biopsy specimens (n=1,119) processed by the Department of Pathology in Hanyang University Hospital from 2005 to 2009 were reviewed. Seven patients with IgA-dominant APIGN were identified, and their clinical data analyzed.
-
Results
- All patients had renal failure, hematuria and proteinuria. One was diabetic, and none of the patients had previous renal diseases. Three had clinical infections at the time of presentation: 2 with methicillin-resistant Staphylococcus aureus and one with rickettsial infection. Light microscopically diffuse endocapillary proliferative and exudative glomerulonephritis was found in all cases. Immunofluorescence microscopy showed granular IgA deposits along peripheral capillary walls and in mesangium. Ultrastructurally, subepithelial 'humps' with mesangial deposits were noted. End-stage renal disease developed in two patients, chronic renal failure was stationary in two, and azotemia improved in three.
-
Conclusions
- Various infections including rickettsiosis preceded IgA-dominant APIGN in both diabetics and nondiabetics. Because the prognosis of IgA-dominant APIGN is poor, early diagnosis based on renal biopsy is required.
- All renal biopsies (n=1,119) processed by the Department of Pathology, Hanyang University Hospital, Seoul, Korea from January 2005 to December 2009 were reviewed retrospectively, and fifteen patients with diagnosis of APIGN were identified. The diagnosis of APIGN was made based on the presence of at least three of the known five criteria.14 We excluded patients with systemic lupus erythematosus, Henoch-Schönlein purpura or IgA nephropathy.15 Among 15 cases diagnosed as APIGN, seven cases showed glomerular dominant or codominant IgA deposits. The medical records of the patients were reviewed for age, gender, underlying diseases, type and cause of infection, clinical presentation, laboratory data including baseline and peak serum creatinine, serum C3 and C4 level, 24-hour urine protein, and urine analysis. We also investigated the modalities of treatment and clinical outcomes. We defined acute renal failure (ARF) as an increase of serum creatinine of at least 0.5 mg/dL above baseline values, and remission of ARF as the normalization of serum creatinine to baseline levels. End-stage renal disease (ESRD) was defined as requiring maintenance dialysis therapy.
- All 7 renal biopsy samples were processed according to standard techniques of LM, IF, and EM. For each patient, 6 slides which were stained with hematoxylin and eosin, Masson's trichrome, periodic acid-Schiff and Jones methenamine silver were reviewed. Systematic analysis on the morphologic changes of glomeruli, tubules, interstitium, and vessels was done according to the practical standardization in renal biopsy reporting.20 IF staining had been performed on 3-µm cryostat sections using polyclonal fluorescein-isothiocyanate-conjugated antibodies to IgG, IgM, IgA, C3, C1q, C4, kappa, lambda, fibrinogen and albumin (DakoCytomation, Glostrup, Denmark). The intensity of IF staining was graded on a scale of 0 to 3+. Samples were prepared for ultrastructural evaluation by standard techniques and examined under a Hitachi H-7600S electron microscope (Hitachi Ltd., Tokyo, Japan) at 80 kV.
MATERIALS AND METHODS
- Clinical features
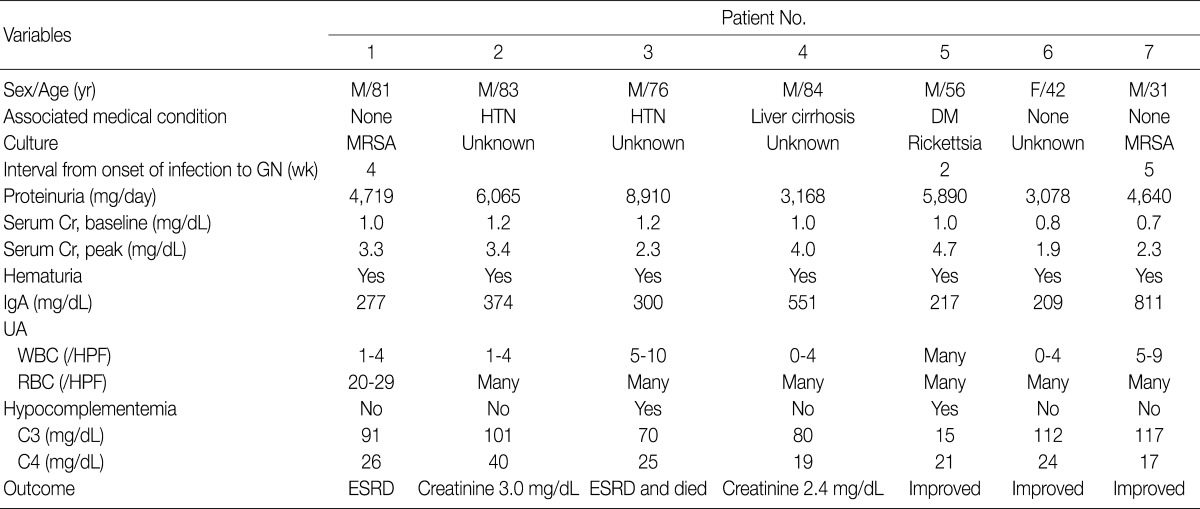
- The clinical features and demographic data of the patients diagnosed as having IgA-dominant PIGN are shown in Table 1. The patients consisted of 6 males and 1 female, with a mean age of 65 years (range, 31 to 84 years). All seven patients presented with ARF, proteinuria and hematuria. The time interval between onset of clinical infection and ARF was 2 to 5 weeks. Basal mean serum creatinine level was 1.0 mg/dL (range, 0.7 to 1.2 mg/dL) and this increased to 3.1 mg/dL (range, 1.9 to 4.7 mg/dL). Hypocomplementemia was present in 2 (patients 3 and 5) out of 7 patients. IgA levels were elevated in patient 4 (551 mg/dL; normal range, 82 to 453 mg/dL) and patient 7 (811 mg/dL). Patient 2 and 3 had hypertension, patient 4 alcoholic liver cirrhosis, and patient 5 diabetes mellitus without nephropathy. None of the patients had underlying renal diseases.
- Clinical infections were documented in three patients at the time of biopsy. Two patients (patient 1 and patient 7) had methicillin-resistant Staphylococcus aureus (MRSA) infections, and one patient (patient 5) had a rickettsial infection. The MRSA infections were infectious arthritis of the knee joint in patient 1 and pneumonia in patient 7. In the remaining 4 patients (patients 2, 3, 4, and 6), no pathogens had been detected.
- Pathology findings
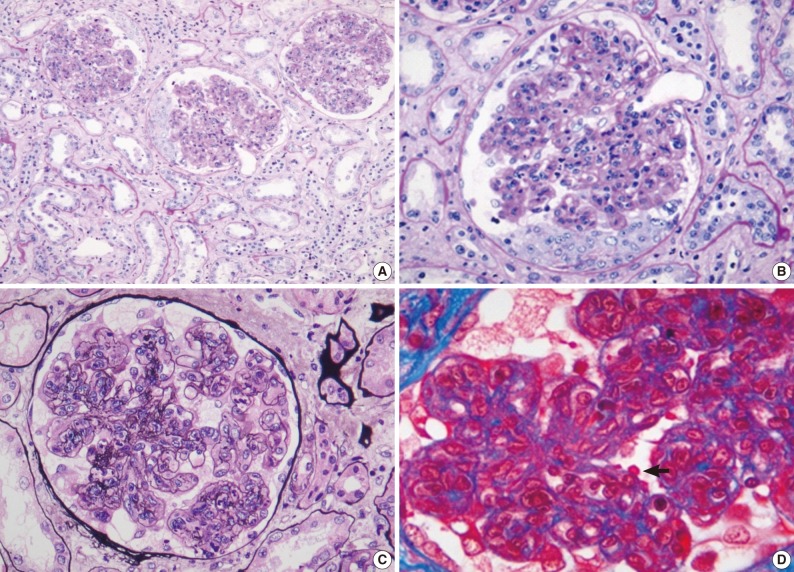
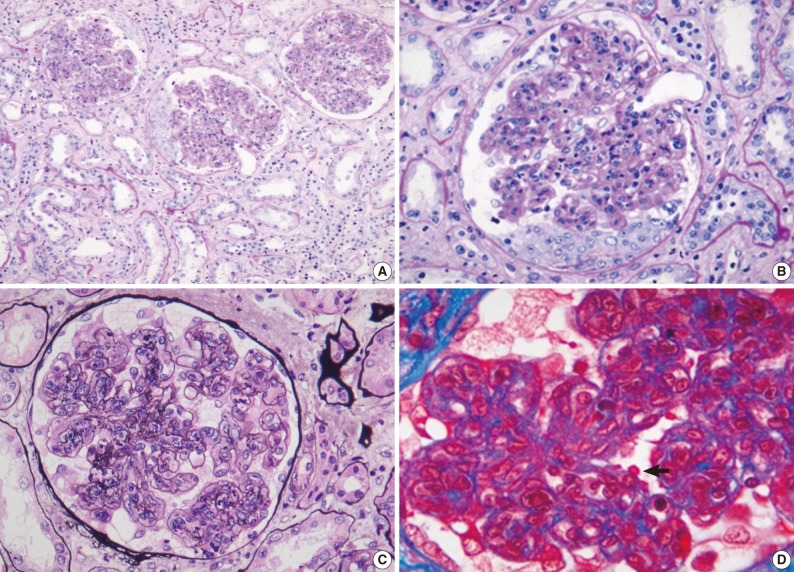
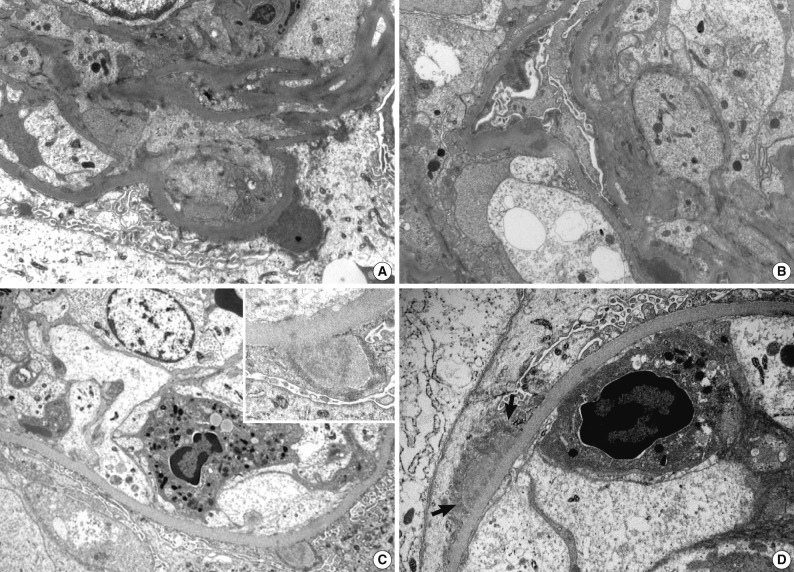
- Renal biopsy findings are summarized in Tables 2 and 3. All seven patients showed acute diffuse proliferative GN with diffuse endocapillary proliferation and prominent intracapillary infiltration of neutrophils and some mononucleated cells (Fig. 1). Small or circumferential cellular crescents associated with segmental fibrinoid necrosis were present in five patients (range, 9 to 67%, affected >50% of glomeruli in one patient), and there was evidence of fibrin thrombi in four cases. Varying degrees of global glomerulosclerosis (6-50%) were noted in four cases. None of the patients had features associated with diabetic nephropathy.
- All seven patients had some degree of interstitial inflammation, consisting predominantly of lymphocytes. Tubular atrophy and interstitial fibrosis were mild in six patients and moderate in the remaining patient (patient 6). Patient 6 also exhibited marked tubulointerstitial inflammation and granular casts, corresponding features associated with acute tubular injury. There was some degree of vascular change in most of the patients. Arterial intimal thickening was mild in five of the patients and moderate in one (patient 5), while mild arteriolosclerosis was noted in four patients (Table 2).
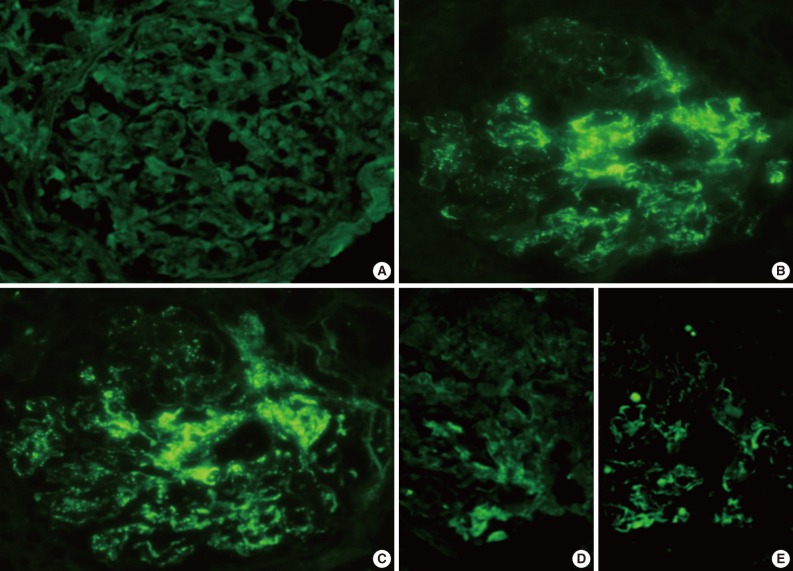
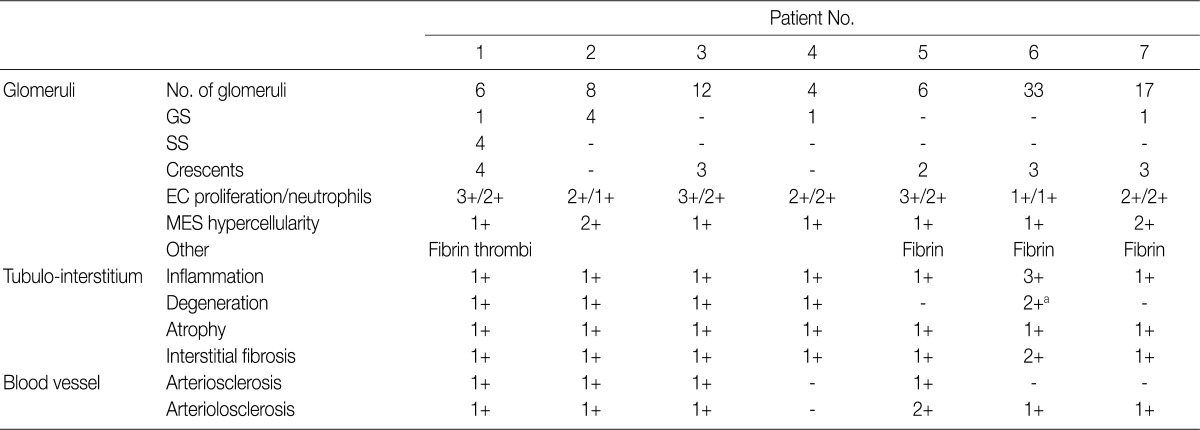
- The IF samples contained about 3 glomeruli (range, 1 to 8). IgA was the dominant class of immunoglobulin deposited in the glomeruli (intensity, 1+ to 3+) (Table 3), and there was a coarsely granular pattern of glomerular capillary wall and/or mesangial staining for IgA and C3 (Fig. 2). Most patients showed no IF staining for C1q and C4, but displayed variable intensities of mesangial and/or glomerular capillary wall staining for IgG. In five patients there was trace to weak IgM staining in the mesangium. In six patients staining for the κ and λ light chains was approximately the same (Table 3).
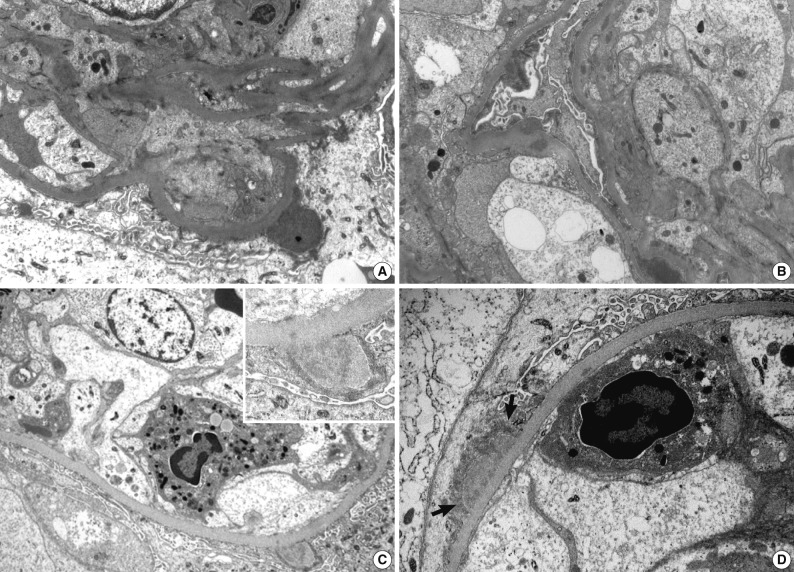
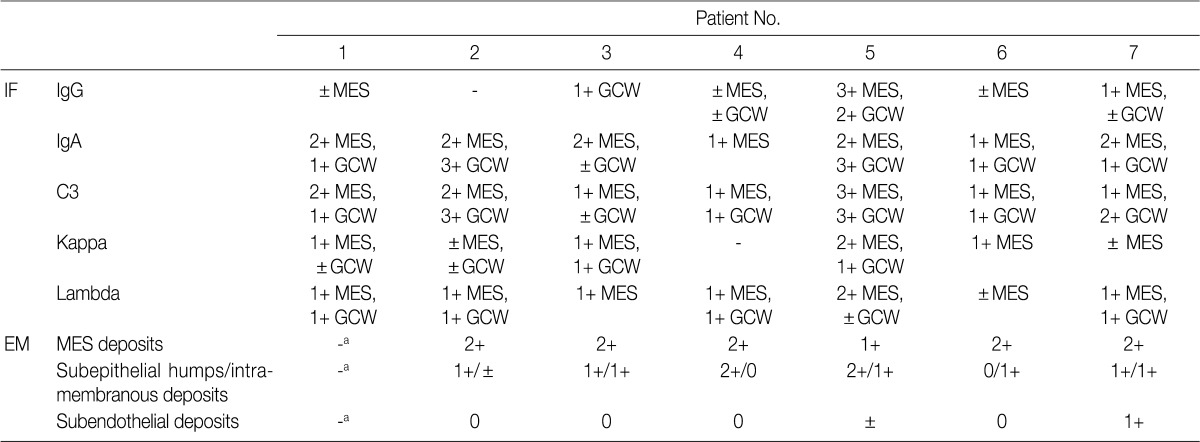
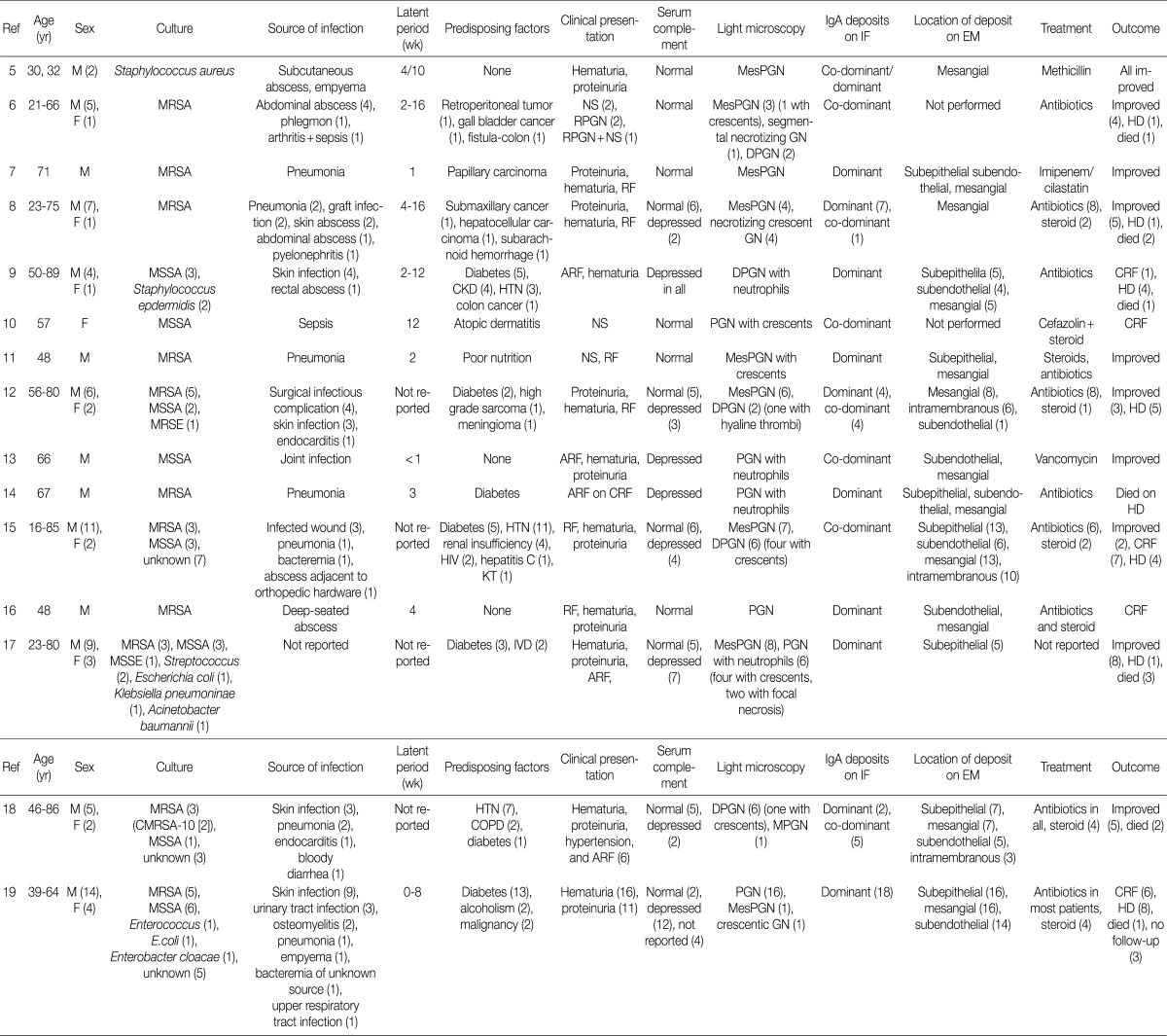
- Ultrastructural analysis was performed in all but one case (patient 1) in which the EM specimen contained no glomerulus. They had varying numbers of typical subepithelial 'humps' and/or intramembranous deposits as well as some mesangial electron dense deposits (Fig. 3). Some subepithelial deposits showed partial or extensive resorption with partial loss of electron density (Fig. 3C). Endocapillary hypercellularity and neutrophilic infiltration were prominent. Foot process effacement was mostly diffuse, estimating about 90% of the external capillary surface except for one (patient 6, 40%) (Table 3).
- Three patients (patients 1, 5, and 7) were treated with antibiotics because they had had definite episodes of infection. Patient 1 progressed to ESRD and had maintenance hemodialysis, while the renal failure in patient 5 improved in response to antibiotic therapy. Patient 7 did not respond to the antibiotics, but his renal function was improved after treatment with high-dose prednisolone. In contrast, patient 3, who was treated with corticosteroid and cyclophosphamide, showed no improvement in renal function and died of complications including necrotizing pneumonia and bacterial meningitis. The remaining three patients (patients 2, 4, and 6), who had had no definite episodes of infection, were conservatively treated. The renal failure in patients 2 and 4 was stable and stationary, whereas azotemia in patient 6 resolved itself.
RESULTS
Light microscopy
IF microscopy
Electron microscopy
Treatment and follow-up
- Recently, a unique form of PIGN characterized by IgA-dominant deposits has been increasingly recognized.5-19 At this time of writing, eight-five cases have been reported in the English literatures.5-19 Their clinical and pathological findings are summarized in Table 4.
- Here we presented seven patients with biopsy-proven IgA-dominant APIGN along with clinical and pathological findings. Four of them were over 60 years of age, and the average age was 65 years. In other reports, the average age at diagnosis was 60 years (reported range, 16 to 89 years), and 61% of the reported patients were 65 years or older.5-19 Thus, the elderly should be predisposed to IgA-dominant PIGN.19 Our patients also had a male predominance of 6:1, similar to other reports.5-19
- In our study, only one patient was diabetic and another had alcoholic liver cirrhosis. Previous studies reported that IgA-dominant APIGN was mainly associated with diabetes mellitus.9,12,14,15,17-19 Thus, diabetes was known to be the important risk factor for IgA-dominant APIGN. However, we showed that many non-diabetic patients could be exposed to IgA-dominant APIGN.
- The infectious agent was identified in 3 patients (43%) within the study, and Staphylococcus aureus was the most common (67%) cause of infection. It took from 2 to 5 weeks between the clinical onset of infection and the renal symptoms. In other studies, the average duration of the incubation period was estimated to be about 4 weeks.5-19 In some cases, the infection may be identified at the time of renal biopsy because infection was unrecognized for some time.21
- All of the patients had renal insufficiency, hematuria, and proteinuria at presentation. More than a half of them showed advanced azotemia (serum creatinine >3 mg/dL). In other reports, the peak serum creatinine ranged from 1.2 to 14.5 mg/dL (mean, 4.0 mg/dL).5-19 Also, all patients were nephrotic, having a mean 24-hour urine protein of 5.2 g. In contrast, previous studies reported 44% of patients having had nephrotic syndrome although proteinuria is a universal presenting feature (reported range, 0.15 to 15 g/day).5-19 Hypocomplementemia may be an important clue to diagnose PIGN. However, it was present in two patients (29%) in this study. Complement levels were not consistently depressed in other studies as well.5-8,10-12,15-19
- Antibiotic therapy is considered mainstay for IgA-dominant APIGN following episodes of infection,8 but it is not always successful. In this study, two of three patients who were treated with antibiotics did not respond to the therapy. One of them showed improvement in renal function after steroid therapy. Steroid treatment is a possible alternative for IgA-dominant APIGN presenting renal insufficiency when the renal function fails to improve after antibiotic therapy.8,11,15,18
- In this study, three patients (42%) had complete recovery of renal function with normalization of serum creatinine and decreasing proteinuria. Two patients (29%) had partial recovery with moderate reduction of proteinuria and serum creatinine. Two patients (29%) remained dialysis-dependent and one patient (14%) died. Before the 1990s, complete remission of PIGN was reported in 60-80% of the patients.21 According to the recent studies, however, it was reported in 43%.19 The prognosis of IgA-dominant PIGN is less favorable than that of typical PIGN.19-22
- Previous studies have shown that endocapillary proliferative and exudative GN is the predominant histological pattern of IgA-dominant PIGN (Table 4). Pure mesangial proliferative and crescentic GN were described in 34% and 6% of cases, respectively.5-19 All our patients had diffuse endocapillary proliferation, and five patients (71%) had small or circumferential cellular crescents with fibrinoid necrosis. On IF, IgA was the dominant immunoglobulin in all our patients, with or without weaker staining for IgG. There was high-intensity staining for C3 as well. This finding is a considerable contrast to IgA nephropathy, in which C3 staining is typically weaker than IgA staining.22 Ultrastructurally, mesangial electron dense deposits were seen in all our cases (100%), subepithelial deposits in five (83%), intramembranous in five (83%), and subendothelial in two (33%). One patient showed extensive resorption of subepithelial deposits. Subepithelial deposits, frequently exhibiting a hump-shaped appearance, were common. Subendothelial deposits were less frequent and small, consistent with other reports.5-19
- In this study, we documented for the first time an association between rickettsial infection and IgA-dominant APIGN. Rickettsial infection is considered as a possible cause of IgA-dominant APIGN, especially in areas where high incidences of rickettsial disease is apparent. The histological features of rickettsial infection generally include interstitial nephritis, vasculitis, acute tubular necrosis and occasionally, APIGN.23 Various humoral immune responses have been suggested to be associated with increased serum IgA levels or IgA-containing circulating immune complexes in rickettsial infection.24,25 In response to the rickettsial infection, interleukin (IL)-6 and IL-10 levels may be increased to stimulate plasma cell growth and differentiation.24,25
- The mechanism of selective IgA deposition in PIGN is unclear. It may be related to increased serum levels of IgA and IgA-containing circulating immune complexes, possibly as a result of subclinical mucosal infection or decreased IgA hepatic clearance caused by serum IgA1 hypersialylation.14,26 A role of superantigens has been suggested.6,7 Superantigens are proteins that may be detected in several pathogenic microbes including Staphylococcus. They are powerful activators of immune systems and stimulate the production of T cells along with a variety of cytokines including tumor necrosis factor-α. They also induce antibody production including IgA. Staphylococcal enterotoxins such as enterotoxin B and C can act as superantigens and have been identified in staphylococcal-associated GN.6,7 Interestingly, IgA-dominant PIGN may also develop following coagulase-negative Staphylococcus which does not produce enterotoxin, suggesting the potential pathogenic role for particular staphylococcal cell surface antigens.22 Regarding nonstaphylococcal pathogens, Endo et al.27 demonstrated that various strains of gram-negative bacteria including Pseudomonas aeruginosa, Escherichia coli, Hemophilus influenza, and Klebsiella pneumoniae, can induce glomerular deposition of IgA and C3 in animal studies. Many patients with IgA-dominant APIGN are diabetic.9,12,14,15,17-19 It is possible that diabetes plays a role in the provocation of an IgA-dominant immune response. Many studies have shown that diabetes have high levels of serum IgA and IgA-containing circulating immune complexes compared with non-diabetes.22 Proposed explanation for the high serum IgA levels in diabetes include decreased IgA hepatic clearance caused by serum IgA1 hypersialylation and increased synthesis of IgA in association with subclinical mucosal infection or as a potential immune response to advanced glycation endproduct.22
- IgA-dominant APIGN may be difficult to differentiate from IgA nephropathy. In several studies, IgA-dominant APIGN can be diagnosed with a high degree of certainty when having ARF at presentation, diffuse endocapillary proliferation with neutrophil infiltration, glomerular capillary wall, and/or when mesangial staining on IF and subepithelial humps are present.5-19,22 However when resolving lesions are present with mesangial proliferation associated with mesangial patterns of immune deposits and loss of subepithelial humps, it is difficult to distinguish IgA-dominant APIGN from IgA nephropathy. In our study, patient 6 had no history of infection and no subepithelial humps on EM. However she was diagnosed as IgA-dominant APIGN rather than IgA nephropathy. Her renal biopsy showed a typical finding of APIGN, particularly in early resolving APIGN that there were IgA and C3 deposits along glomerular capillary wall and mesangium, and localized electron dense deposits on notch regions overlying mesangial areas. The histological findings largely depend on the timing of renal biopsies. The acute phase of APIGN may be subclinical, and detected only on renal biopsy performed enthusiastically because of asymptomatic renal failure or microscopic urinary abnormalities.
- In summary, extensive use of renal biopsy has demonstrated the presence of an atypical feature of APIGN, glomerular IgA-dominant deposition. It is most common in the elderly and occurs in both diabetics and non-diabetics. It typically occurs in association with staphylococcal infections, and presents with renal failure, hematuria, and proteinuria. Hypocomplementemia may be an important clue to the diagnosis of PIGN, but inconsistently present. Antibiotic therapy is considered mainstay, and steroid therapy may be a possible alternative when IgA-dominant APIGN is accompanied by renal insufficiency and does not respond to antibiotic treatments. Prognosis is less favorable than typical APIGN. Histologically, most cases exhibit endocapillary hypercellularity and neutrophil infiltration. In renal biopsies, IgA is the dominant or co-dominant immunoglobulin deposited in the glomeruli. Rickettsial infection can cause IgA-dominant APIGN and should be considered as a possible cause of APIGN in areas where high incidence of rickettsial disease occur. Greater awareness of the presentation and course of IgA-dominant APIGN is necessary to ensure prompt diagnosis and treatment.
DISCUSSION
- 1. Montseny JJ, Meyrier A, Kleinknecht D, Callard P. The current spectrum of infectious glomerulonephritis: experience with 76 patients and review of the literature. Medicine (Baltimore) 1995; 74: 63–73. PMID: 7891544. ArticlePubMed
- 2. Nasr SH, Markowitz GS, Stokes MB, Said SM, Valeri AM, D'Agati VD. Acute postinfectious glomerulonephritis in the modern era: experience with 86 adults and review of the literature. Medicine (Baltimore) 2008; 87: 21–32. PMID: 18204367. ArticlePubMed
- 3. Keller CK, Andrassy K, Waldherr R, Ritz E. Postinfectious glomerulonephritis: is there a link to alcoholism? Q J Med 1994; 87: 97–102. PMID: 8153294. PubMed
- 4. Nadasdy T, Silva FG. In : Jennette JC, Olson JL, Schwartz MM, Silva FG, eds. Acute postinfectious glomerulonephritis and glomerulonephritis caused by persistent bacterial infection. Heptinstall's pathology of the kidney. 2009; 6th ed. Philadelphia: Lippincott Williams & Wilkins, 321–396.
- 5. Spector DA, Millan J, Zauber N, Burton J. Glomerulonephritis and Staphylococcal aureus infections. Clin Nephrol 1980; 14: 256–261. PMID: 7226584. PubMed
- 6. Koyama A, Kobayashi M, Yamaguchi N, et al. Glomerulonephritis associated with MRSA infection: a possible role of bacterial superantigen. Kidney Int 1995; 47: 207–216. PMID: 7731148. ArticlePubMed
- 7. Yoh K, Kobayashi M, Hirayama A, et al. A case of superantigen-related glomerulonephritis after methicillin-resistant Staphylococcus aureus (MRSA) infection. Clin Nephrol 1997; 48: 311–316. PMID: 9403216. PubMed
- 8. Nagaba Y, Hiki Y, Aoyama T, et al. Effective antibiotic treatment of methicillin-resistant Staphylococcus aureus-associated glomerulonephritis. Nephron 2002; 92: 297–303. PMID: 12218306. ArticlePubMed
- 9. Nasr SH, Markowitz GS, Whelan JD, et al. IgA-dominant acute poststaphylococcal glomerulonephritis complicating diabetic nephropathy. Hum Pathol 2003; 34: 1235–1241. PMID: 14691907. ArticlePubMed
- 10. Handa T, Ono T, Watanabe H, Takeda T, Muso E, Kita T. Glomerulonephritis induced by methicillin-sensitive Staphylococcus aureus infection. Clin Exp Nephrol 2003; 7: 247–249. PMID: 14586723. ArticlePubMed
- 11. Kai H, Shimizu Y, Hagiwara M, et al. Post-MRSA infection glomerulonephritis with marked Staphylococcus aureus cell envelope antigen deposition in glomeruli. J Nephrol 2006; 19: 215–219. PMID: 16736424.
- 12. Satoskar AA, Nadasdy G, Plaza JA, et al. Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol 2006; 1: 1179–1186. PMID: 17699345. ArticlePubMed
- 13. Long JA, Cook WJ. IgA deposits and acute glomerulonephritis in a patient with staphylococcal infection. Am J Kidney Dis 2006; 48: 851–855. PMID: 17060008. ArticlePubMed
- 14. Nasr SH, Share DS, Vargas MT, D'Agati VD, Markowitz GS. Acute poststaphylococcal glomerulonephritis superimposed on diabetic glomerulosclerosis. Kidney Int 2007; 71: 1317–1321. PMID: 17311069. ArticlePubMed
- 15. Haas M, Racusen LC, Bagnasco SM. IgA-dominant postinfectious glomerulonephritis: a report of 13 cases with common ultrastructural features. Hum Pathol 2008; 39: 1309–1316. PMID: 18619648. ArticlePubMed
- 16. Okuyama S, Wakui H, Maki N, et al. Successful treatment of post-MRSA infection glomerulonephritis with steroid therapy. Clin Nephrol 2008; 70: 344–347. PMID: 18826861. ArticlePubMed
- 17. Wen YK, Chen ML. Discrimination between postinfectious IgA-dominant glomerulonephritis and idiopathic IgA nephropathy. Ren Fail 2010; 32: 572–577. PMID: 20486840. ArticlePubMed
- 18. Worawichawong S, Girard L, Trpkov K, Gough JC, Gregson DB, Benediktsson H. Immunoglobulin A-dominant postinfectious glomerulonephritis: frequent occurrence in nondiabetic patients with Staphylococcus aureus infection. Hum Pathol 2011; 42: 279–284. PMID: 21111456. ArticlePubMed
- 19. Nasr SH, Fidler ME, Valeri AM, et al. Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol 2011; 22: 187–195. PMID: 21051737. ArticlePubMed
- 20. Jin SY, Jeong HJ, Sung SH, et al. Practical standardization in renal biopsy reporting. Korean J Pathol 2010; 44: 613–622. Article
- 21. Kanjanabuch T, Kittikowit W, Eiam-Ong S. An update on acute postinfectious glomerulonephritis worldwide. Nat Rev Nephrol 2009; 5: 259–269. PMID: 19384327. ArticlePubMed
- 22. Nasr SH, D'Agati VD. IgA-dominant postinfectious glomerulonephritis: a new twist on an old disease. Nephron Clin Pract 2011; 119: c18–c25. PMID: 21659781. ArticlePubMed
- 23. Kim DM, Kang DW, Kim JO, et al. Acute renal failure due to acute tubular necrosis caused by direct invasion of Orientia tsutsugamushi. J Clin Microbiol 2008; 46: 1548–1550. PMID: 18003808. ArticlePubMedPMC
- 24. Park DS, Cho JH, Lee JH, Lee KE. Clinical course of monoclonal and oligoclonal gammopathies in patients infected with Orientia tsutsugamushi. Am J Trop Med Hyg 2009; 81: 660–664. PMID: 19815883. ArticlePubMed
- 25. Cho JH, Park DS. Incidence and type of monoclonal or biclonal gammopathies in scrub typhus. Korean J Lab Med 2009; 29: 116–121. PMID: 19411777. ArticlePubMed
- 26. Vázquez-Moreno L, Candia-Plata MC, Robles-Burgueño MR. Hypersialylated macromolecular serum immunoglobulin A1 in type 2 diabetes mellitus. Clin Biochem 2001; 34: 35–41. PMID: 11239513. ArticlePubMed
- 27. Endo Y, Kanbayashi H, Hara M. Experimental immunoglobulin A nephropathy induced by gram-negative bacteria. Nephron 1993; 65: 196–205. PMID: 8247180. ArticlePubMed
References
M, male; MesPGN, mesangial proliferative glomerulonephritis; F, female; MRSA, methicillin-resistant Staphylococcus aureus; NS, nephrotic syndrome; GN, glomerulonephritis; RPGN, rapidly progressive glomerulonephritis; DPGN, diffuse proliferative (mesangial and endocapillary) glomerulonephritis; HD, hemodialysis; MSSA, methicillin-sensitive Staphylococcus aureus; CKD, chronic kidney disease; HTN, hypertension; ARF, acute renal failure; CRF, chronic renal failure; PGN, endocapillary proliferative glomerulonephritis; MRSE, methicillin-resistant Staphylococcus epidermidis; HIV, human immunodeficiency virus; KT, kidney transplantation; MSSE, methicillin-sensitive Staphylococcus epidermidis; IVD, iv drug use; CMRSA, community-associated methicillin-resistant Staphylococcus aureus-10; COPD, chronic obstructive pulmonary disease.
Figure & Data
References
Citations

- Staphylococcus aureus Infection-Related Glomerulonephritis with Dominant IgA Deposition
Mamiko Takayasu, Kouichi Hirayama, Homare Shimohata, Masaki Kobayashi, Akio Koyama
International Journal of Molecular Sciences.2022; 23(13): 7482. CrossRef - A rare case of Immunoglobulin A dominant post-infectious glomerulonephritis (IgA PIGN) in a young patient
A. Saghar, G. Klaus, B. Trutnau, M. Kömhoff, H. J. Gröne, S. Weber
BMC Nephrology.2022;[Epub] CrossRef - IgA-Dominant Infection-Associated Glomerulonephritis Following SARS-CoV-2 Infection
Aurora Pérez, Isidro Torregrosa, Luis D’Marco, Isabel Juan, Liria Terradez, Miguel Ángel Solís, Francesc Moncho, Carmen Carda-Batalla, María J. Forner, Jose Luis Gorriz
Viruses.2021; 13(4): 587. CrossRef - Relationship between blood neutrophil‐lymphocyte ratio and renal tubular atrophy/interstitial fibrosis in IgA nephropathy patients
Lingxiong Chai, Kedan Cai, Kaiyue Wang, Qun Luo
Journal of Clinical Laboratory Analysis.2021;[Epub] CrossRef - The Continuing Need for Electron Microscopy in Examination of Medical Renal Biopsies: Examples in Practice
Michifumi Yamashita, Mercury Y. Lin, Jean Hou, Kevin Y.M. Ren, Mark Haas
Glomerular Diseases.2021; 1(3): 145. CrossRef - Clinicopathologic features of infection-related glomerulonephritis with IgA deposits: a French Nationwide study
Elodie Miquelestorena-Standley, Charlotte Jaulerry, Marie-Christine Machet, Nolwenn Rabot, Christelle Barbet, Aurélie Hummel, Alexandre Karras, Cyril Garrouste, Thomas Crepin, Didier Ducloux, Maud Cousin, Catherine Albert, Joseph Rivalan, Emilie Cornec-Le
Diagnostic Pathology.2020;[Epub] CrossRef - IgA nephropathy and infections
Cristiana Rollino, Gisella Vischini, Rosanna Coppo
Journal of Nephrology.2016; 29(4): 463. CrossRef - <i>Staphylococcus</i>-associated Glomerulonephritis
Dong Yeol Shin, Sung Han Kim, Ji Wan Lee, Ki Ju Chang, Seung Ha Hwang, Yong Mee Cho, Soon Bae Kim
The Korean Journal of Medicine.2016; 90(2): 148. CrossRef - Use of steroid therapy in immunoglobulin A-dominant poststaphylococcal glomerulonephritis
Mahesh Eswarappa, Vijay Varma, K.C. Gurudev
Hong Kong Journal of Nephrology.2015; 17(2): 46. CrossRef - Clinicopathologic Features of IgA-Dominant Infection-Associated Glomerulonephritis: A Pooled Analysis of 78 Cases
Ru Bu, Qian Li, Zhi-yu Duan, Jie Wu, Pu Chen, Xiang-mei Chen, Guang-yan Cai
American Journal of Nephrology.2015; 41(2): 98. CrossRef - Garland-pattern postinfectious glomerulonephritis with IgA-dominant deposition
Makoto Kanno, Kenichi Tanaka, Hiroshi Kimura, Kimio Watanabe, Yoshimitsu Hayashi, Koichi Asahi, Masaaki Nakayama, Kensuke Joh, Tsuyoshi Watanabe
CEN Case Reports.2014; 3(1): 56. CrossRef

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article