Rare Case of Anal Canal Signet Ring Cell Carcinoma Associated with Perianal and Vulvar Pagetoid Spread
Article information
Abstract
A 61-year-old woman was referred to surgery for incidentally found colonic polyps during a health examination. Physical examination revealed widespread eczematous skin lesion without pruritus in the perianal and vulvar area. Abdominopelvic computed tomography showed an approximately 4-cm-sized, soft tissue lesion in the right perianal area. Inguinal lymph node dissection and Mils’ operation extended to perianal and perivulvar skin was performed. Histologically, the anal canal lesion was composed of mucin-containing signet ring cells, which were similar to those found in Pagetoid skin lesions. It was diagnosed as an anal canal signet ring cell carcinoma (SRCC) with perianal and vulvar Pagetoid spread and bilateral inguinal lymph node metastasis. Anal canal SRCC is rare, and the current case is the third reported case in the English literature. Seven additional cases were retrieved from the world literature. Here, we describe this rare case of anal canal SRCC with perianal Pagetoid spread and provide a literature review.
According to the recent World Health Organization classification of the digestive system, patients with mucinous adenocarcinoma have a significantly high survival rate compared to those with signet ring cell carcinoma (SRCC) [1]. By definition, SRCC has more than 50% intracellular mucin and mucinous carcinoma has more than 50% extracellular mucin. SRCC can arise from virtually all organs, including stomach, colon, breasts, gallbladder, or even urinary bladder [2]. When an extragastric SRCC is found in an unusual site, metastasis from other primary sites should be excluded. Among colorectal SRCCs of the digestive tract, anal canal SRCCs are even rarer [3]. Only two cases of anal canal SRCC have been described in the English literature [4,5].
We recently encountered a rare case of anal canal SRCC and extramammary Paget’s disease. Extramammary Paget’s disease commonly appears in the vulvar area, followed by the perianal region, scrotum, penis, and axillae [6]. In most cases, Paget’s disease is not accompanied by cancer; however, cancer hidden in the adjacent organs, including the vulva, vagina, uterus, ovary, bladder, and colorectal area, should be kept in mind.
Here, we report on a case of anal canal SRCC and Pagetoid spread, and provide a literature review.
CASE REPORT
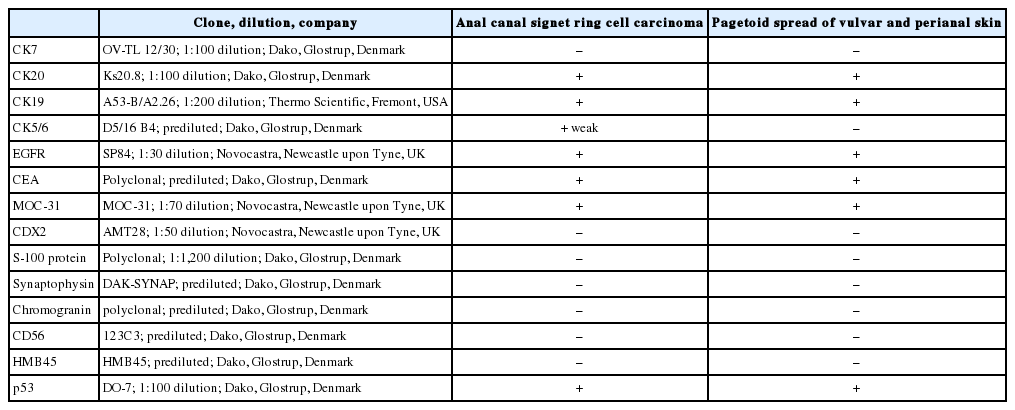
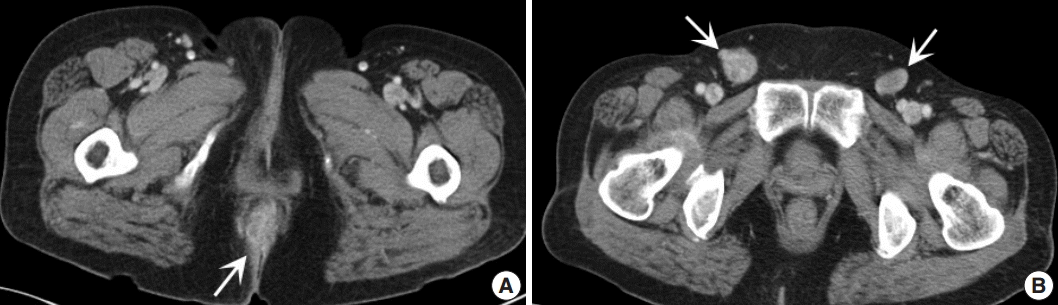
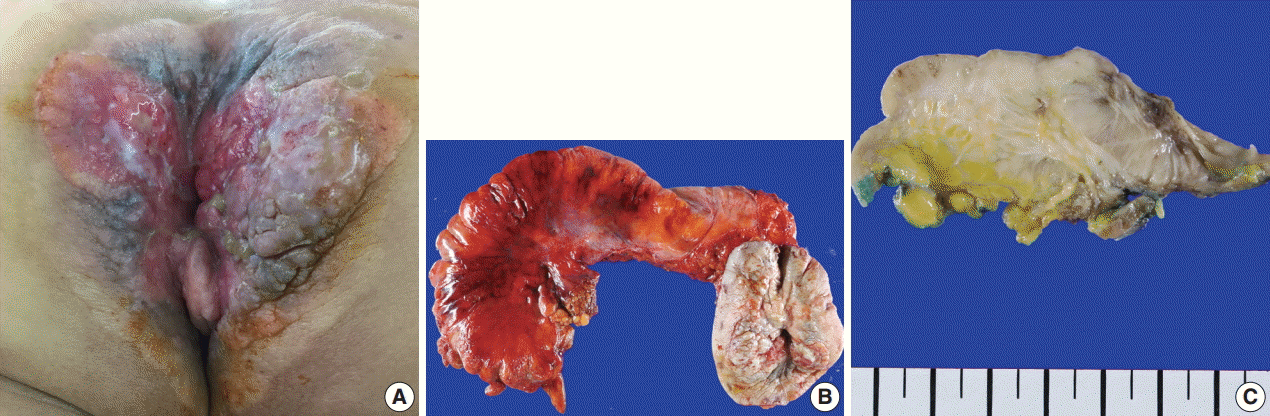
A 61-year-old woman visited our hospital for alleged colon polyps found during a colonoscopy examination as part of a routine health examination. Endoscopic submucosal dissection was performed. Four polyps were diagnosed as tubular adenoma. Abdominopelvic computed tomography (CT) showed an approximately 4-cm-sized, well-enhancing soft tissue lesion in the right perianal area (Fig. 1A) and a well-enhancing enlarged lymph node in both inguinal areas, suggesting hypervascular tumor metastasis such as melanoma in those lymph nodes (Fig. 1B). Upon physical examination, however, a widespread erythematous lichenified skin lesion, measuring 12×9.7 cm, was noted in the perianal and vulvar area (Fig. 2A), which had developed over the previous 3 years according to the patient. She did not complain of pruritus. She had no history of anal fissure, fistula, or change in bowel habit. Skin biopsy was taken from the perianal and vulvar area, which showed infiltrating Pagetoid cells and SRCC cells. There was neither abnormal bowel wall thickening nor masses in the sigmoid colon. No other lesion was observed on upper esophagogastroscopy. The preoperative carcinoembryonic antigen (CEA) level was elevated to 39.75 ng/mL (reference, 0 to 5 ng/mL). Carbohydrate antigen 19-9 was within normal ranges. Inguinal lymph node dissection with an extended Miles’ operation was performed; traditional Miles’ operation with extended resection around bilateral labium majora, followed by skin reconstruction with gluteal flap, was performed. The resected specimen was composed of the anal canal and rectum with skin and soft tissue of the perianal region and vulva (Fig. 2B). Upon sectioning, the anal canal showed an ulcerative firm mass measuring 4.0×3.0×2.7 cm, which invaded the perianal sphincter muscle and subcutaneous fat of the anal skin (Fig. 2C). Light microscopy showed that the anal canal was totally replaced by singly scattered intracytoplasmic mucin-containing signet ring cells and some extracellular mucin (Fig. 3A, B). Focally, well-formed glands were also found in less than 5% of the tumor. Most of the surface epithelium was denuded. The focal residual transformation zone showed a focus of adenocarcinoma in situ (Fig. 3C), and was not contiguous with the anal glands. Anal canal SRCC was diagnosed. The erythematous skin around the anus and labium majora showed linear infiltration of Pagetoid cells as well as infiltration of signet ring cells in the dermis and subcutis (Fig. 3D). Involved skin area measured 8.2×8.0×0.5 cm. Lymphatic permeation was noted in the dermis. Enlarged bilateral inguinal lymph nodes also showed infiltration of the signet ring cells.
Radiologic findings. (A) Abdominal computed tomography reveals a well-enhancing lesion (arrow) at the right perianal portion. (B) Enlarged bilateral inguinal lymph nodes (arrows) are found.
Gross pictures. (A) Perianal and vulvar skin shows elevated erythematous changes. (B) Extended Miles’ operation specimen consists of distal rectal segment and excision of perianal region and vulva. (C) Cross-section of the resected specimen shows a firm anal canal mass infiltrating to the perianal skin and levator ani muscle.
(A) A transmural, yellowish gray firm mass is composed of singly scattered signet ring cells and some extracellular mucin. Note the surface mucosal erosion. (B) The majority of the mass is infiltrated by signet ring carcinoma cells, and poorly differentiated mucinous glands are floating in the mucin pool. Inset indicates high magnification of signet ring cells showing intracytoplasmic mucin compressing the hyperchromatic nuclei to the periphery. (C) The transformation zone of the anal canal shows infiltration of multifocal signet ring cell carcinoma (white arrows) and a focus of atypical gland beneath the mucosa (black arrow). Inset shows adenocarcinoma in situ with a gland having enlarged hyperchromatic nuclei with coarse chromatin and increased nuclear/cytoplasmic ratio. (D) Large Paget cells showing clear cytoplasm with eccentric hyperchromic nuclei are located along the basal layer of the squamous epithelium. Inset indicates Paget cells in the epidermis.
Immunohistochemically, both signet ring cells and Pagetoid cells were positive for cytokeratin (CK) 20, CEA, MOC-31, and CK19. Intracellular and extracellular mucin was eosinophilically stained with mucicarmine. Both signet ring cells and Paget’s cells were positive for epidermal growth factor receptor and weakly positive for CK5/6. They were negative for CK7, human melanoma black 45, S-100 protein, caudal-related homeobox gene nuclear transcription factor (CDX2), p53, synaptophysin, chromogranin, and CD56. These results are shown in Table 1.
A mutation study of the KRAS gene (codon 12 and codon 13) was performed by means of the peptide nucleic acid-mediated real-time polymerase chain reaction clamping method using genomic DNA isolated from formalin fixed paraffin-embedded tissue. Mutations in codon 12 and 13 were not detected in anal canal SRCC.
Microsatellite instability (MSI) was tested using five Bethesda markers (D2S123, D5S346, D17S250, BAT25, and BAT26). MSI-high (MSI-H) was defined if they differed in at least two of the five markers and MSI-low was defined if they differed in only one of the five markers. Anal canal mass was microsatellite stable (MSS).
Positron emission tomography CT and chest CT showed no evidence of systemic metastases. The case was diagnosed as anal canal SRCC with Pagetoid spread in perianal and vulva skin, stage IIIB according to the TNM staging system of the American Joint Committee on Cancer.
The patient was scheduled to undergo chemotherapy with mitomycin-C and 5-fluorouracil (5-FU) and subsequent radiotherapy. The patient’s postoperative course was unremarkable.
Institutional review board (IRB) approval was obtained for this case report.
DISCUSSION
Colorectal SRCC is a rare histologic subtype of adenocarcinoma, accounting for 0.1% to 2.4% of all colorectal malignancies [1]. Anal canal SRCC is even rarer; only two cases of anal canal SRCCs have been reported in the English literature [4,5]. Seven additional cases have been retrieved from the Japanese literature [3,7-12].
The current case showed anal canal SRCC. Anal canal carcinoma is histologically and pathogenetically divided into squamous cell carcinoma, cloacogenic (basaloid or transitional cell) carcinoma and adenocarcinoma [13]. Anal canal squamous cell carcinoma originates from the non-keratinizing squamous epithelium below the dentate line of the anal canal, while anal canal adenocarcinoma is regarded as arising from the upper part lined by the columnar epithelium. Anal canal adenocarcinomas are rare. According to the World Health Organization classification, anal canal adenocarcinomas are subclassified according to adenocarcinoma arising from anal mucosa and extramucosa, and adenocarcinoma arising from anorectal fistula or adenocarcinoma of anal glands [14]. No description of in situ lesion was found in the previously reported cases of anal canal SRCCs. Based on the findings of the overlying ulcerated anal surface mucosa, remnants of anal gland adenocarcinoma in situ without continuity to SRCC portion, the present anal canal SRCC belongs to extramucosal perianal adenocarcinoma with wide Pagetoid spread along the perianal soft tissues and skin.
We have summarized the clinicopathologic characteristics in Table 2. Similar to the current case, eight out of 10 anal canal SRCCs (80%) were accompanied by perianal or vulvar Pagetoid spread [3-5,7-12]. Extramammary perianal Paget’s disease is a rare and heterogeneous neoplasm, which is frequently combined with underlying hidden adenocarcinoma [6]. Immunohistochemical panels such as CK7, CK20, CEA, gross cystic disease fluid protein-15 (GCDFP-15), MUC1, MUC2, MUC5AC, and CDX2 have been used to distinguish primary from secondary Paget’s disease [15]. In anal SRCCs with Pagetoid spread, i.e., not primary extramammary Paget’s disease, the tumor cells were positive for CK20, MUC1, or MOC-31, while they were negative for GCDFP-15, and negative for CK7, whereas primary Paget’s disease is commonly positive for CK7 [16]. However, none of these were a specific and confirmative diagnostic clue; further accumulative data may be needed. SRCC is most commonly encountered in the stomach, but it can also arise in various organs, such as breast, urinary bladder, or colon. Immunohistochemical profiles for CK7 and CK20 have also been used to differentiate the sites of origin for SRCCs; CK20 is normally expressed in the gastrointestinal epithelium, urothelium, and in Merkel’s cells. While CK7 is uncommonly expressed in the lower gastrointestinal tract, it is commonly expressed in lung, ovary, endometrium, and breast [15].
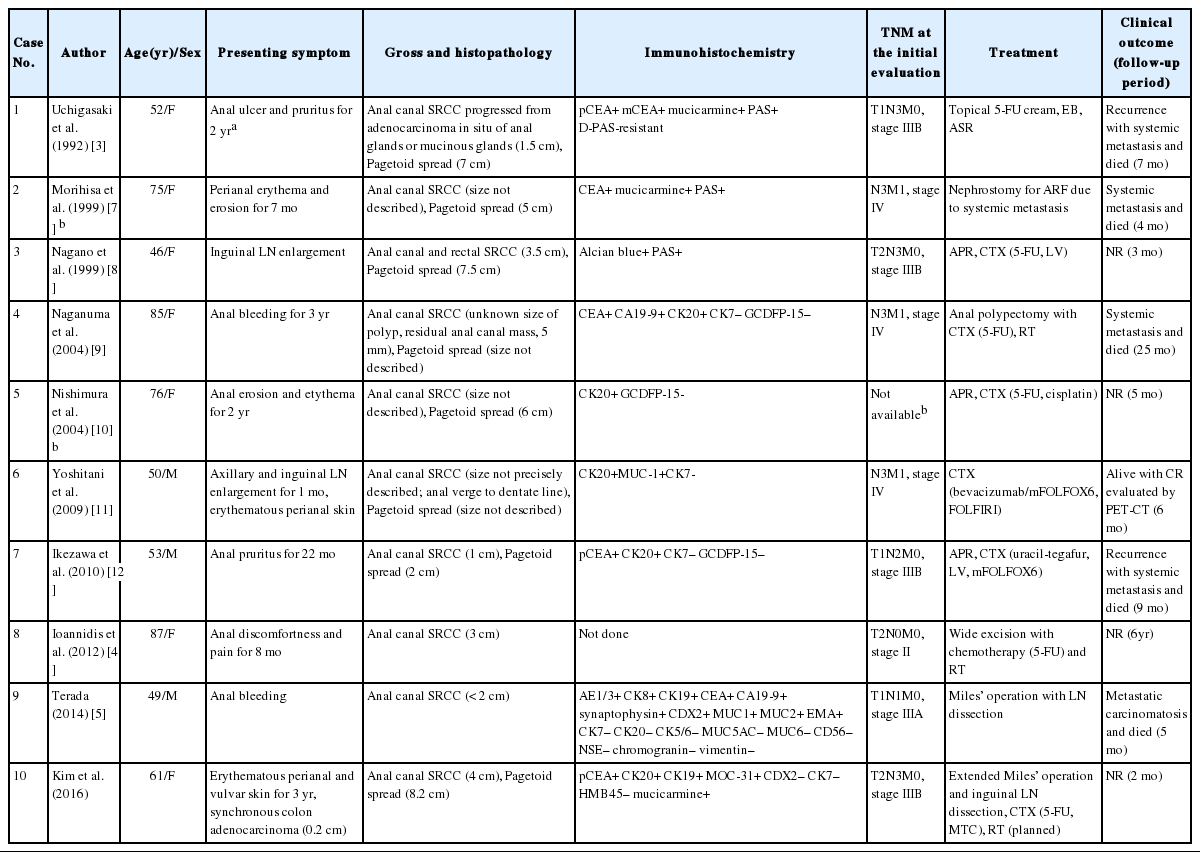
Clinicopathologic summary of the reported cases of primary anal canal signet ring cell carcinomas with or without Paget’s disease
As the term “signetring cells” implies, the tumor cells have intracellular mucin vacuoles displacing the nucleus to one side of the cells scattered as single cells or in loose clusters. This is caused by disrupted cell-to-cell adhesion and diffuse spreading, and results in more frequent lymphovascular invasion and node metastasis of SRCC as compared with other mucinous adenocarcinoma.
In view of the clinical aspects, colorectal SRCC has a very high mortality rate compared to mucinous carcinoma and nonmucinous colorectal adenocarcinomas. Even a minor signet-ring cell component, i.e., 50% or less, in colorectal carcinomas was independently associated with a high mortality rate, regardless of molecular or other clinicopathological factors [17]. Anal canal SRCC grows insidiously underneath the mucosa, and thus it may not be found before the late advanced stage. It is uncertain whether anal SRCCs have an adverse prognosis like that of colorectal SRCC [18]. Inguinal nodes should be examined for anal cancer staging. In a review of 10 cases of anal canal SRCCs, the ages ranged from 46 to 87 years (mean, 63.4 years) [3-5,7-12]. Two cases were proven as primary anal canal SRCCs by autopsy [7,9]. SRCC has a shorter patient survival. During the follow-up period, ranging from 4 to 25 months, death caused by lymphatic or peritoneal carcinomatosis or distant metastases occurred in 50% of cases (5/10) [3,5,7,9,12]. The five remaining cases were alive with a follow-up period ranging from 2 months to 6 years. None of the previous cases underwent moleculocytogenetic application. For the past decade, molecular prognostic markers in colorectal carcinoma have been studied; MSI-H is a well-established reliable predictive marker for chemotherapeutic efficacy in colorectal carcinoma. However, contrary to colorectal SRCCs that take an aggressive course, colorectal mucinous carcinoma takes a favorable course, although both are associated with MSI-H [1]. Data on anal canal SRCC concerning MSI-H as a predictive factor is lacking. The current case showed MSS, albeit it is only one case. Due to the rarity of anal canal SRCCs, the MSI status of anal canal SRCC relating to prognosis has not been determined. Further accumulative investigation is required.
Traditional treatment of anal canal carcinoma is surgery including abdominopelvic resection with or without chemoradiotherapy [19]. Radiation therapy for anal canal carcinoma reaches a cure rate of up to 70% in selected low-stage patients. However, there is no general agreement regarding the treatment of anal canal SRCC due to the extremely rare incidence and limited data on cancer treatment, as shown in Table 2. In a case reported by Yoshitani et al. [11], a patient with distant metastatic foci achieved complete remission after chemotherapy using bevacizumab/mFOLFOX6, FOLFIRI. Ioannidis et al. [4] reported on a long-survived stage II patient with no recurrent tumor who underwent chemoradiotherapy using 5-FU for a 6-year follow-up period.
In summary, anal canal SRCC is extremely rare. If there is perianal Paget’s disease raising the possibility of underlying hidden adenocarcinoma, careful evaluation is necessary for early diagnosis of anal canal SRCC.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.