Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(6); 2012 > Article
-
Case Report
Multiple Jejunal Myeloid Sarcomas Presenting with Intestinal Obstruction in a Non-leukemic Patient: A Case Report with Ultrastructural Observations - Na Rae Kim, Woon Kee Lee1, Jong In Lee2, Hyun Yee Cho
-
Korean Journal of Pathology 2012;46(6):590-594.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.590
Published online: December 26, 2012
Department of Pathology, Gachon University Gil Medical Center, Incheon, Korea.
1Department of General Surgery, Gachon University Gil Medical Center, Incheon, Korea.
2Division of Hematooncology, Department of Internal Medicine, Wonju Christian Hospital, Yonsei University College of Medicine, Wonju, Korea.
- Corresponding Author: Hyun Yee Cho, M.D. Department of Pathology, Gachon University Gil Medical Center, 21 Namdong-daero 774beon-gil, Namdong-gu, Incheon 405-760, Korea. Tel: +82-32-460-3865, Fax: +82-32-460-2394, 'hicho@gilhospital.com'
• Received: March 6, 2012 • Revised: April 16, 2012 • Accepted: April 25, 2012
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Myeloid sarcoma is a rare extramedullary myeloid tumor, which is frequently misdiagnosed when no evidence of leukemia is initially observed. Here, we report on a peculiar case of a 49-year-old man afflicted with multiple masses in the jejunum, the superior mesentery, and the serosa of the transverse colon, without leukemic manifestation. The tumor was composed of undifferentiated small round cells containing eosinophilic cytoplasm, which were negative for myeloperoxidase, nonspecific esterase, lysozyme, terminal deoxynucleotidyl transferase, leukocyte common antigen, CD3, CD4, CD15, CD20, CD30, CD43, CD56, CD68/PG-M1, CD79a, human melanoma black-45, c-kit, and CD34 with positivity only for CD68/KP1, CD99, and vimentin. Under electron microscopy, those cells had abundant membrane-bound cytoplasmic granules that measured 200 to 300 nm in diameter, which were consistent with granulocytic azurophilic granules. The tumor was finally diagnosed as a myeloid sarcoma. The presence of non-leukemic myeloid sarcomas showing immunonegativity for conventional myeloid-leukemic markers necessitated a diagnosis by ultrastructural observation.
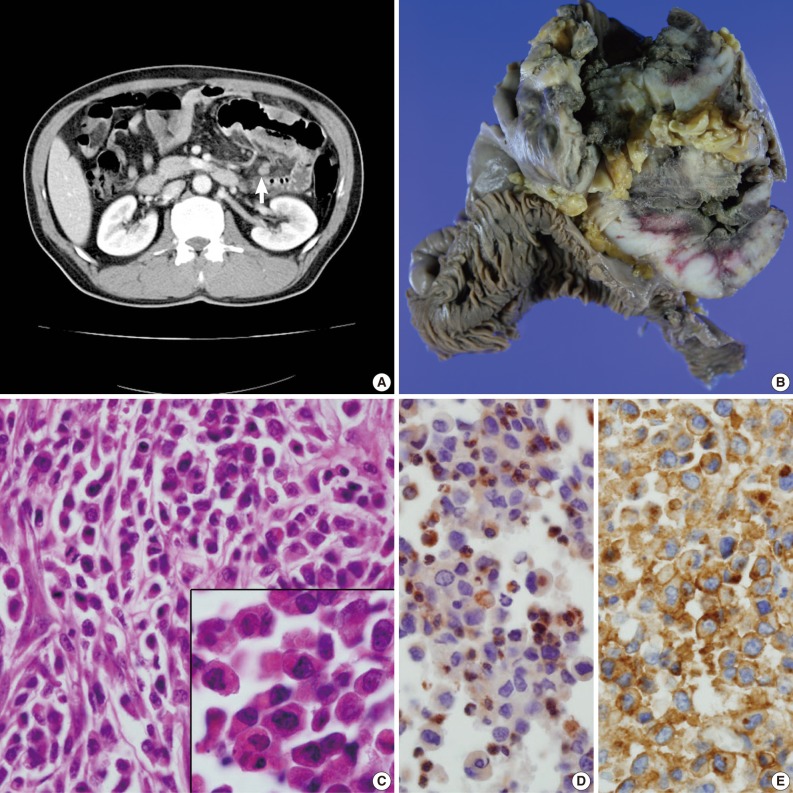
- A 49-year-old man presented with recurrent central colicky abdominal pain, which had commenced 2 weeks earlier. Abdominopelvic computed tomography (CT) showed a mass-like lesion at the left upper abdominal region (Fig. 1A). Segmental resection of the jejunum 30 cm below the Treitz ligament was performed. Intraoperatively, the obstructed portion was conglomerated and adhered to other segments of the distal small intestine. The mass-like portion demonstrated thick adhesion to the transverse colon and superior mesentery.
- The resected segment measured 106 cm in length. The cut surface revealed two portions of circumferential stricture by two separate ulceroinfiltrating masses. The masses measured 15×10 cm and 12×10 cm each. The masses were separated from each other by 21 cm. Grossly, the masses showed transmural involvement and had invaded through the proper muscle layer of the adhered segment of the jejunum. The cut surface had a brown-colored homogeneous and fish-fleshy appearance (Fig. 1B). The mucosal surfaces of the masses were coated by necrotic exudates. Histologically, each tumor was diffusely infiltrated by round small to moderately sized cells containing eosinophilic granular cytoplasm (Fig. 1C). The tumor cells were irregularly shaped with angulated nuclei, irregular nuclear contours, and fine chromatin. Immunohistochemically, the tumor cells were cytoplasmically stained with CD68 (1:100, KP1, Lab Vision Co., Cheshire, UK) (Fig. 1D), CD99 (prediluted, 12E7, Dako, Glostrup, Denmark) (Fig. 1E) and vimentin (prediluted, V9, Dako). The cells produced negative results for myeloperoxidase (MPO), toluidine blue, lysozyme, terminal deoxynucleotidyl transferase (TdT), nonspecific esterase, leukocyte common antigen (prediluted, Dako), CD3 (prediluted, polyclonal, Dako), CD4 (4B12, prediluted, Dako), CD15 (1:50, Carb-3, Dako), CD20 (prediluted, polyclonal, L26, Dako), CD30 (prediluted, Ber-H2, Dako), CD43 (prediluted, Dako), CD56 (1:100, 123C2, Dako), CD68 (1:50, PG-M1, Dako), CD79a (1:50, JCB117, Dako), human melanoma black-45 (prediluted, HMB-45, Dako), CD117 (prediluted, c-kit, Dako), and CD34 (prediluted, QBEnd10, Dako). To exclude the remote possibility of a gastrointestinal stromal tumor (GIST), platelet-derived growth factor receptor-alpha (PDGFRA) and c-kit mutation studies were performed using polymerase chain reaction sequencing. No PDGFRA or c-kit mutations were observed.
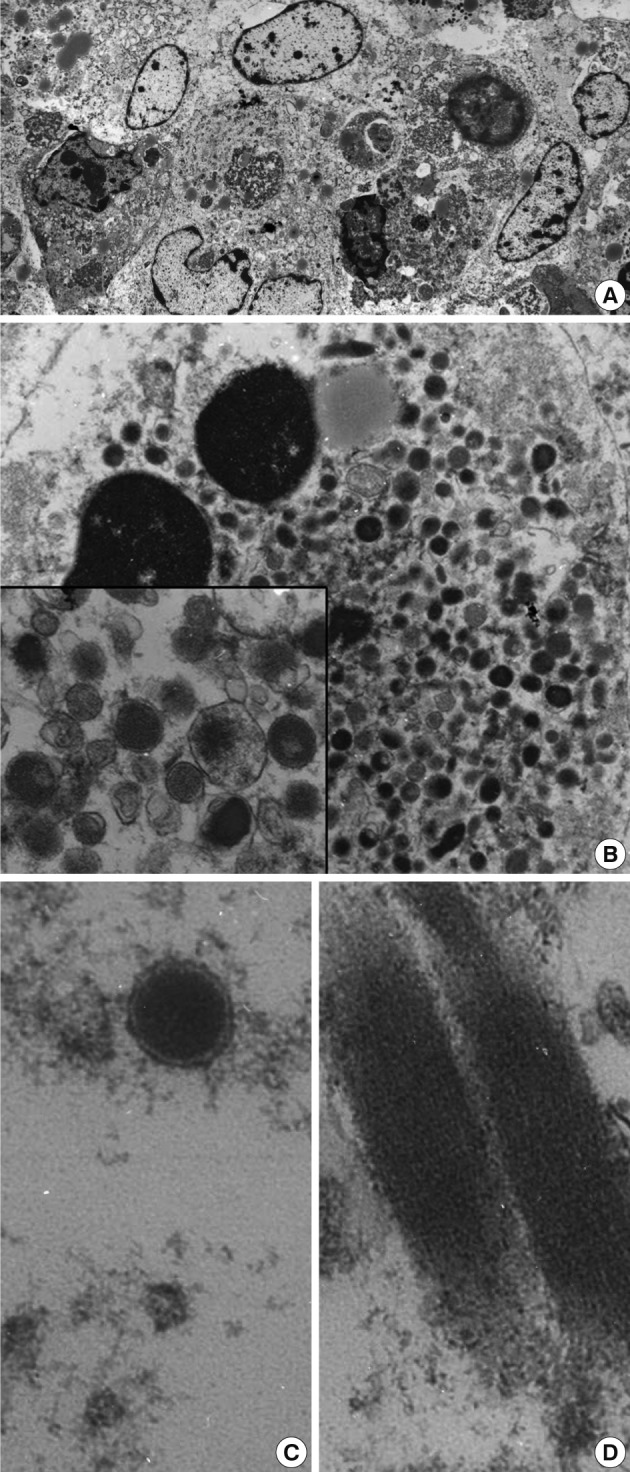
- Upon examination of their ultrastructure, the ovoid-shaped tumor cells possessed peripherally condensed chromatin with occasional involuted configurations (Fig. 2A, B). The tumor cells were packed with abundant intracytoplasmic membrane-bound granules that measured from 200 to 300 nm in diameter. The rectangular inclusions revealed a fine lamellar substructure, and the periodicity of the filamentous striations was about 10 nm (Fig. 2C, D). The peripheral blood smears failed to demonstrate any abnormal hematopoietic cells or blasts. Myeloid sarcoma was diagnosed. A bone marrow study was not performed due to the patient's request. After the operation, a chest CT showed a 7 cm heterogeneous enhancing cavitary mass within low density in the right upper lobe. At that time, two enhancing small nodules were found at the liver. After 7 months, the size of the masses of the lung and liver had enlarged slightly. The peripheral blood smears indicated that no abnormal myeloid cells or blasts remained. Biopsies were not taken from these organs. The patient experienced severe recurrent hemoptysis. His condition continued to decline and then he died. An autopsy was not performed.
CASE REPORT
- Non-leukemic myeloid sarcoma can manifest initially as an isolated intestinal mass, and chemotherapy is the treatment of choice. Surgery is indicated only if there are complications such as bowel obstruction, bleeding, or perforation.7 Because of frequent misdiagnosis as other undifferentiated high grade tumors, an accurate diagnosis may be made only after acute myeloid leukemia (AML) has developed (mean, 9 months).8 Therefore, an early and accurate diagnosis will enable proper treatment, which has the potential to be successful despite the aggressive clinical course that is typical of myeloid sarcomas. Microscopically, a dense infiltrate of relatively small, undifferentiated high grade tumor cells showing an unfamiliar histology and uncertain origin are the first presentation of a hematologic malignancy.9 The diagnosis can be challenging to pathologists when there is a non-leukemic phase or in the absence of a known hematological disorder. From light microscopic morphology, the differential diagnoses of myeloid sarcoma include a GIST, malignant lymphoma, melanoma, and an undifferentiated tumor such as Ewing's sarcoma/PNET. Once the possibility of a myeloid sarcoma is considered, immunohistochemistry can reliably make this distinction in nearly all cases. Immunohistochemical panels for myeloid markers such as MPO, lysozyme, c-kit, CD43, and CD68/KP1 are all sensitive and helpful, although markers for CD43, CD68, and c-kit are nonspecific, particularly CD43.5,10,11 Among them, CD68/KP1 is a good marker and even appears more sensitive than MPO for granulocytic precursors, while CD68/PG-M1 is more sensitive in monocytic differentiation.11,12 CD30 and CD56 are seldom recorded in myeloid sarcoma cases.13 Our case showed negativity for conventional immunohistochemical markers such as chloroacetate esterase, MPO, and lysozyme, and showed positivity only for CD68/KP1 and CD99. These results had the potential to be misdiagnosed as Ewing's sarcoma/PNET because CD99 is a transmembrane glycoprotein p30/32mic2, a product of the MIC2 gene, and is positive in Ewing's sarcoma/PNET and lymphoblastic lymphoma.14 However, CD99 has also been expressed by monocytes, B cells, and granulocyte-lineage cells such as those from myeloid sarcoma, lymphoblastic lymphoma, or TdT-positive AML.15 Immunoreactivity for CD99 has been reported in more than 50% of the myeloid sarcomas, which is in line with its not infrequent expression in hematopoietic tumors.5,11-14 TdT is expressed in approximately 90% of acute lymphoblastic lymphoma cases, which represents a small subset of AML cases. If the disease develops in the gastrointestinal tract, differential diagnoses of GIST and gastrointestinal lymphoma should be considered. Although immunopositivity for c-kit and CD34 are important diagnostic markers, shared features exist between GIST and myeloid sarcoma.16 In confusing cases showing negativity for a myeloid marker including MPO, a mutation analysis for PDGFR and c-kit, coupled with electron microscopic investigation, may be required.
- In the present case, the presence of membrane-bound cytoplasmic granules in the ultrastructural analysis offered a diagnostic clue. Differential diagnoses from the various types of secretory granules included membrane-bound granulocytic granules, dense-core granules, zymogen granules, mucin granules, Birbeck granules, melanosomes, surfactant granules, laminated granules of the mast cells, lipofuscin granules, and Weibel-Palade bodies.17 Lipofuscin granules are aggregates of osmiophilic granules and appear as an electron-dense layer. Although there have been rare reports of granular variants of some tumors such as mammary lobular carcinoma, which may contain such cytoplasmic granules, granules are not commonly found even in the myeloid metaplasia of tumors such as those secondary to carcinoma, leukoerythroblastosis, or tuberculosis.18 Investigation of the nuclear morphology of the tumors cells is helpful for distinguishing their differentiation and maturation. As granulocytes mature, their nuclei become flattened, indented, and then lobulated.19 Cytoplasmic granules appear and then lose cytoplasmic basophilia. Ultrastructural changes of the granule content, with observed loss of electron density, indicates secretory activity. Giant granules up to 3 µm that are commonly observed in diseases such as Chediak-Higashi syndrome are of rare occurrence in myeloid sarcoma.20 Identification of the cytoplasmic granules offered a diagnostic clue in the present case.
- In conclusion, the application of a screening panel for high grade undifferentiated tumors should be broad in order to avoid a diagnostic misinterpretation due to occasionally aberrant or unexpected antigen expression. Even in cases of negative clinical results and unusual immunohistochemical observations, a high index of suspicion is required to avoid overlooking the diagnostic findings, and in these cases, the use of electron microscopic examination is vital for the diagnosis of myeloid sarcomas.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Pressler H, Horny HP, Wolf A, Kaiserling E. Isolated granulocytic sarcoma of the ovary: histologic, electron microscopic, and immunohistochemical findings. Int J Gynecol Pathol 1992; 11: 68–74. PMID: 1563910. ArticlePubMed
- 2. Ghafoor T, Zaidi A, Al Nassir I. Granulocytic sarcoma of the small intestine: an unusual presentation of acute myelogenous leukaemia. J Pak Med Assoc 2010; 60: 133–135. PMID: 20209703. PubMed
- 3. Kohl SK, Aoun P. Granulocytic sarcoma of the small intestine. Arch Pathol Lab Med 2006; 130: 1570–1574. PMID: 17090205. ArticlePubMed
- 4. Corpechot C, Lémann M, Brocheriou I, et al. Granulocytic sarcoma of the jejunum: a rare cause of small bowel obstruction. Am J Gastroenterol 1998; 93: 2586–2588. PMID: 9860434. ArticlePubMed
- 5. Markoc F, Bozdogan N, Yükrük FA, Gumuc EB, Akdur NC. Granulocytic sarcomas: difficulties in diagnosis. Tumori 2010; 96: 149–153. PMID: 20437873. ArticlePubMedPDF
- 6. Menasce LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology 1999; 34: 391–398. PMID: 10231412. ArticlePubMed
- 7. Ioannidis O, Cheva A, Kakoutis E, et al. Primary myeloid sarcoma of the jejunum and greater omentum causing small intestine obstruction. Acta Gastroenterol Belg 2009; 72: 369–372. PMID: 19902875. PubMed
- 8. Arnal-Monreal FM, Alvarez Fernandez JC, Sanchez Varela JM, Marini Diaz M. Meningeal granulocytic sarcoma without evidence of leukemia: light and ultrastructural study of one case. Virchows Arch A Pathol Anat Histol 1981; 392: 111–118. PMID: 6944946. ArticlePubMed
- 9. Bahrami A, Truong LD, Ro JY. Undifferentiated tumor: true identity by immunohistochemistry. Arch Pathol Lab Med 2008; 132: 326–348. PMID: 18318577. ArticlePubMed
- 10. Brunning RD, Matutes E, Flandrin G, et al. In : Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Acute myeloid leukaemia not otherwise categorised. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. 2001; Lyon: IARC Press, 104–105.
- 11. Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007; 21: 340–350. PMID: 17170724. ArticlePubMed
- 12. Chang CC, Eshoa C, Kampalath B, Shidham VB, Perkins S. Immunophenotypic profile of myeloid cells in granulocytic sarcoma by immunohistochemistry. Correlation with blast differentiation in bone marrow. Am J Clin Pathol 2000; 114: 807–811. PMID: 11068557. ArticlePubMed
- 13. Kurata H, Okukubo M, Fukuda E, Ichihashi M, Ueda M. Myeloid markers should be undertaken in cases of CD56 positivity to exclude granulocytic sarcoma. Br J Dermatol 2002; 147: 609–611. PMID: 12207615. ArticlePubMed
- 14. Kang LC, Dunphy CH. Immunoreactivity of MIC2 (CD99) and terminal deoxynucleotidyl transferase in bone marrow clot and core specimens of acute myeloid leukemias and myelodysplastic syndromes. Arch Pathol Lab Med 2006; 130: 153–157. PMID: 16454553. ArticlePubMed
- 15. Levy R, Dilley J, Fox RI, Warnke R. A human thymus-leukemia antigen defined by hybridoma monoclonal antibodies. Proc Natl Acad Sci U S A 1979; 76: 6552–6556. PMID: 316541. ArticlePubMedPMC
- 16. Goldstein NS, Ritter JH, Argenyi ZB, Wick MR. Granulocytic sarcoma: potential diagnostic clues from immunostaining patterns seen with anti-lymphoid antibodies. Int J Surg Pathol 1995; 2: 177–186. Article
- 17. Toner PG, McCormick FW. In : McGee JO, Issacson PG, Wright NA, Dick HM, Slack MP, eds. Cellular organization and cellular interrelationships. Theb Oxford textbook of pathology: Vol. 1. Principles of pathology. 1992; Oxford: Oxford University Press, 3–33.
- 18. Andre J, Schwartz R, Dameshek W. Tuberculosis and myelosclerosis with myeloid metaplasia: report of three cases. JAMA 1961; 178: 1169–1174. PMID: 13861374. ArticlePubMed
- 19. Banik S, Grech AB, Eyden BP. Granulocytic sarcoma of the cervix: an immunohistochemical, histochemical, and ultrastructural study. J Clin Pathol 1989; 42: 483–488. PMID: 2732341. ArticlePubMedPMC
- 20. Golomb HM, Rowley JD, Vardiman JW, Testa JR, Butler A. "Microgranular" acute promyelocytic leukemia: a distinct clinical, ultrastructural, and cytogenetic entity. Blood 1980; 55: 253–259. PMID: 6928105. ArticlePubMedPDF
References
Fig. 1(A) Abdominopelvic computed tomography reveals multifocal enhancing concentric wall thickening (arrow) with a skipped lesion in the jejunal loop. (B) A gross picture of the bisected jejunal segment reveals two separate encircling masses. (C) Primitive, undifferentiated, round-shaped tumor cells infiltrate the bowel wall. The inset indicates plump eosinophilic granular cytoplasm. (D, E) The tumor cells are cytoplasmically stained with CD68/KP1 (D) and CD99 (E).
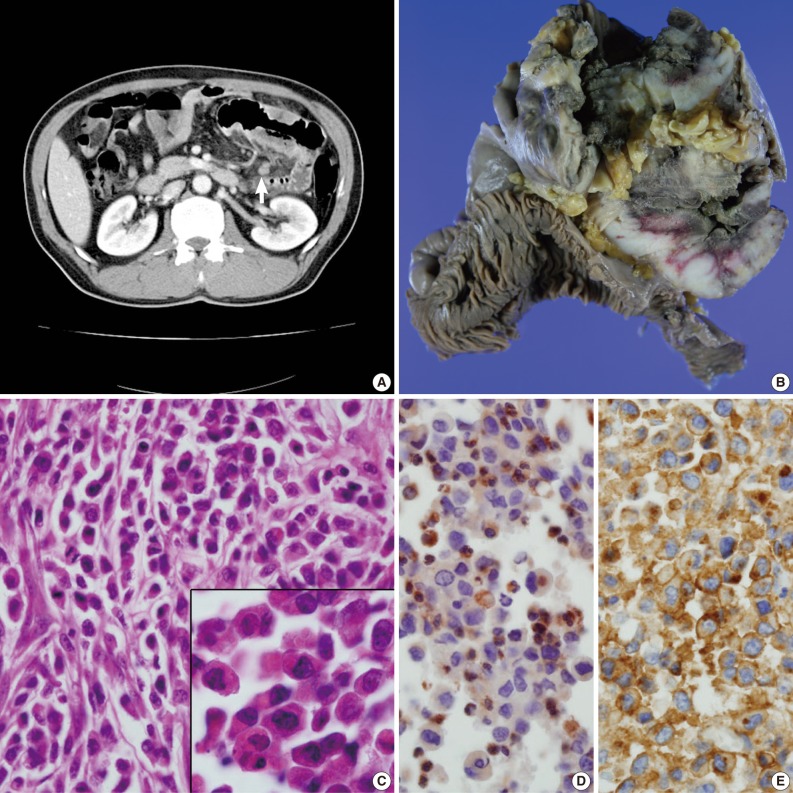

Fig. 2(A) Low power view shows the closely apposed ovoid-shaped cells containing voluminous granular cytoplasm and nuclei of heterochromatin. Note the slightly indented nuclei with marginally condensed chromatin (×1,500). (B) The cytoplasm of the ovoid-shaped cells is filled with abundant cytoplasmic granules (×6,000). The inset indicates the membrane-bound granules of variable sizes (×15,000). (C, D) A high power examination indicates the membrane-bound primary azurophilic granules measuring 220 nm in diameter (C, ×20,000) and rectangular cytoplasmic inclusions (D, ×20,000).
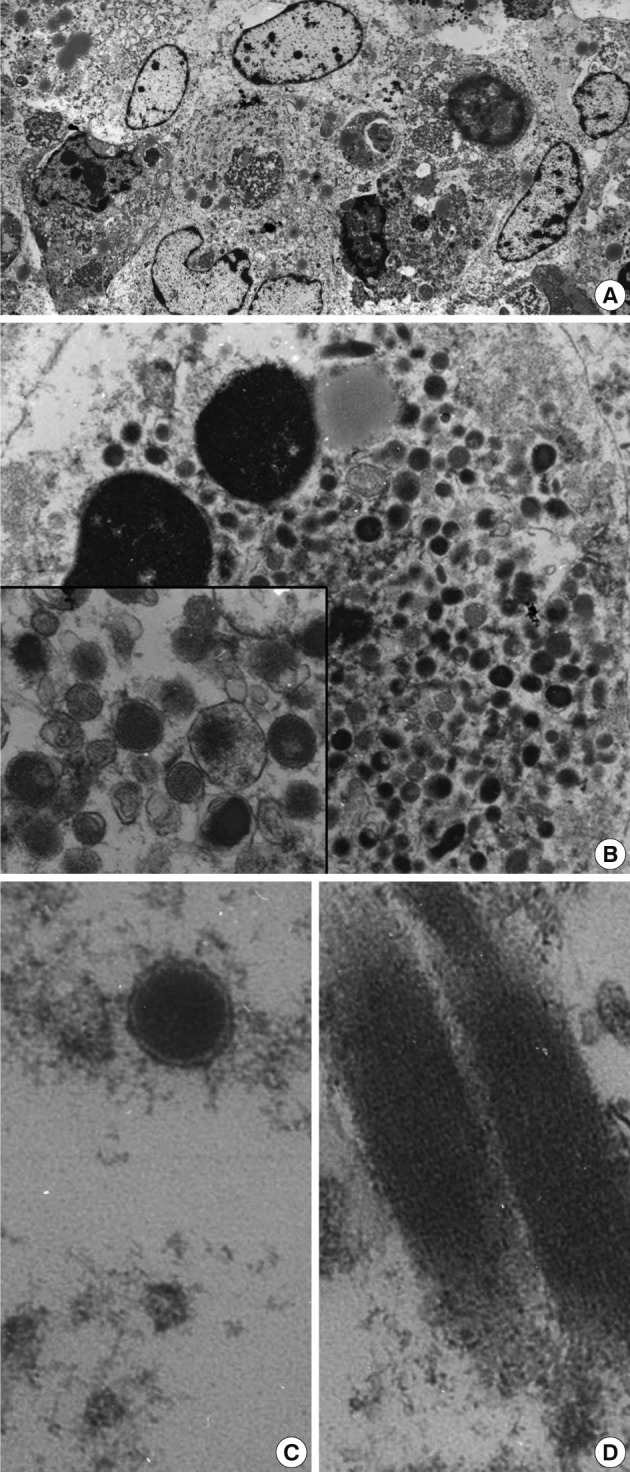

Figure & Data
References
Citations
Citations to this article as recorded by 

- Isolated myeloid sarcoma presenting with small bowel obstruction: a case report
Rie Mizumoto, Masanori Tsujie, Tomoko Wakasa, Kotaro Kitani, Hironobu Manabe, Shuichi Fukuda, Kaoru Okada, Shumpei Satoi, Hajime Ishikawa, Toshihiko Kawasaki, Hitoshi Hanamoto, Masao Yukawa, Masatoshi Inoue
Surgical Case Reports.2020;[Epub] CrossRef - Primary Myeloid Sarcoma of the Ileum and Mesentery Causing Small Bowel Obstruction: Case Report and Literature Review
Andrej Nikolovski, Dragoslav Mladenovikj, Aleksandra Veljanovska, Gordana Petrusevka
Lietuvos chirurgija.2020; 19(1-2): 55. CrossRef - Utility of Transmission Electron Microscopy in Small Round Cell Tumors
Na Rae Kim, Seung Yeon Ha, Hyun Yee Cho
Journal of Pathology and Translational Medicine.2015; 49(2): 93. CrossRef

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article