Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(6); 2012 > Article
-
Original Article
Construction of High-Density Tissue Microarrays at Low Cost by Using Self-Made Manual Microarray Kits and Recipient Paraffin Blocks - Chang Hwan Choi, Kyu Ho Kim, Ju Young Song, Suk Jin Choi, Lucia Kim, In Suh Park, Jee Young Han, Joon Mee Kim, Young Chae Chu
-
Korean Journal of Pathology 2012;46(6):562-568.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.562
Published online: December 26, 2012
Department of Pathology, Inha University Hospital, Inha University College of Medicine, Incheon, Korea.
- Corresponding Author: Suk Jin Choi, M.D. Department of Pathology, Inha University Hospital, Inha University College of Medicine, 27 Inhang-ro, Jung-gu, Incheon 400-711, Korea. Tel: +82-32-890-3972, Fax: +82-32-890-3464, 'sukjinchoi007@gmail.com'
• Received: August 17, 2012 • Revised: November 6, 2012 • Accepted: November 14, 2012
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Advances of tissue microarray (TMA) technology have enabled simultaneous in situ analysis of biomarker expression in a large number of archived pathology specimens. However, the relatively high cost of TMA construction may hamper many researchers from using this essential tool of modern pathology research. We discuss methods for making TMA kits and recipient blocks for manual construction of high-density TMAs at low cost.
-
Methods
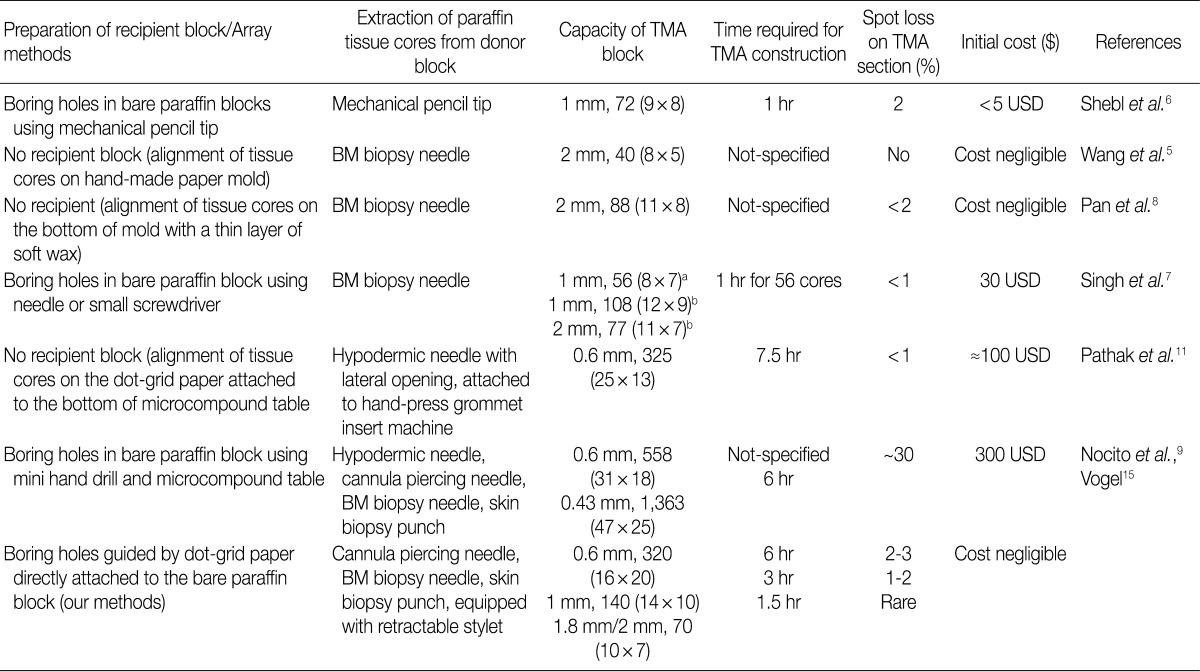
- Ordinary cannula piercing needles, hypodermic needles, bone marrow biopsy needles, metallic ink cartridges of ballpoint pens, and disposable skin biopsy punches were used to construct self-made manual TMA kits. The recipient blocks were manufactured by boring holes in the conventional bare paraffin blocks. A mini electric hand drill and a microcompound table assembled on a drill stand were used to maximize the capacity of the recipient blocks.
-
Results
- By using TMA kits made from cannula piercing needles (16- and 18-gauge), it was possible to construct TMAs with 1 mm×140 cores, 0.6 mm×320 cores, 2 mm×70 cores, 3 mm×35 cores, and 5 mm×12 cores. The capacity of the recipient blocks could be dramatically increased by drilling holes.
-
Conclusions
- Construction of TMAs using self-made TMA kits is an inexpensive alternative to construction of TMAs using commercial devices.
- Self-made manual TMA kits with punch extractor equipped with retractable stylet
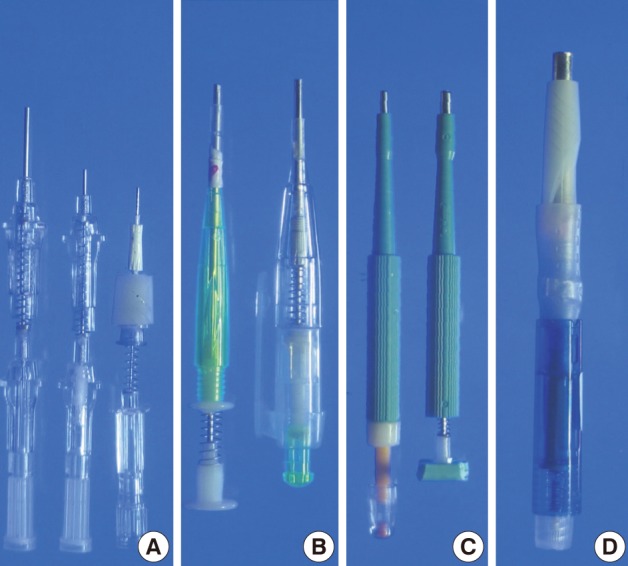
- We used ordinary cannula piercing needles, metallic ink cartridges of ballpoint pens, ordinary skin biopsy punches, and a bone marrow biopsy needle to make self-made manual TMA kits, which consisted of a punch extractor equipped with a retractable stylet (Fig. 1).
- The ordinary cannula piercing needles were cut to the appropriate length so that they could be used as punch extractors to punch out paraffin cores (16-gauge needle for 1 mm, 18-gauge needle for 0.6 mm) from paraffin blocks. To remove the paraffin cores lodged inside the 16-gauge piercing needle, we used a stylet made from an 18-gauge cannula piercing needle which was blunted and plugged with ordinary commercial silicone adhesives in advance. A 22-gauge cannula piercing needle was modified in the same way to be used for a stylet for punch extractor made from the 18-gauge cannula piecing needle. A 22-gauge hypodermic needle (inner diameter 0.5 mm) was modified to make a punch extractor. The stylet for the 22-gauge hypodermic needle was made from a 24-gauge cannula piercing needle.
- To construct a TMA kit for a 1.8 mm core diameter, a cannula for 11-gauge bone marrow biopsy needle kit was cut shorter and sharpened to be used as a hollow needle to punch paraffin cores. A trocar-tip needle from a bone marrow biopsy needle kit was cut and blunted to be used as a stylet for the punch extractor made from the cannula. Metallic ink cartridges from ballpoint pens with an inner diameter of 2 mm and 5 mm were modified to be used as punch extractors for 2 mm and 5 mm core diameters, respectively. A short segment of plastic tube (outer diameter about 4.5 mm) and a cotton swab were used to construct a stylet for a punch extractor made from the metallic ink cartridge with a 5 mm inner diameter. Cotton from a cotton swab was dampened with liquid paraffin and was used to plug the plastic tube. Ordinary springs from the ordinary ballpoint pens were used to keep the stylet withdrawn while the self-made punch extractors were empty. Plastic body parts of ordinary retractable ballpoint pens were used to house the punch extractor with the stylet and the spring. Disposable skin biopsy punches (2 mm or 3 mm diameter) were modified to have retractable stylets made from the trocar-tip needle of the bone marrow biopsy needle kits and springs from the ballpoint pens.
- Self-made recipient paraffin blocks with high capacity
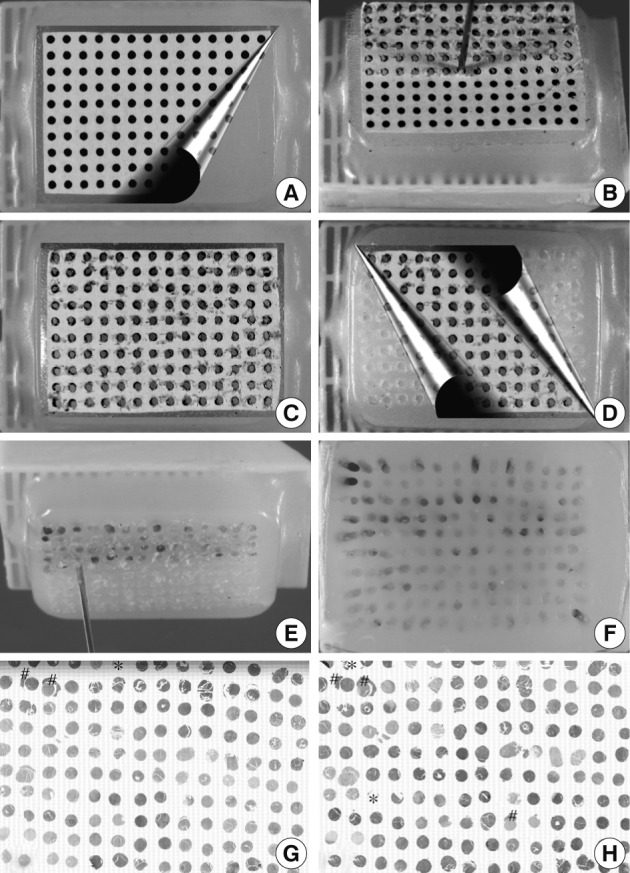
- The conventional bare paraffin blocks were used to make recipient blocks. The bare paraffin blocks were prepared by using the melt paraffin, plastic cassettes, and conventional metal block mold that are routinely used in a histology lab. To construct self-made recipient paraffin blocks for a core diameter of 1 mm and 0.6 mm, the conventional bare paraffin blocks were bored to make holes using a 18-gauge cannula piercing needle and 22-gauge hypodermic needle, respectively. As a guide for boring the holes, dot-grid paper was attached to the surface of the bare paraffin block with a double-faced adhesive tape (Fig. 2A-D, 3A-D); The grid paper with regularly aligned dots was designed and printed on plain paper using Graph Paper Printer software ver. 4.0, a free software designed by Dr. Phillippe Marquis (Metz, France; available from: http://graph-paper-printer.en.softonic.com/).
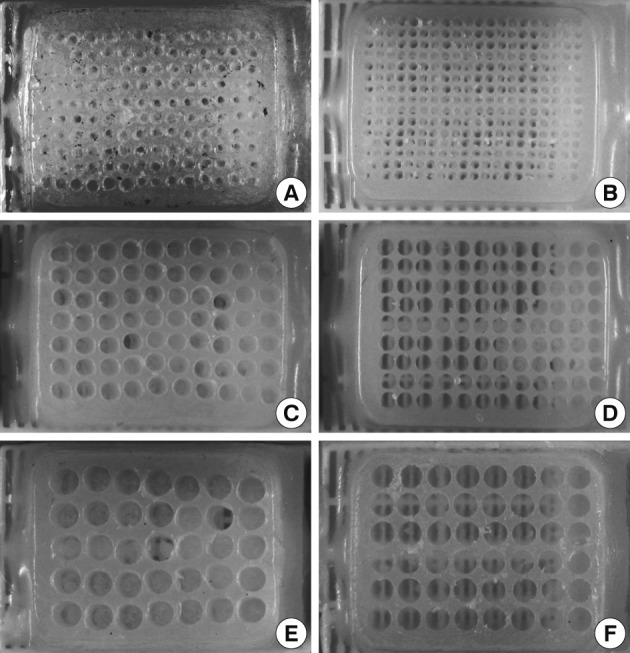
- To prepare the recipient paraffin blocks for the paraffin cores of larger diameters, the holes were bored using self-made punch extractors constructed from a cannula (for 1.8 mm cores) of an 11-gauge bone marrow biopsy needle kit, disposable skin biopsy punches (for 2 mm and 3 mm cores), and ballpoint pen metallic ink cartridges (for 2 mm and 5 mm cores). In order to maximize the capacity of the recipient blocks with holes in precise coordinates, we have used methods described by Vogel and Bueltmann.10 Conventional bare paraffin blocks were drilled to bore holes using a mini electric hand drill (Boramae mini drill, model DS-2000CH, Dongsung Electronics, Seoul, Korea; cost, US $50 including shipping fee) assembled on a mini drill stand (MICROMOT Drill Stand MB 140/S, Proxxon, Föhren, Germany; cost, US $80.00 including shipping fee). A microcompound table (K70, Proxxon; cost, US $140 including shipping fee) mounted on a drill stand was used for precise coordinate drilling. A cassette clamp from a microtome was used to hold the bare paraffin block while it was being drilled. The drill bits of various diameters (0.6, 1, 1.8, 2, and 3 mm; cost, US $1.00-2.00 each) were purchased from a local hardware shop.
- Construction of high density and low-cost TMAs
- We used redundant tissue from human tonsils to make donor paraffin blocks. Redundant tissue of female breast and malignant melanoma was also used to make donor paraffin blocks. The punch extractors were used to punch out paraffin tissue cores from donor paraffin blocks. The paraffin tissue cores were gently injected into the holes in the recipient blocks using the stylets. On completion of the assembly of each TMA, a clean microscope slide was attached to the face of the TMA block to apply firm yet gentle pressure in order to press down any protrusion from the surface of the block. To facilitate the integration of the donor tissue cores into the recipient block, the block was placed in a 50℃ oven for 60 minutes or in a 60℃ oven for 10 minutes face down on a microscope slide. Then, the irregularity of the block surface was flattened by applying gentle and even pressure to the microscope slide. The sections of the TMA blocks were routinely processed for hematoxylin and eosin (H&E) stain and CD20-immunostain.
MATERIALS AND METHODS
- By using self-made TMA kits and recipient paraffin blocks, we successfully constructed self-made high density TMAs with varying core diameters (0.6 mm, 1 mm, 2 mm, 3 mm, and 5 mm) at low cost. The arrays were fairly well established in terms of alignment of the tissue cores, so that we were able to appreciate the histologic and immunohistochemical features of the TMA sections without substantial difficulty. When we used self-made manual TMA kits made from cannula piercing needles, it was possible to construct TMAs that had as many as 140 cores (1 mm, 14×10 grid) and 320 cores (0.6 mm, 20×16 grid). There was no substantial (less than 2% and 3%, respectively) loss of tissue cores on 100 consecutive TMA sections for H&E or immunohistochemical staining. This degree of spot loss on sections is comparable to that described in the literature.4-9 We initially experienced some cross contamination between tissue cores (Figs. 2E, F, 3E, F), which was preventable by more thorough cleaning the needles. By using TMA kits made from bone marrow biopsy needles, disposable skin biopsy punches and metallic ink cartridges of ballpoint pens, we were able to construct TMAs that had 70 tissue cores (2 mm, 10×7 grid), 35 cores (3 mm, 7×5 grid), and 12 holes (5 mm, 4×3 grid). Compared to a conventional commercial manual TMA kit (tissue array kit, Microdigital, Gunpo, Korea) that we have previously used for construction of small-format TMAs with a 2 mm core diameter, our self-made manual TMA kits did not cause noticeable damage to the paraffin blocks when they were punched out; the self-made kit with a punch extractor made from the metallic ink cartridges (5 mm diameter) of ballpoint pens caused minor cracks in the paraffin block due to the thickness of the wall of metallic ink cartridge. By drilling to bore holes in conventional bare paraffin blocks to make self-made recipient blocks, we successfully constructed recipient blocks for 1 mm×320 holes in a 20×16 grid, 1.8 mm×140 holes in a 14×10 grid, 2 mm×108 holes in a 12×9 grid, and 3 mm×48 holes in a 8×6 grid without significant damage to the recipient paraffin blocks (Fig. 4).
RESULTS
- The TMA is one of the essential tools in biomedical research that requires large-scale, high-throughput in situ analysis of biomarker expression using archived oncologic specimens which encompass immunohistochemistry, in situ hybridization, and in situ reverse transcription polymerase chain reaction.9 The high-cost for high-density TMA construction, however, deters some researchers in small institutions saddled with limited budgets or insufficient manpower from using commercial automated or semiautomatic tissue microarrayers for construction of TMAs. An easy and relatively inexpensive alternative is manual construction of TMAs using commercial manual TMA instruments such as Quick Ray (Unitma, Seoul, Korea), costing more than US $3,500. The Quick Ray consists of punch extractors with spring action and changeable punch tips of different diameters (1, 1.5, 2, 3, and 5 mm). Also commercially available are array mold kits (Untima; each costs, US $700-800) that can be used to make precast recipient paraffin blocks for variable hole diameters.
- An alternative to using expensive commercial devices or kits for TMA construction is manual construction TMAs using self-made manual TMA kits and self-made recipient blocks. No special skill was needed to manipulate the needles or biopsy kits to craft the manual TMA kits equipped with retractable stylets. It was easy and quick to assemble each manual TMA kit after a short period of practice (about 30 minutes for each). In addition, the initial total cost for TMA construction using our manual TMA kits was negligible (Table 1). Differing from those that have been described in the literature,4-8 the self-made manual TMA kits we have designed and constructed are equipped with spring action to keep the stylets withdrawn while the punch extractors are empty. The spring action for retractable stylets makes it easy to periodically clean any residual paraffin from the punch extractors to prevent cross contamination between tissue cores from different donor blocks.
- To make data collection and analysis of biomarker expression easy, it is a prerequisite to construct a TMA that has tissue cores in well-aligned coordinate axes. By using sheets of dot-grid papers to guide boring holes in the bare paraffin block, it was possible for us to construct self-made recipient blocks for construction of TMAs with 0.6 mm×320 cores and 1 mm×140 holes in a precise spacing pattern. It seems to us that these capacities and tissue core diameters of the TMAs are satisfactory relative to the cost. It has been suggested that construction of TMAs containing duplicates of donor tissue with core diameters of a 0.6 or 1 mm is optimal in that the density of tissue cores in the TMAs could be maximized without severe damage to the donor blocks.1,2,9 We initially experienced cracking of TMA blocks during cutting for H&E staining and immunohistochemical staining, which was prevented by securing a sufficient edge free of tissue cores (about 3 mm in width) in the TMA block. It is arguable that our method for construction of a high-density TMA is labor intensive. However, we believe that our method could be a basic alternative to expensive arrayers or commercial TMA kits in laboratories with limited funding for TMA construction.
- In the manual construction of TMAs containing 2 mm tissue cores we used a self-made punch extractor constructed from an 11-gauge bone marrow biopsy needle (the inner diameter of which was 1.8 mm) to make holes in the recipient blocks. In our experience, the paraffin tissue cores taken from donor blocks by using a 2 mm skin biopsy punch just fitted the holes bored by using the punch extractor with inner diameter of 1.8 mm. In this way, we could prevent crescentic gaps between the holes in the recipient paraffin and donor tissue cores which can cause dropouts of tissue cores during the cutting of TMA blocks. On the other hand, when we used the 2 mm skin biopsy punch both to make holes in the recipient blocks and to take paraffin tissue cores from the donor blocks, complete integration of donor paraffin tissue cores into the recipient block was not possible because the actual diameter of the holes in the recipient block are apt to be slightly larger than the diameter of the donor tissue cores. This resulted in substantial loss of tissue cores during the sectioning of TMA blocks for H&E and immunohistochemical staining.
- Manual construction of TMAs without using recipient blocks has also been described, in which tissue cores are attached to the bottom of the block mold using double-sided adhesive tape on a thin layer of soft wax.5,8,11,12 In this method, all the tissue cores from the donor blocks are vertically attached to the bottom of the block mold before melted paraffin is poured in to fill the residual spaces. However, the size of the cores should be large enough that they can be firmly attached to the adhesive tape. Another drawback of this method is that air bubbles get easily trapped between the tissue cores while the melted paraffin is poured into the mold, resulting in a brittle TMA block or significant loss of tissue cores during cutting of the TMA block. Furthermore, punch extractors with larger diameters could cause damage to the donor blocks. This is not desirable for cases with limited material only, although one can avoid or minimize this damage in the original donor block by pre-warming the donor blocks at 45℃ for 15 minutes before punching.8
- To increase the density of holes in a recipient block compared to those made by manual boring, we first attempted to make recipient blocks by drilling holes in conventional bare paraffin blocks using a mini electric hand drill mounted on an ordinary drill stand. We found, however, that it is not easy to have well aligned holes with regular and precise spaces in coordinate axes. Therefore, we tried to use a mini X-Y table for precision motion and alignment as Vogel and Bueltmann10 first described. He has also updated methods to maximize the capacity of homemade recipient blocks.13-16 By using an electric mini hand drill and microcompound table, it was possible for us to dramatically increase the density of a TMA at low cost.
- A key consideration in preparation of bare paraffin blocks to be used as recipient blocks is to avoid any air bubbles or cracks created within the bare paraffin blocks. At the beginning, we experienced significant loss of tissue cores and even collapse of TMA blocks on subsequent sectioning. These problems can be minimized by using paper or plastic block molds instead of using metal block molds during preparation of bare paraffin blocks.17 In this study, however, we could prevent these problems by cooling the melted paraffin in the conventional metal block molds at room temperature for more than 1 hour instead of on a cooling plate.
- In conclusion, we introduced simple and inexpensive methods for constructing self-made manual TMA kits and self-made recipient blocks for assembly of high density TMAs, which are simple and cost-effective alternatives to expensive commercial devices.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol 2001; 195: 72–79. PMID: 11568893. ArticlePubMed
- 2. Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet 2001; 10: 657–662. PMID: 11257096. ArticlePubMed
- 3. Singh A, Sau AK. Tissue microarray: a powerful and rapidly evolving tool for high-throughput analysis of clinical specimens. Int J Case Rep Images 2010; 1: 1–6. Article
- 4. Hidalgo A, Piña P, Guerrero G, Lazos M, Salcedo M. A simple method for the construction of small format tissue arrays. J Clin Pathol 2003; 56: 144–146. PMID: 12560397. ArticlePubMedPMC
- 5. Wang SL, Yang CH, Chen HH, Chai CY. A simple and economical method for the manual construction of well-aligned tissue arrays. Pathol Res Pract 2006; 202: 485–486. PMID: 16563652. ArticlePubMed
- 6. Shebl AM, Zalata KR, Amin MM, El-Hawary AK. An inexpensive method of small paraffin tissue microarrays using mechanical pencil tips. Diagn Pathol 2011; 6: 117PMID: 22132713. ArticlePubMedPMC
- 7. Singh DK, Sakhuja P, Gondal R. Making and using inexpensive manually constructed tissue micro-array: experience of a tertiary care hospital in India. Indian J Pathol Microbiol 2009; 52: 304–309. PMID: 19679948. ArticlePubMed
- 8. Pan CC, Chen PC, Chiang H. An easy method for manual construction of high-density tissue arrays. Appl Immunohistochem Mol Morphol 2004; 12: 370–372. PMID: 15536340. ArticlePubMed
- 9. Nocito A, Kononen J, Kallioniemi OP, Sauter G. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer 2001; 94: 1–5. PMID: 11668471. ArticlePubMed
- 10. Vogel UF, Bueltmann BD. Simple, inexpensive, and precise paraffin tissue microarrays constructed with a conventional microcompound table and a drill grinder. Am J Clin Pathol 2006; 126: 342–348. PMID: 16880136. ArticlePubMed
- 11. Pathak GS, Deshmukh SD, Ashturkar AV. Construction of tissue arrays without prefabricated recipient paraffin block experience of a novel technique in resource poor settings. Indian J Pathol Microbiol 2011; 54: 654–655. PMID: 21934258. ArticlePubMed
- 12. Pires AR, Andreiuolo Fda M, de Souza SR. TMA for all: a new method for the construction of tissue microarrays without recipient paraffin block using custom-built needles. Diagn Pathol 2006; 1: 14PMID: 16869973. ArticlePubMedPMC
- 13. Vogel UF. Depositing archived paraffin tissue core biopsy specimens in paraffin tissue microarrays using a paraffin tissue punch modified with a countersink. J Clin Pathol 2007; 60: 206–207. PMID: 17079355. ArticlePubMedPMC
- 14. Vogel UF. Inexpensive and precise paraffin tissue microarrays constructed with a computer numerical control (CNC) drilling machine. Histopathology 2007; 51: 136–137. PMID: 17532771. ArticlePubMed
- 15. Vogel UF. The construction of high-density paraffin tissue microarrays with 0.43-mm-diameter paraffin tissue core biopsies is technically feasible. Virchows Arch 2008; 453: 43–46. PMID: 18551310. ArticlePubMed
- 16. Vogel UF, Bode J, Bueltmann B. Increasing the efficiency of paraffin tissue microarrays by packing the paraffin tissue core biopsies in a honeycomb pattern. Appl Immunohistochem Mol Morphol 2007; 15: 343–345. PMID: 17721282. ArticlePubMed
- 17. Parsons M, Grabsch H. How to make tissue microarrays. Diagn Histopathol 2009; 15: 142–150. Article
References
Fig. 1Manufacture of self-made manual tissue microarray (TMA) kits equipped with spring action for retractable stylets. Cannula piercing needles (16-, 18-, and 22-gauge) are modified into punch extractors (A). A cannula of an 11-gauge bone marrow biopsy needle and a metallic ink cartridge from an ordinary ballpoint pen are modified to make TMA kits with 1.8 mm- and 2 mm-punch extractor, respectively (B). They are equipped with retractable stylets and housed in the body parts of regular ballpoint pens. Disposable skin biopsy punches (2- and 3-mm) are modified to make TMA kits equipped with retractable stylets made from the trocar tip-needle of a bone marrow biopsy needle kit (C). A punch extractor made from a metallic ink cartridge with an inner diameter of 5 mm is equipped with a retractable stylet. It is housed in the body parts of a ballpoint pen (D).
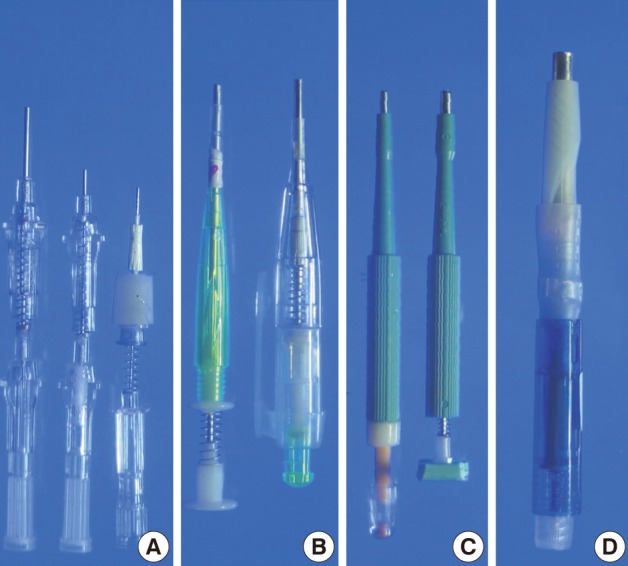

Fig. 2Manual construction of tissue microarray (TMA) with 1 mm core diameter. To guide the boring of holes of 1 mm in diameter in conventional bare paraffin blocks, dot-grid paper is attached to the surface of the bare paraffin block by means of double-faced adhesive tape (A). An 18-gauge cannula piercing needle is used to bore holes (B). After completion of boring (C), the dot-grid paper is removed (D) and tissue cores from the donor blocks are transferred (E) into the holes using a punch extractor made from a 16-gauge needle to make a TMA containing 140 tissue cores (F). There is no substantial loss of the cores or cross contamination between samples (G, hematoxylin and eosin staining; H, CD20 immunostaining; loss of cores designated by *; cross contamination designated by #).
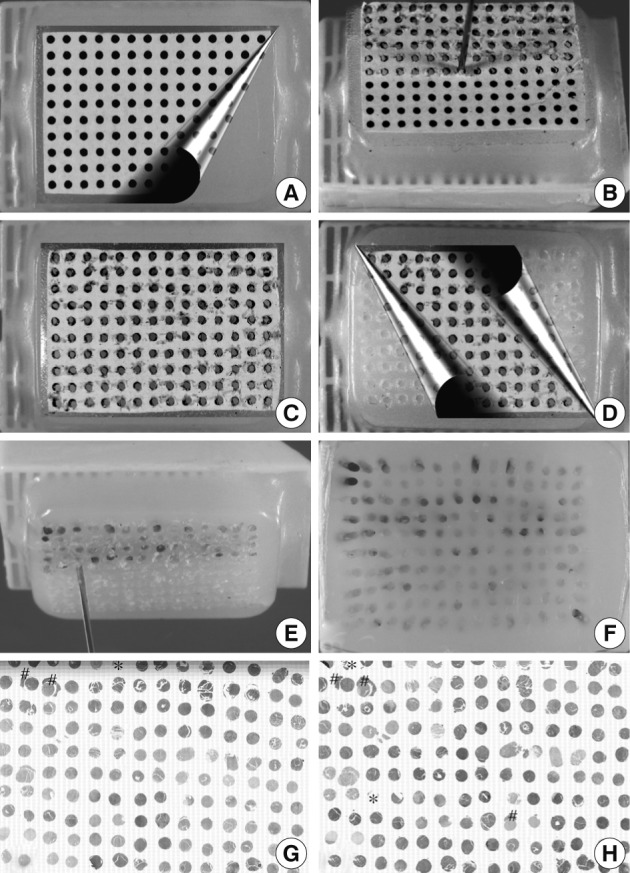

Fig. 3Manual construction of tissue microarray (TMA) with 0.6 mm core diameter. To guide the boring of holes of 0.6 mm in diameter in conventional bare paraffin blocks, the dot-grid paper in Fig. 2 is reduced by 60% and attached to the surface of the bare paraffin block by means of a double-face adhesive tape (A). A 22-gauge hypodermic needle is used to bore holes (B) in the recipient block followed by insertion of a donor tissue core (C). Then, dot-grid paper is removed (D) to make a TMA with 320 cores of 0.6 mm diameter (E). An hematoxylin and eosin-stained section shows minor loss of tissue cores (F, core loss designated by *).
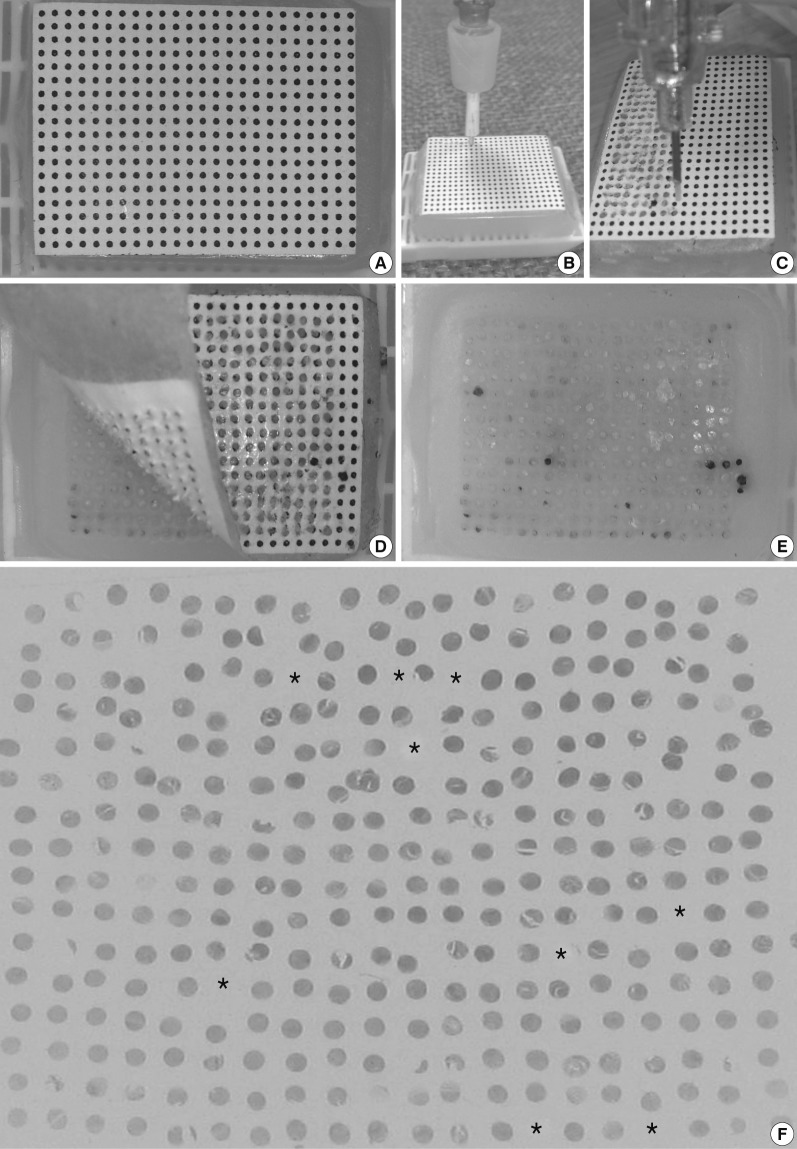

Fig. 4Self-made recipient blocks constructed by boring the holes with manual tissue microarray (TMA) kit or mini electric hand drill assembled with mini X-Y table. Conventional bare paraffin blocks are used to construct recipient blocks. Compared to boring holes using self-made manual TMA kits (A, 18-gauge needle; C, 2 mm disposable skin biopsy punch; E, 3 mm disposable skin biopsy punch), drilling the holes with a mini electric hand drill produces recipient blocks with higher density and better-aligned arrays of holes of different diameters (B, 1 mm; D, 2 mm; F, 3 mm).
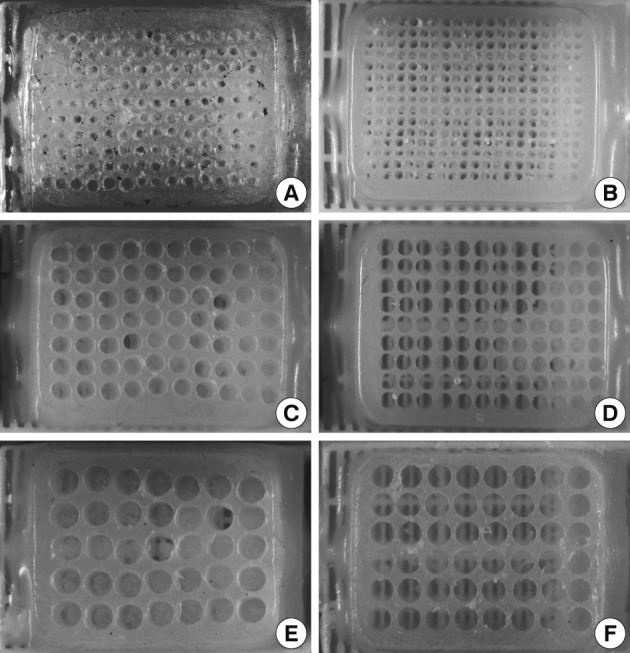

Figure & Data
References
Citations
Citations to this article as recorded by 

- Constructing high-density tissue microarrays with a novel method and a self-made tissue-arraying instrument
Ping Qin, Liu Li, Li Zhao, Piaopiao Bian, Zhongtang Xiong
Pathology - Research and Practice.2023; 245: 154430. CrossRef - The correlation of PD-L1 expression in cytological and histological material of serous high-grade ovarian cancer
Ljubiša Jovanović, Anđa Ćirković, Ljubinka Nikolić, Milena Jović, Darko Mikić, Svetlana Milenković, Radmila Janković
Srpski medicinski casopis Lekarske komore.2023; 4(3): 246. CrossRef - Expression of estrogen and progesterone receptors, HER2 protein and Ki-67 proliferation index in breast carcinoma in both tumor tissue and tissue microarray
UP Hacısalihoğlu, MA Dogan
Biotechnic & Histochemistry.2022; 97(4): 298. CrossRef - PD-L1 Expression in High-Grade Serous and Clear Cell Ovarian Cancer
Ljubiša Jovanović, Andja Ćirković, Milena Jović, Radmila Janković
Indian Journal of Gynecologic Oncology.2022;[Epub] CrossRef - PD-L1 Expression in Different Segments and Histological Types of Ovarian Cancer According to Lymphocytic Infiltrate
Ljubiša Jovanović, Radmila Janković, Andja Ćirković, Milena Jović, Tijana Janjić, Slaviša Djuričić, Svetlana Milenković
Medicina.2021; 57(12): 1309. CrossRef - Optimization of Tissue Microarrays from Banked Human Formalin-Fixed Paraffin Embedded Tissues in the Cancer Research Setting
Tammy Sexton, Gregory L. Kucera, Edward A. Levine, Kounosuke Watabe, Stacey S. O'Neill
Biopreservation and Biobanking.2019; 17(5): 452. CrossRef - Peripheral nerve sheath tumor invading the nasal cavities of a 6-year-old female Pointer dog
Alessandra Sfacteria, Laura Perillo, Francesco Macrì, Giovanni Lanteri, Claudia Rifici, Giuseppe Mazzullo
Veterinary Quarterly.2015; 35(3): 170. CrossRef - High Quality Tissue Miniarray Technique Using a Conventional TV/Radio Telescopic Antenna
Mohamed A. Elkablawy, Abdulkader M. Albasri
Asian Pacific Journal of Cancer Prevention.2015; 16(3): 1129. CrossRef - Overview on Techniques to Construct Tissue Arrays with Special Emphasis on Tissue Microarrays
Ulrich Vogel
Microarrays.2014; 3(2): 103. CrossRef - Tissue Microarray
Kathleen Barrette, Joost J. van den Oord, Marjan Garmyn
Journal of Investigative Dermatology.2014; 134(9): 1. CrossRef - Altered Expression of PTEN and Its Major Regulator MicroRNA-21 in Pulmonary Neuroendocrine Tumors
Hyoun Wook Lee, Seung Yeon Ha, Mee Sook Roh
Korean Journal of Pathology.2014; 48(1): 17. CrossRef - Optimizing tissue microarray construction procedure to improve quality of sections
Hua Chang, Diane Peluso, Sadiq Hussain, Michail Shipitsin, Peter Blume-Jensen
Journal of Histotechnology.2014; 37(3): 95. CrossRef - In-house Manual Construction of High-Density and High-Quality Tissue Microarrays by Using Homemade Recipient Agarose-Paraffin Blocks
Kyu Ho Kim, Suk Jin Choi, Yeon Il Choi, Lucia Kim, In Suh Park, Jee Young Han, Joon Mee Kim, Young Chae Chu
Korean Journal of Pathology.2013; 47(3): 238. CrossRef

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article