Evaluation of the VE1 Antibody in Thyroid Cytology Using Ex Vivo Papillary Thyroid Carcinoma Specimens
Article information
Abstract
Background:
Recently, VE1, a monoclonal antibody against the BRAFV600E mutant protein, has been investigated in terms of its detection of the BRAFV600E mutation. Although VE1 immunostaining and molecular methods used to assess papillary thyroid carcinoma in surgical specimens are in good agreement, evaluation of VE1 in thyroid cytology samples is rarely performed, and its diagnostic value in cytology has not been well established. In present study, we explored VE1 immunoexpression in cytology samples from ex vivo papillary thyroid carcinoma specimens in order to minimize limitations of low cellularity and sampling/targeting errors originated from thyroid fineneedle aspiration and compared our results with those obtained using the corresponding papillary thyroid carcinoma tissues.
Methods:
The VE1 antibody was evaluated in 21 cases of thyroid cytology obtained directly from ex vivo thyroid specimens. VE1 immunostaining was performed using liquid-based cytology, and the results were compared with those obtained using the corresponding tissues.
Results:
Of 21 cases, 19 classic papillary thyroid carcinomas had BRAFV600E mutations, whereas two follicular variants expressed wild-type BRAF. VE1 immunoexpression varied according to specimen type. In detection of the BRAFV600E mutation, VE1 immunostaining of the surgical specimen exhibited 100% sensitivity and 100% specificity, whereas VE1 immunostaining of the cytology specimen exhibited only 94.7% sensitivity and 0% specificity.
Conclusions:
Our data suggest that VE1 immunostaining of a cytology specimen is less specific than that of a surgical specimen for detection of the BRAFV600E mutation, and that VE1 immunostaining of a cytology specimen should be further evaluated and optimized for clinical use.
BRAF, a serine/threonine kinase and the v-RAF murine sarcoma viral oncogene homolog B1, is an activator of the mitogen-activated protein kinase (MAPK) pathway [1]. Mutations in BRAF constitutively activate the MAPK pathway, allowing human cancers to develop and progress [1,2]. Of the various BRAF mutations, BRAFV600E, a valine to glutamic acid substitution at codon 600, is the most common [1,3]. In clinical practice, the BRAFV600E mutation is of major interest because it is considered a critical diagnostic, prognostic, and predictive biomarker of many types of cancer [3-6]. Among the many endocrine malignancies, the BRAFV600E mutation is a reliable diagnostic marker of papillary thyroid carcinoma (PTC), as it is detected in 40%–80% of PTCs but virtually never in benign tumors [3,7]. Currently, the BRAFV600E mutation in PTC is typically identified using DNA-based methods such as direct sequencing, allele-specific polymerase chain reaction (PCR), or real-time PCR [7,8]. Although these methods all afford high sensitivity and specificity, expensive equipment and rigorous quality control are required [8-10].
Recently, the VE1 antibody, a monoclonal antibody against the BRAFV600E mutant protein, was investigated in terms of its detection of the BRAFV600E mutation [9]. Although VE1 immunostaining revealed a high concordance rate with molecular methods in surgical specimens of PTC [10-12], evaluation of VE1 in thyroid cytology samples is rarely performed, and its diagnostic value in cytology has not been well established [13-16]. In the present study, we evaluated the use of the VE1 antibody in cytology samples from ex vivo thyroid PTC specimens in order to overcome the drawbacks of fine-needle aspiration (FNA) including low cellularity and sampling/targeting errors [13,15,16], and the results were compared to the data from corresponding PTC tissues.
MATERIALS AND METHODS
This study was approved by the Ajou University Hospital Institutional Review Board (AJIRB-BMR-OBS-13-342). Cytology samples were obtained from fresh ex vivo PTC tissues immediately following surgical resection in cases that provided informed consent. After gross examination of fresh PTC specimens, cytology samples were obtained by scraping representative cancerous areas. Smear slides were prepared and stained with hematoxylin and eosin to explore the adequacy of liquid-based cytology (LBC). In later evaluations, LBC slides were prepared using the BD SurePath method employing CytoRich Red (TriPath Inc., Burlington, NC, USA). PTC tissues were fixed in 4% buffered formalin and, after embedding in paraffin, processed for histology and ancillary tests.
Immunohistochemistry and immunocytochemistry
Formalin-fixed, paraffin-embedded tissue blocks that included the cytology-sampled lesion were sectioned at a 4-μm slice thickness and deparaffinized for immunohistochemistry (IHC). VE1 immunostaining was performed using the aid of a Benchmark XT automated IHC platform (Ventana Medical Systems, Tucson, AZ, USA), as described previously [16]. Briefly, after cell conditioning (conditioner 1) for 64 minutes and inhibition of the preprimary peroxidase, slides were incubated with the VE1 antibody (1:50, Spring Bioscience, Pleasant, CA, USA) at 37°C for 32 minutes. Primary antibodies were detected using an OptiView DAB IHC Detection kit (Ventana Medical Systems) following incubation with hematoxylin and a bluing reagent (4 minutes each). For immunocytochemistry (ICC), unstained LBC slides were fixed in 95% ethyl alcohol for a minimum of 30 minutes. The ICC protocol was identical to that of IHC, except that the cells were not conditioned.
Two pathologists (J.-H.K. and Y.H.K), blinded to the molecular findings, assessed all IHC and ICC data independently; any difference in the interpretation was resolved by consensus. The extent of VE1 staining was graded from 0 to 3: 0, negative; 1, VE1 staining in <30% of cells; 2, VE1 staining in 30%–80% of cells; and 3, VE1 staining in >80% of cells. In terms of cytoplasmic staining of follicular cells, intensity was also graded from 0 to 3: 0, negative; 1, weak; 2, moderate; and 3, strong. In defining BRAFV600E mutation, VE1 immunostaining was considered positive if the intensity of cytoplasmic staining was grade 2 or 3, regardless of the overall extent of staining [13,16].
In cases of discrepancy between immunostaining and molecular results, we repeated immunostaining with a different method, the Ultravision LP Detection System (Thermo Fisher Scientific, Fremont, CA, USA), and re-evaluated the results.
Detection of the BRAFV600E mutation
For genomic DNA isolation, formalin-fixed, paraffin-embedded tissue blocks were sectioned at 10-μm thickness. Genomic DNA was extracted from manually microdissected tumor areas from each tissue section using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. To detect the BRAFV600E mutation, mutant enrichments with 3’-modified oligonucleotide sequencing were performed to confirm the presence or absence of the BRAFV600E mutation, employing primers and PCR conditions as described previously [7]. Results were analyzed using Sequencher 4.10 software (Gene Codes, Ann Arbor, MI, USA).
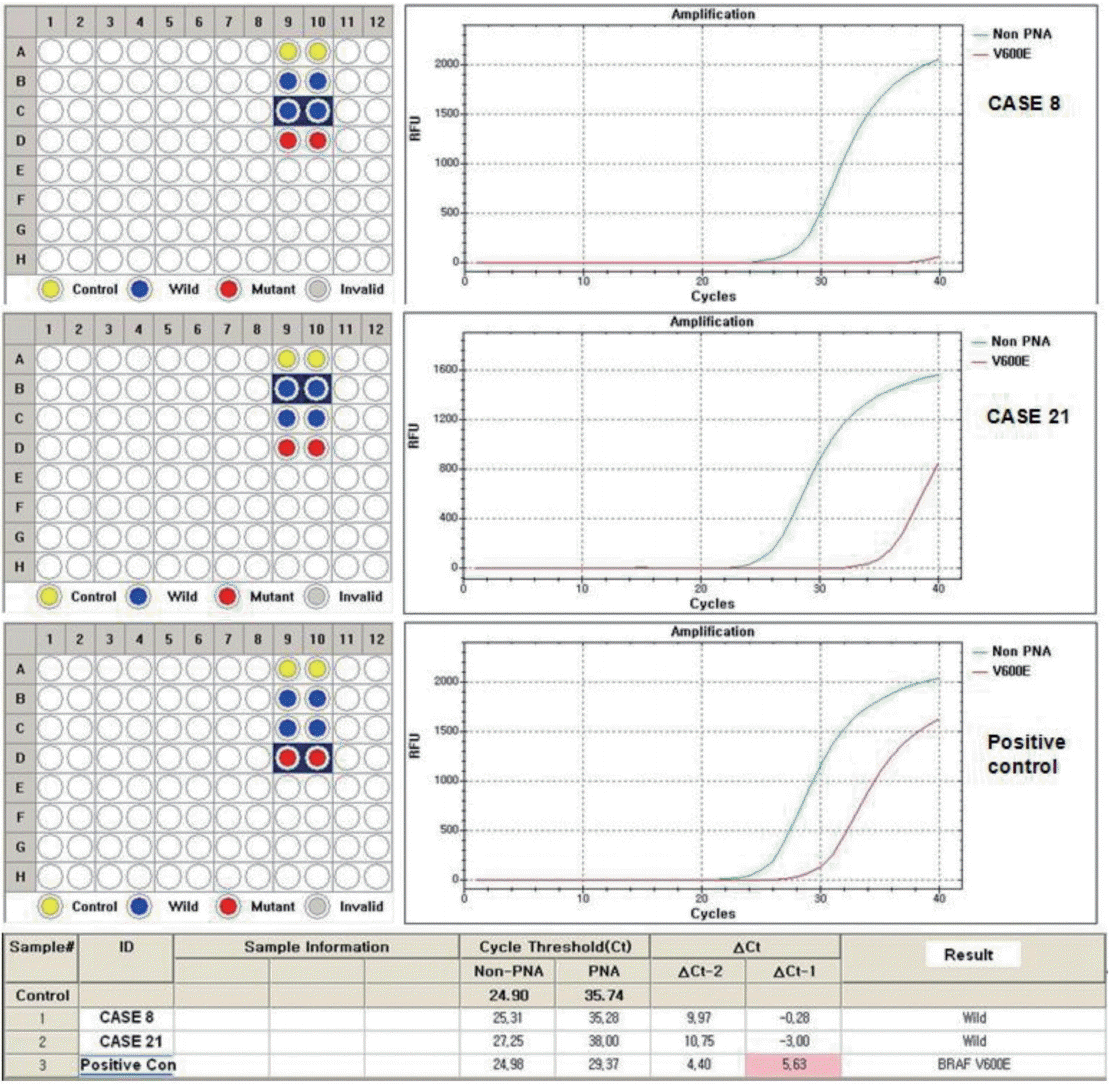
In cases of discrepancy between immunostaining and molecular results, we repeated molecular testing with a different method, the PNAClamp Technology (Panagene, Daejeon, Korea), and re-evaluated the results.
RESULTS
VE1 immunostaining of LBC material and formalin-fixed, paraffin-embedded tissue sections of the corresponding areas was performed in 21 ex vivo PTC specimens. Clinicopathological characteristics of the 21 cases are summarized in Table 1. Of these, 19 were classic PTC cases, and two were follicular variants of PTC. The results of VE1 immunostaining according to BRAFV600E mutation status are shown in Table 2. Of the 21 cases, VE1 IHC of the 19 classic PTC cases exhibited diffuse immunoexpression with moderate or strong intensity, whereas staining in the two follicular variants was weak. Upon VE1 ICC, however, only 11 PTC cases (52.4%) exhibited diffuse immunoexpression (Table 3). The remaining cases yielded focal (2 cases, 9.5%) or multifocal (8 cases, 38.1%) immunostaining patterns (Fig. 1). Of the 21 cases with VE1 ICC, only 11 (52.4%) exhibited immunostaining intensity as strong as that of the corresponding VE1 IHC staining. In six cases (28.6%), immunostaining intensity was weaker than VE1 IHC staining, and in four cases (19.4%), VE1 immunostaining intensity was stronger than VE1 IHC staining (Table 4). VE1 immunostaining was interpreted as positive in 19 IHC and 20 ICC specimens (Table 5). We varied the molecular and immunohistochemical methods in cases of discrepancy between VE1 immunostaining and molecular results, but the results were similar (Appendices 1–3). In terms of the BRAFV600E mutation, VE1 immunostaining exhibited 100% sensitivity and 100% specificity with IHC but 94.7% sensitivity and 0% specificity with ICC.
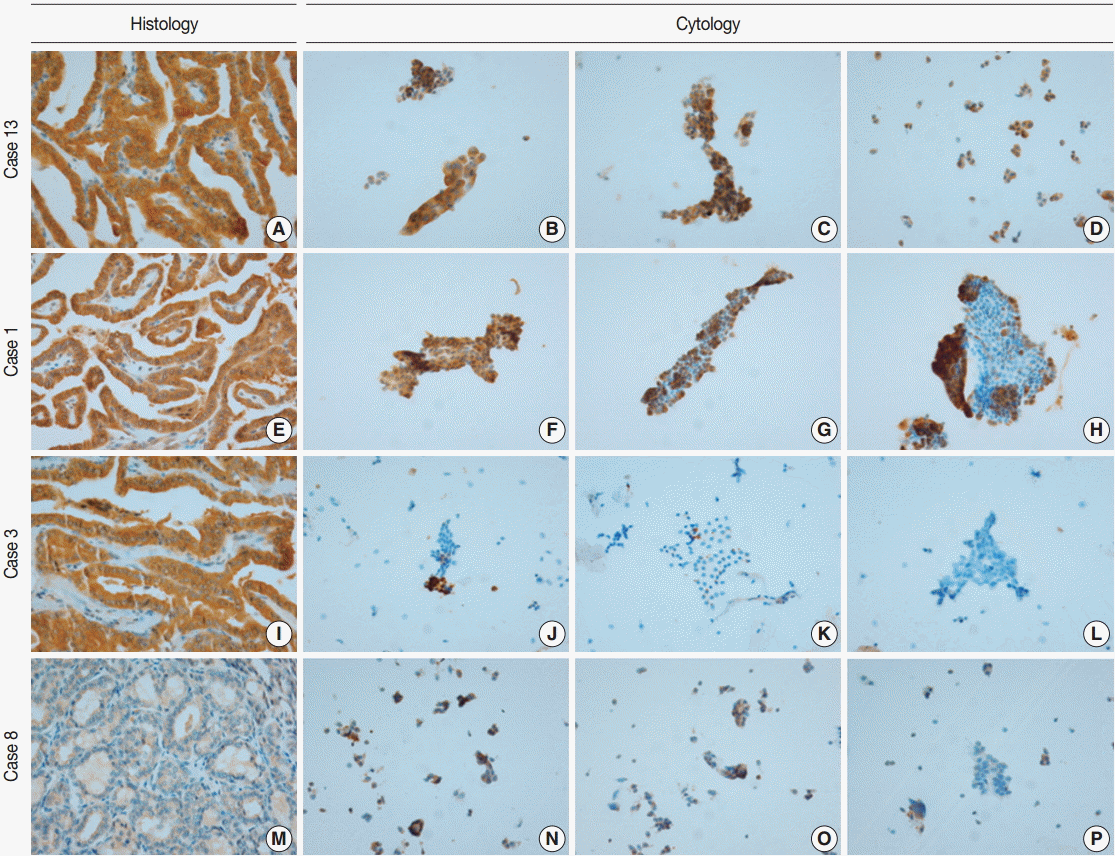
The extent of VE1 immunoexpression in representative cases evaluated histologically (A, E, I, M) and cytologically (B–D, F–H, J–L, N–P). (A, E, I) Diffuse VE1 positivity in classic papillary thyroid carcinomas. (M) VE1 negativity in follicular variant papillary thyroid carcinoma. (B–D) Diffuse VE1 positivity in corresponding cytology. (F–H) Multifocal VE1 positivity in corresponding cytology. (J–L) Focal VE1 positivity in corresponding cytology. (N–P) Multifocal VE1 positivity in corresponding cytology.
DISCUSSION
Clinical applications of VE1 immunostaining in terms of thyroid cytology evaluation are of great interest because PTC diagnosis in daily clinical practice is generally based on thyroid FNA cytology; immunostaining is simple, inexpensive, and routinely performed in most pathology laboratories. Moreover, VE1 immunostaining is not dependent on DNA quality or the proportion of tumor cells in a FNA sample and allows for in situ assessment of tumor cells expressing the BRAFV600E mutant protein at a single-cell level [13,16].
In the present study, we evaluated the VE1 antibody in thyroid cytology using LBC specimens obtained directly from surgically resected ex vivo PTC specimens, because previous studies have suggested that the lower sensitivity and specificity of VE1 ICC compared to those of VE1 IHC might be related to the limitations of thyroid FNA cytology such as the extent of cellularity and the representative nature of the obtained thyroid tissue [13,15,16]. In the present study, all 21 LBC samples contained predominantly tumor cells, representing the cancerous area of each PTC specimen, and had an optimal cellularity for evaluation with VE1 ICC. Our data showed that the VE1 antibody had a higher sensitivity (94.7%) than that afforded by FNA cytology. Rossi et al. [15] and Lee et al. [13] reported sensitivities of 82.0% and 88.8%, respectively, when the VE1 antibody was evaluated in LBC samples. Wobker et al. [14] evaluated the VE1 antibody using smears of thyroid FNA material, but the detection sensitivity (63.6%) was less than that afforded by LBC samples.
Although the cytology specimen in the present study was more representative of the corresponding histology than the cytology of FNA samples, the VE1 immunostaining patterns in ICC differed from those in IHC. All PTCs with BRAFV600E mutation showed diffuse positivity in VE1 IHC, as in previous studies [10,12,17], suggesting that the BRAFV600E mutation represents a clonal event during PTC development [17]. Using ICC, however, only 11 PTCs (57.9%) with the BRAFV600E mutation revealed diffuse positivity; other cases exhibited focal or multifocal positivity. Variations in the intensities and proportions of VE1-positive tumor cells in the same samples were also noted in earlier studies using FNA material [14-16]. Staining variability can be influenced by storage duration, technical problems, or fixation type [14,15,18]. In the present study, ICC on LBC was performed within 48 hours after sampling. In an attempt to eliminate technical problems, VE1 ICC was performed using different methods, but the results were similar (Appendix 1). It has been suggested that ethanol-based fixation destabilizes proteins not only in histology [19], but also in cytology [18]. We used a methanol- and isopropanol-based preservative (CytoRich Red) containing 1% formalin as a fixative, which is known to be more compatible with ICC than an ethanol-based fixative [18]. Nonetheless, we found that VE1 ICC was less sensitive than IHC in detecting BRAFV600E mutation. Previous studies found that the extent of disagreement between ICC and IHC was 7.2%–34.7% and suggested that differences in fixation methods might explain the observed discrepancies [14-16]. Our results also indicate that differences in fixation between ICC and IHC are a major contributing factor resulting in different VE1 immunoexpression in the same tissue samples.
Upon IHC, VE1 was detected with high specificity, but the ICC specificity was 0% because one PTC harboring the BRAFV600E mutation was negative for VE1, while two follicular variant PTCs lacking the BRAFV600E mutation were positive for VE1 immunostaining. We varied the molecular and immunohistochemical methods used, but the results were similar (Appendices 2, 3). To evaluate the specificity, the number of wild-type PTC samples in the present study was too small. Nonetheless, false positivity of VE1 in thyroid cytology should not be underestimated. Nonspecific staining of colloids, macrophages, and follicles containing colloids or stroma has been suggested to hamper the interpretation of VE1 ICC [13-16]. One recent study showed that the VE1 antibody cross-reacted with certain ciliary structural proteins, inducing VE1 false positivity [20]. Some proteins expressed in endocrine organs, including α-ketoglutarate-dependent dioxygenase alk-B homolog 7, eukaryotic translation initiation factor 2-α kinase 4, polo-like kinase-1δ, potassium channel tetramerization domain-containing 4, and solute carrier family 4 (anion exchanger) member 3, also share sequence similarities with the peptide immunogen used to generate the VE1 antibody [20]. Such cross-reactivities might possibly explain the nonspecific staining of, or false-positivity for, VE1 in thyroid PTC samples.
This study has several limitations, mostly stemming from its small number of cases. Nonetheless, the results from the present study suggest that VE1 ICC is less specific than VE1 IHC in detecting the BRAFV600E mutation. For clinical application of the VE1 antibody in thyroid cytology, further evaluation and optimization of VE1 immunostaining in cytology specimens are essential.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We thank So Hyun Park for her contribution of immunostaining, Seung Jung Jo for her kind help in cytologic preparations, and Se Hwa Son for her assistance in molecular works. Finally, we acknowledge the late Ga-Young Lee, M.D., a former member of our pathology department, for her substantial contribution during the early stages of this work.
This study was supported by a grant (2012R1A1A2004721) of the National Research Foundation of Korea (NRF) to Jang-Hee Kim.
References
Appendices
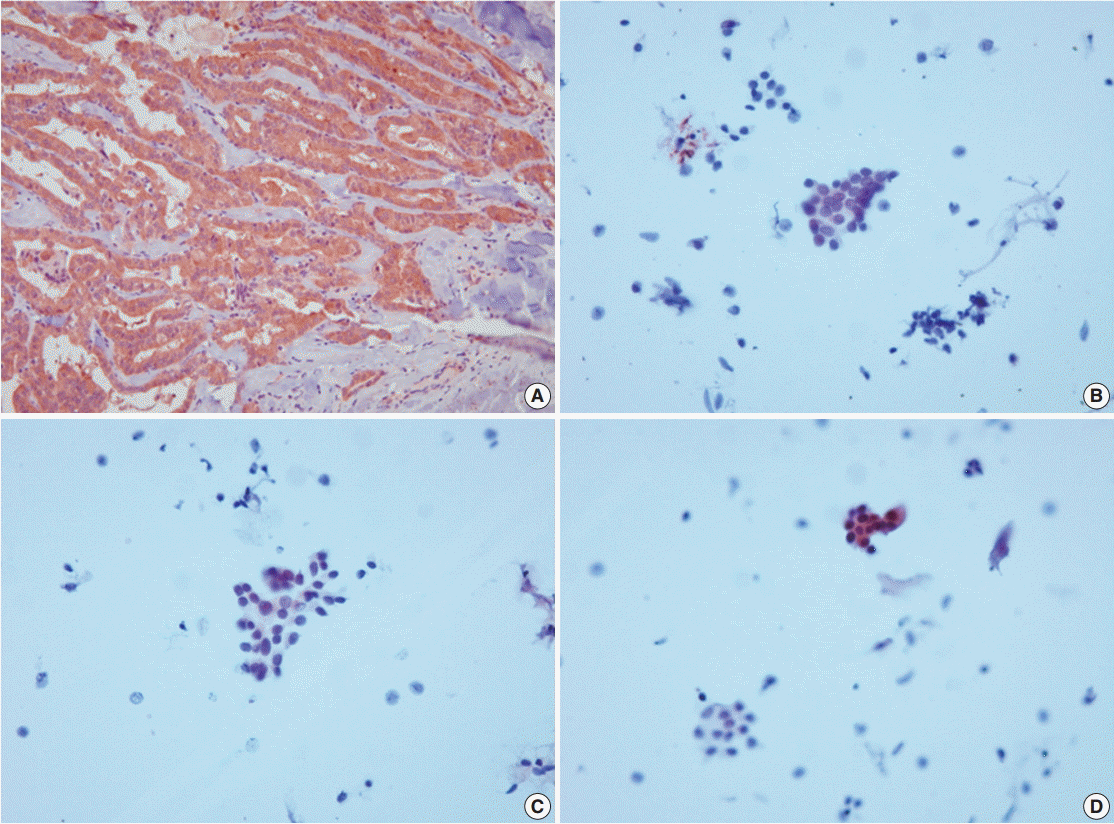
Appendix 1.
VE1 immunoexpression in histology and cytology of case 3 with manual method using the Ultravision LP Detection System (Thermo Fisher Scientific). (A) Diffuse and strong VE1 expression in histology of case 3. (B–D) Focal VE1 expression in corresponding cytology.
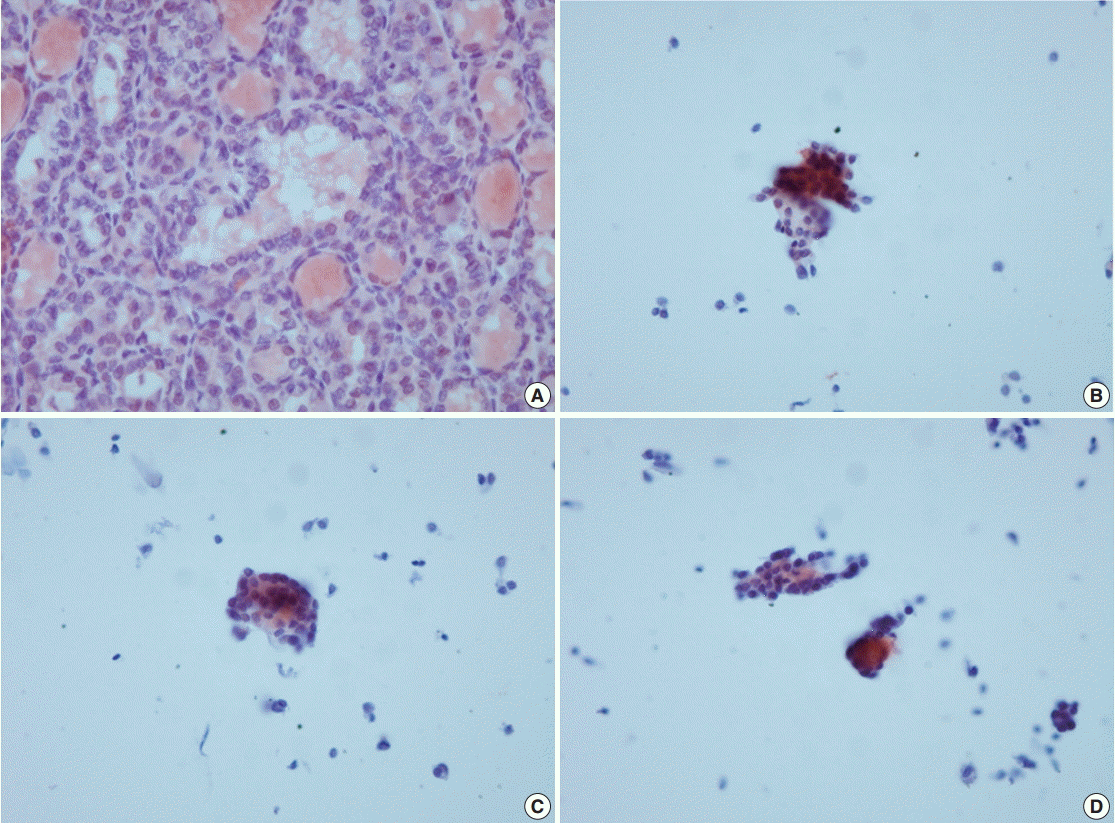
Appendix 2.
VE1 immunoexpression in histology and cytology of case 8 with manual method using the Ultravision LP Detection System (Thermo Fisher Scientific). (A) Negative VE1 expression in histology of case 8. (B–D) Multifocal VE1 expression in corresponding cytology.
Appendix 3.
No BRAFV600E mutation in case 8 and case 21 by peptide nucleic acid clamping method.