Expressions of E-cadherin, Cortactin and MMP-9 in Pseudoepitheliomatous Hyperplasia and Squamous Cell Carcinoma of the Head and Neck: Their Relationships with Clinicopathologic Factors and Prognostic Implication
Article information
Abstract
Background
E-cadherin, cortactin, and matrix metalloproteinase (MMP)-9 have roles in tumor development or progression, but their expression has not been fully investigated in pseudoepitheliomatous hyperplasia (PEH) and squamous cell carcinoma (SCC) of the head and neck.
Methods
We evaluated the immunohistochemical expression of E-cadherin, cortactin, and MMP-9 in 29 cases of PEH and 97 cases of SCC. Additionally, we evaluated their relationship with clinicopathologic factors and prognostic implications in SCC.
Results
Thirty-five cases of SCC showed reduced expression of E-cadherin, whereas none of the PEH did. A total of 20 cases and 11 cases of SCC were immunoreactive for cortactin and MMP-9, respectively, whereas none of the PEH did. In SCC, reduced expression of E-cadherin was correlated with cortactin expression and invasion depth. Cortactin expression was correlated with differentiation, T classification, and recurrence and/or metastasis. MMP-9 expression was correlated with invasion depth. Cortactin expression was correlated with poor overall survival and relapse-free survival and it was an independent prognostic factor.
Conclusions
The reduced expression of E-cadherin and the expression of cortactin may be helpful for the differential diagnosis of PEH and SCC. Furthermore, cortactin expression in association with reduced E-cadherin expression is correlated with poor prognosis in SCC.
Pseudoepitheliomatous hyperplasia (PEH) is a reactive epithelial proliferation that occurs in response to underlying infections, inflammations, or neoplasms.1 Histologically, PEH is characterized by proliferating strands of thin, markedly elongated, anastomosing epithelium, and heavy infiltration of inflammatory cells, as well as varying degrees of hyperkeratosis and papillomatosis. Squamous cell carcinoma (SCC) of the head and neck region represents a major worldwide health problem, with patients exhibiting a 5-year survival rate of <50%.2 Compared with SCC, PEH lacks pronounced nuclear atypia, abundant or abnormal mitosis, and prominent dyskeratosis.3 However, the histological features of PEH may be similar to well-differentiated SCC and their differential diagnosis may be difficult.4
E-cadherin is an intercellular homophilic calcium-dependent cell adhesion molecule that plays a critical role in cell adhesion and the suppression of tumor invasion.5 Loss of E-cadherin expression has been reported in various malignant tumors, including SCC, and immunohistochemistry for E-cadherin may be a useful tool to differentiate SCC from PEH.4 Loss of E-cadherin expression may be a promising prognostic marker for SCC of the head and neck region.6
Cortactin is an F-actin-binding protein that binds and activates the actin-related protein (Arp) 2/3 complex to regulate dynamic actin networks in cell migration and adhesion.7 Cortactin is recruited to cell-cell adhesive contacts in response to hemophilic E-cadherin ligation.8 Therefore, cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. In addition, it has been reported to be an essential regulator of matrix metalloproteinase (MMP) secretion and extracellular matrix degradation in invadopodia of head and neck SCC,9 and its overexpression has been reported in several human cancers of the esophagus, liver, stomach, and breast.10-13
MMPs are a large family of proteases that collectively can degrade virtually all extracellular matrix components and can regulate the tumor microenvironment by processing substrates including growth factors and their receptors, cell adhesion molecules, cytokines, chemokines, apoptotic ligands, and angiogenic factors.14 MMP-9 degrades type IV collagen, which is major component of the basement membrane in human tissues, and allows the tumor cells to escape the site of the primary tumor, leading to invasion and metastasis.15 An increased concentration of serum MMP-9 has been detected in many patients with head and neck SCC16 and MMP-9 expression has been shown to be a prognostic indicator for shortened survival in patients with head and neck SCC.17 In addition, overexpression of cortactin may increase extracellular matrix degradation and secretion of MMP-2 and MMP-9, correlated with cortactin expression level.9
Recent studies of E-cadherin, cortactin, and MMP-9 have suggested that the proteins play roles in tumor development or progression, although their expressions has not been fully investigated in PEH and SCC of the head and neck region. In this study, we evaluated the immunohistochemical expression of E-cadherin, cortactin, and MMP-9 in PEH and SCC of the head and neck region and the correlations between cortactin, MMP-9, and E-cadherin expression and clinicopathologic factors and their prognostic significance.
MATERIALS AND METHODS
Patients and tissue samples
This study included 29 cases of PEH obtained from mucosal biopsy, 21 cases of SCC from biopsy, and 97 cases of SCC from excision or resection in the head and neck region obtained between June 2000 and December 2008 in Chonbuk National University Hospital. We obtained clinical data as well as survival data from the medical records of PEH and SCC patients. Histologic findings for all cases were reviewed by two authors (T.K. You and H.S. Park), with consensus. We considered a case to be PEH when there was a downward proliferation of epithelium characterized by thin, markedly elongated, ananstomosing strands into the submucosa, and heavy infiltration by inflammatory cells.4 The individual cells were mature with only occasional dyskeratosis. Hematoxylin and eosin (H&E) stained slides of SCC were reviewed and were graded according to the World Health Organization classification.18 Pathologic staging was reviewed based on the tumor, node, metastasis staging system of the American Joint Committee on Cancer.19 SCC patients were grouped according to age, sex, differentiation, invasion depth, T classification, perineural invasion, lymphovascular invasion, recurrence and/or metastasis, and stage. The study was approved by the local ethics committee of the Institutional Review Board of Chonbuk National University Hospital.
Immunohistochemical staining and scoring
The tissue sections were de-paraffinized and treated in sodium citrate buffer (pH 6.0) for 10 minutes using a pressure-cooker antigen-retrieval procedure. Endogenous peroxidase was blocked by incubation with a peroxidase blocker (Dako, Carpinteria, CA, USA) for 10 minutes. Then the tissue sections were incubated for 2 hours at room temperature with a monoclonal anti-cortactin antibody (1 : 50, clone 30, BD Bioscience, Franklin Lakes, NJ, USA) and monoclonal anti-E-cadherin (1 : 200, clone 36, BD Bioscience). Another tissue section was incubated overnight at 4℃ with a polyclonal anti-MMP-9 antibody (1 : 100, Thermo Fisher Scientific, Fremont, CA, USA). For the negative controls, sections were treated in the same manner but incubated using Tris-buffered saline without the primary antibody. Reaction products were visualized by immersing the section in 3-amino-9-ethyl-carbazole (AEC) chromogen (Dako), and the samples were counterstained with Meyer's hematoxyline. Immunohistochemical analysis was performed by consensus of two authors (T.K. You and H.S. Park) who were blinded to clinicopathologic information. Immunoreactivity of E-cadherin, cortactin, and MMP-9 was evaluated by multiplying the staining intensity and stained area scores. Staining intensity was rated as: weak, 1; moderate, 2; and strong, 3. The stained area of E-cadherin was categorized into four groups: negative, 0; ≤50% of cells, 1; 51-75% of cells, 2; and >75% of cells, 3.20 The stained area of cortactin was classified into four groups: negative, 0; <25% of cells, 1; 25-50% of cells, 2; and >50% of cells, 3.21 The stained area of MMP-9 was divided into four groups: negative, 0; <10% of cells, 1; 10-50% of cells, 2; >50% of cells, 3.22 The immunoreactivity of E-cadherin was divided into two groups based on the product of the scores; a score >6 was defined as preserved immunoreactivity and a score of 0-6 were defined as reduced immunoreactivity. The immunoreactivity of cortactin and MMP-9 was divided into two groups in the same manner; a score of 0-3 was defined as negative immunoreactivity and a score ≥4 was defined as positive immunoreactivity.
Statistical analysis
The endpoints of interest were overall survival and relapse-free survival. The endpoint of follow-up was the date of the last contact or the date of death through December 2011. Overall survival was calculated as the time from diagnosis to the date of death or last contact. Patients who were alive at last contact were censored for overall survival analysis. Relapse-free survival was calculated from the time of diagnosis to the date of recurrence, metastasis, death, or last contact. Patients who were alive at last contact and who did not have recurrence or metastasis were censored for relapse-free survival analysis. The correlations between clinicopathologic factors and the expressions of E-cadherin, cortactin, and MMP-9 were analyzed using Pearson's χ2 test. Univariate and multivariate Cox proportional hazards regression analyses were performed to estimate the impact of the clinicopathologic factors and the expression of each marker on overall survival and relapse-free survival. Kaplan-Meier survival curves were constructed to further illustrate the impact of overall survival and relapse-free survival when indicated. SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and p<0.05 was considered statistically significant.
RESULTS
Patient characteristics
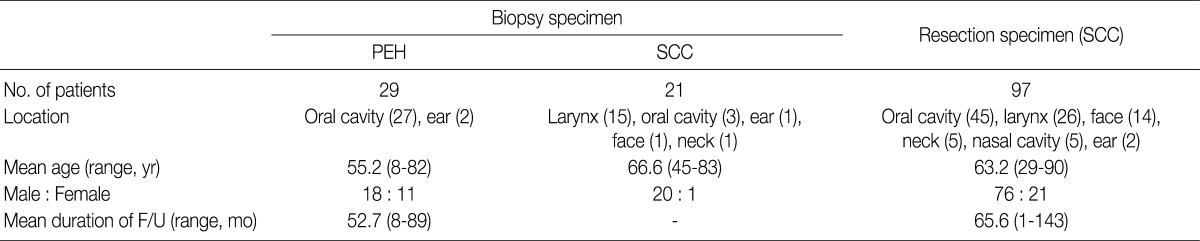
Patient characteristics are summarized in Table 1. In 29 cases of PEH, the age of patients ranged from 8 to 82 years (mean age, 55.2 years). Eighteen patients were males and 11 were females. Most cases were located in the oral cavity (27 cases) and 2 were located in the ear. The duration of follow-up ranged from 8 to 89 months (mean duration, 52.7 months), and no cases of recurrence were observed during the follow-up period. In 21 cases of SCC from biopsy, the age of patients ranged from 45 to 83 years (mean age, 66.6 years). Twenty patients were males and 1 was female. There were 15 cases located in the larynx, 3 cases in the oral cavity, and 1 each in the ear, face, and neck. In 97 cases of SCC from resection, the age of patients ranged from 29 to 90 years (mean age, 63.2 years). Seventy-six patients were males and 21 were females. The locations were the oral cavity (45 cases), larynx (26), face (14), neck (5), nasal cavity (5), and ear (2). The duration of follow-up ranged from 1 to 143 months (mean duration, 65.6 months); 45 patients died and 38 patients developed recurrences and/or metastases during the follow-up period.
Associations of E-cadherin, cortactin, and MMP-9 expression in PEH and SCC with clinicopathologic characteristics
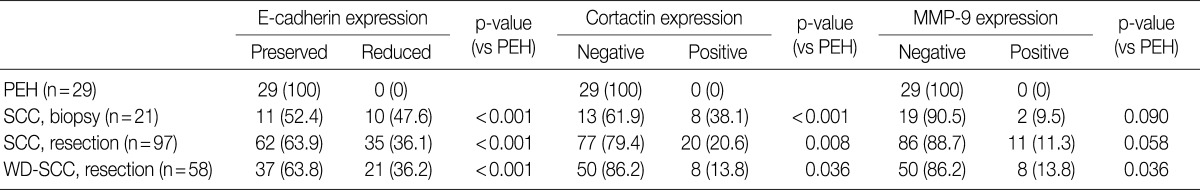
Representative images of H&E stain in PEH and SCC are shown in Fig. 1. E-cadherin was detected in the cell membranes of PEH and SCC specimens. All of the PEH cases showed preserved expression of E-cadherin, whereas 47.6% (10 of 21 cases from biopsy) and 36.1% (35 of 97 cases from resection) of SCC specimens showed reduced E-cadherin expression, respectively (Table 2, Fig. 1C, D). Compared with PEH, 36.2% of well-differentiated SCC also showed reduced E-cadherin expression. None of the PEH cases expressed cortactin, whereas 38.1% (8 of 21 cases from biopsy) and 20.6% (20 of 97 cases from resection) of SCC cases expressed cortactin, including 13.8% of well-differentiated SCC cases (Table 2, Fig. 1E, F). Cortactin was detected in the cytoplasm of the SCC cells. None of the PEH cases expressed MMP-9, whereas 9.5% (2 of 21 cases from biopsy) and 11.3% (11 of 97 cases from resection) of SCC cases expressed MMP-9, including 13.8% of well-differentiated cases (Table 2, Fig. 1G, H). MMP-9 was detected in the cytoplasm of the SCC cells.
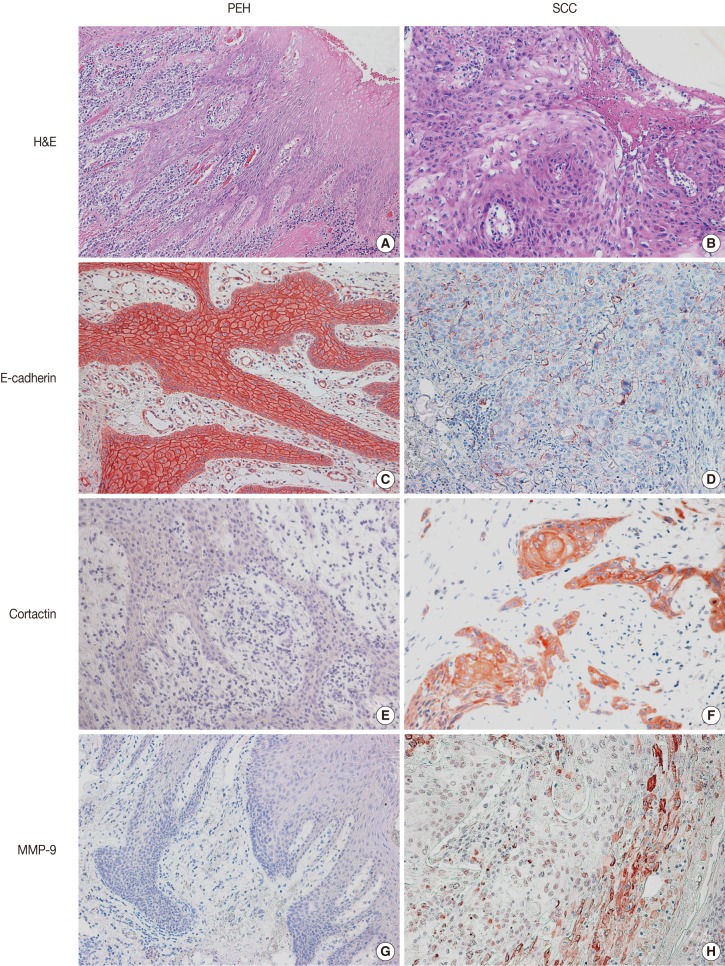
Histologic findings and immunohistochemical staining for E-cadherin, cortactin, and matrix metalloproteinase 9 (MMP-9) in pseudoepitheliomatous hyperplasia (PEH) (A, C, E, G) and in squamous cell carcinoma (SCC) (B, D, F, H). PEH shows thin, elongated, reactive epithelial proliferation with intense subepithelial inflammation (A). SCC shows atypical squamous cells with a loss of polarity in the superficial portion (B). Expression of E-cadherin is preserved in PEH (C) but is reduced in SCC (D). PEH is negative for cortactin (E) and MMP-9 (G), whereas SCC shows cytoplasmic expression of cortactin (F) and MMP-9 (H). H&E, hematoxylin and eosin.
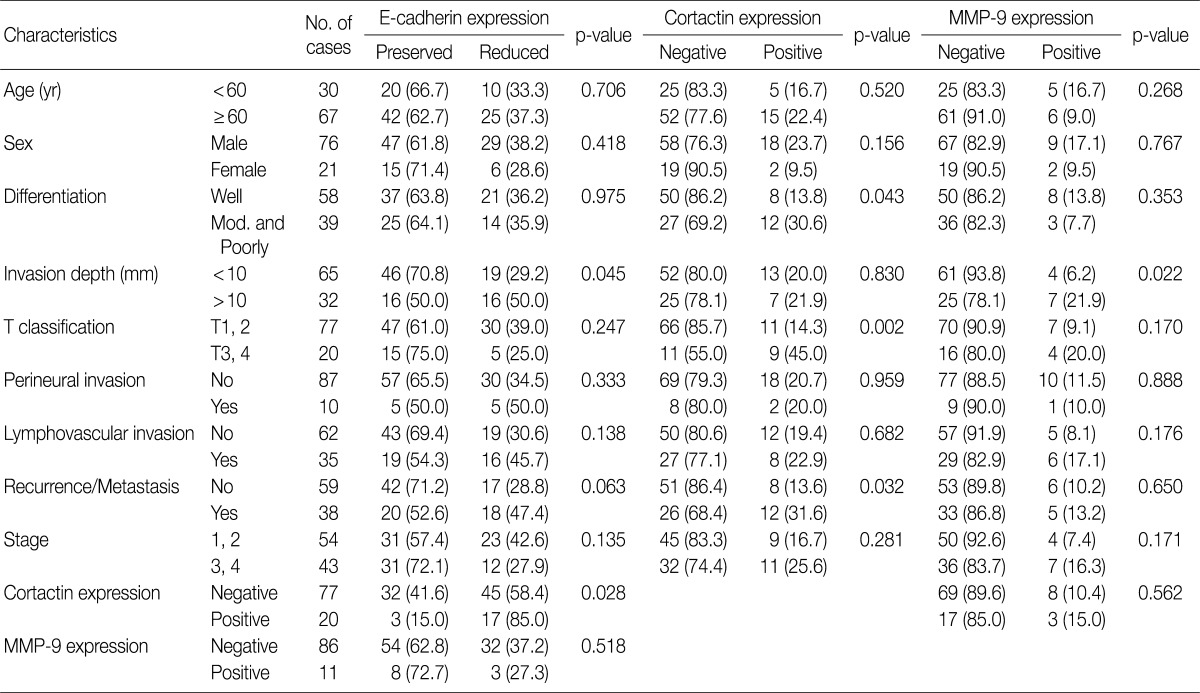
In SCC cases from resection, reduced E-cadherin expression was associated with invasion depth (p=0.045) (Table 3). Cortactin expression was associated with differentiation (p=0.043), T classification (p=0.002) and recurrence and/or metastasis (p=0.032) (Table 3). MMP-9 expression was associated with invasion depth (p=0.022) (Table 3). However, other clinicopathologic factors including age, sex, perineural invasion, lymphovascular invasion, and stage had no association with reduced expression of E-cadherin, expression of cortactin and MMP-9. There was an association between reduced E-cadherin expression and cortactin expression (p=0.028), however neither was associated with MMP-9 expression (Table 3).
Associations of E-cadherin, cortactin, and MMP-9 expression with overall survival and relapse-free survival in SCCs
Univariate Cox proportional hazard analysis of E-cadherin, cortactin, and MMP-9 expression and their association with overall survival and relapse-free survival is shown in Table 4. Perineural invasion (p<0.001) (Fig. 2A), lymphovascular invasion (p=0.028), recurrence and/or metastasis (p<0.001), stage (p=0.008) (Fig. 2B), postoperative radiation (p=0.022), and cortactin expression (p<0.001) (Fig. 2C) predicted shorter overall survival. In addition, perineural invasion (p=0.027) (Fig. 2D), stage (p=0.002) (Fig. 2E), postoperative radiation (p<0.001), and cortactin expression (p<0.001) (Fig. 2F) predicted shorter relapse-free survival. In addition, the combined expressions of E-cadherin and cortactin was analyzed to evaluate its relationship with overall survival and relapse-free survival. In this analysis, 94 cases of SCC were divided into three groups: E-cadherin-preserved/cortactin-negative, E-cadherin-reduced/cortactin-negative, and E-cadherin-reduced/cortactin-positive. Cases of E-cadherin-preserved/cortactin-positive SCC were excluded because of their small number. The E-cadherin-reduced/cortactin-positive group had the worst prognosis in overall survival (p=0.001) (Fig. 3A) and relapse-free survival (p=0.001) (Fig. 3B).
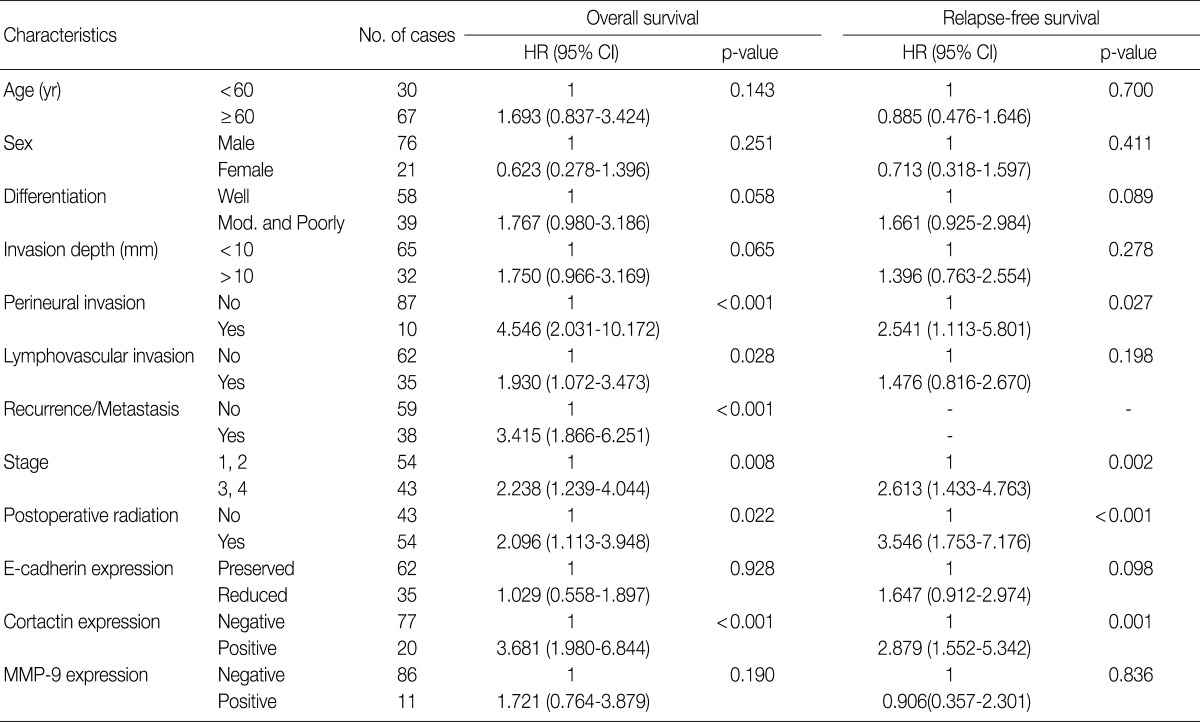
Clinicopathologic factors and their effects on overall survival and relapse-free survival by univariate Cox proportional hazard regression analysis
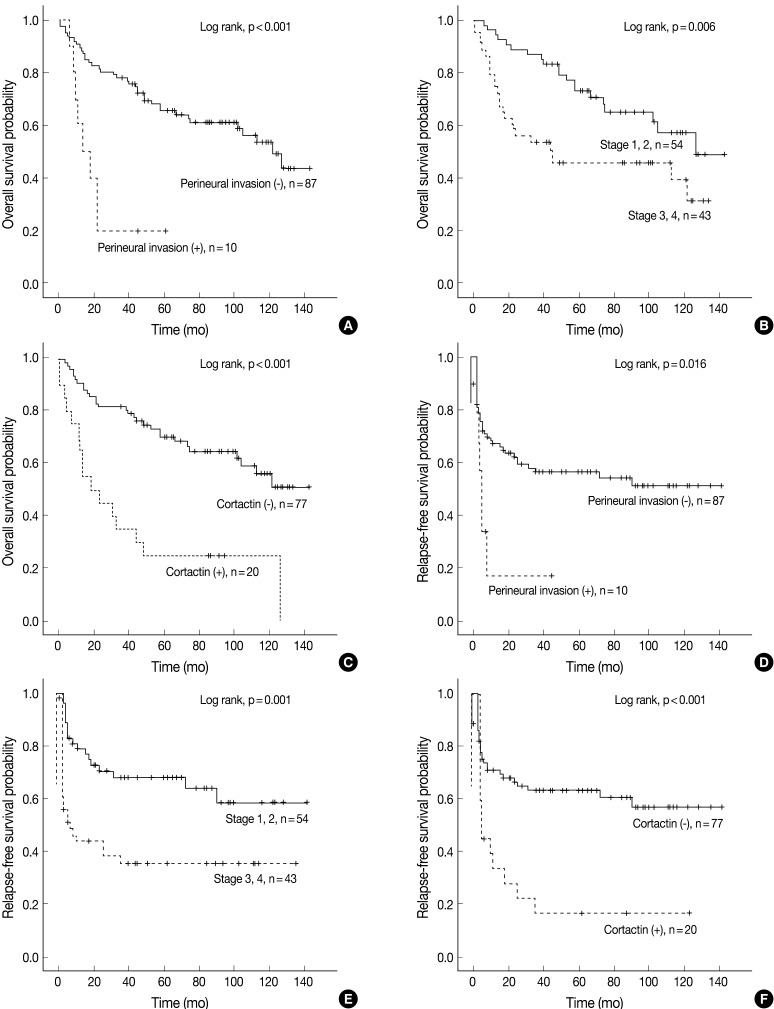
Kaplan-Meier survival analysis of squamous cell carcinoma in the head and neck region. Overall survival in groups according to perineural invasion (A), stage (B), and cortactin expression (C). Relapse-free survival in groups according to perineural invasion (D), stage (E), and cortactin expression (F). p-values are determined by comparing survival distributions using the log rank test.
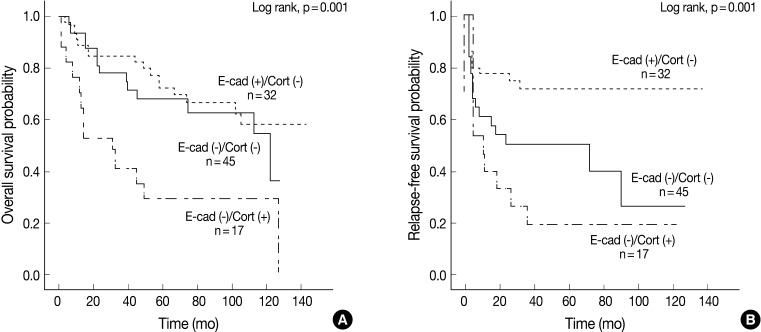
Kaplan-Meier survival analysis of 94 cases of squamous cell carcinoma grouped by combined expression patterns of E-cadherin and cortactin. Overall survival (A) and relapse-free survival (B) in three groups: E-cadherin-preserved/cortactin-negative group [E-cad (+)/Cort (-)], E-cadherin-reduced/cortactin-negative group [E-cad (-)/Cort (-)], and E-cadherin-reduced/cortactin-positive group [E-cad (-)/Cort (+)]. p-values are determined by comparing survival distributions using the log rank test.
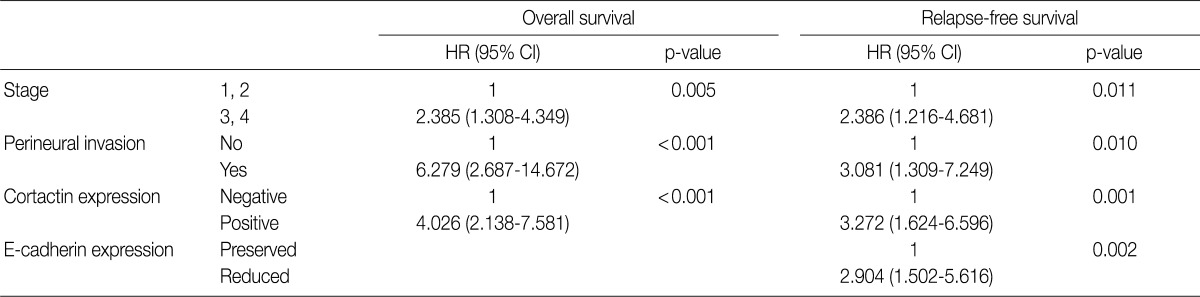
Multivariate analysis was performed in all 97 cases of SCC patients with complete information for all variables. Variables considered in the analysis were age, sex, stage, postoperative radiation, differentiation, perineural invasion, cortactin expression, MMP-9 expression, and E-cadherin expression. From the multivariate analysis, the independent prognostic factors that were significantly associated with overall survival and relapse-free survival were stage (p=0.005 and p=0.011, respectively), perineural invasion (p<0.001 and p=0.010, respectively), and cortactin expression (p<0.001 and p=0.001, respectively) (Table 5). In addition, reduced E-cadherin expression (p=0.002) was an independent prognostic factor significantly associated with poor relapse-free survival.
DISCUSSION
We investigated the immunohistochemical expressions of E-cadherin, cortactin, and MMP-9 in PEH and SCC specimens of the head and neck region, as well as the correlations between expression of E-cadherin, cortactin, and MMP-9 and clinicopathologic factors and their prognostic significance. We found that 1) 47.6% of SCCs from biopsy and 36.1% of SCCs from resection showed reduced E-cadherin expression, whereas none of the PEH specimens did, and the reduced preservation of E-cadherin expression was significantly correlated with an invasion depth of more than 10 mm; 2) 38.1% of SCCs from biopsy and 20.6% of SCCs from resection expressed cortactin, whereas none of the PEH specimens did, and cortactin expression was significantly correlated with moderate or poor differentiation, advanced T stage (T3, 4), and recurrence and/or metastasis; 3) 9.5% of SCCs from biopsy and 11.3% of SCCs from resection expressed MMP-9, whereas none of the PEH specimens did, and its expression was significantly correlated with an invasion depth of more than 10 mm; 4) reduced E-cadherin expression was associated with cortactin expression in SCCs of the head and neck region; 5) cortactin expression was associated with significantly shorter overall survival and relapse-free survival in SCCs of the head and neck region.
Histologically, PEH does not demonstrate cytological features of malignancy such as nuclear pleomorphism, maturation atypia, mitosis, and stromal invasion, although it may show reactive atypia.4 However, it is sometimes extremely difficult to distinguish PEH from SCC, especially from well-differentiated SCC. Moreover, mistaking PEH for SCC may lead to the unnecessary removal of additional tissue or to unnecessary therapy, such as radiation therapy. For this reason, some studies have tried to distinguish PEH from SCC using immunohistochemical markers including E-cadherin; p53; MMP-1, -7, -12, -13, and -19; and p16.4,23 The increased expressions of p53 and MMP-1, -7, -12, and -13, and decreased expression of E-cadherin, p16, and MMP-19 has been shown in SCC.4,23 In this study, SCCs from both biopsy and resection showed reduced immunoexpression of E-cadherin and immunoreactivity for cortactin and MMP-9, whereas this was not true of the PEH specimens. Moreover, well-differentiated SCCs showed a different immunoexpression pattern from those of PEH.
E-cadherin is a transmembrane molecule that plays an important role in both cell-cell adhesion and cell signal transduction. Decreased expression of E-cadherin has been reported in various malignant tumors including carcinomas of the breast, esophagus, stomach, and colon. Moreover, it has been associated with vascular invasion and poor prognosis in SCCs of the head and neck.24 Our results showed that reduced expression of E-cadherin is associated with invasion depth and relapse-free survival. In addition, the E-cadherin-reduced/cortactin-positive SCC group had a worse prognosis for overall survival and relapse-free survival than the other two groups. This suggests that reduced expression of E-cadherin may play a role in tumor progression in association with cortactin expression in SCCs of the head and neck.
Cortactin contributes to the formation of the adherens junction, which is formed upon homophilic binding of E-cadherin molecules on juxtaposed cells. In MDCK cell monolayers, cortactin localizes to E-cadherin-based cell-cell contacts, and cortactin is recruited along with the Arp2/3 complex to the extending outer margins of cadherin-based lamellipodia.8 These data indicate that cortactin plays a role in the actin re-organization required for the formation of stable cadherin-based contact zones. A regulator of Arp 2/3-mediated actin polymerization contributes to tumor cell growth and cancer progression.7 The amplification of cortactin has been reported in 30% of SCCs in the head and neck region,25 and it is correlated with metastasis of esophageal SCC and poor prognosis of SCC in the head and neck.26,27 Our results are concordant to those of previous studies in that 20.6% of SCCs expressed cortactin, and its expression was an independent prognostic factor associated with poor overall survival and relapse-free survival.
MMPs are a family of proteolytic enzymes involved in the degradation of extracellular matrix components that play a role in several steps of tumor progression including invasion, angiogenesis, and metastasis. In some studies that investigated the expression of MMPs of SCC in the head and neck, these enzymes were up-regulated in SCC, and their levels were correlated with tumor invasion, metastases, and patient survival.28 It has also been reported that MMP-9 expression is associated with the invasion in SCCs of the esophagus29 and with poor survival in SCCs of the head and neck.30 However, in the present study, MMP-9 expression was associated with invasion depth only. Moreover, MMP-9 expression was not associated with other clinicopathologic factors or prognosis. We believe that the different results between the present and others may be due to differences in sample size, methods of scoring criteria, and the antibodies used to evaluate expression. Furthermore, enzymatic activity is more important to proper function than is immunohistochemical expression. More studies are needed to evaluate the correlations between MMP-9 expression and clinicopathological factors in SCCs of the head and neck region.
In conclusion, the expression of E-cadherin, cortactin, and MMP-9 may play a role in the progression of SCCs in the head and neck region. Moreover, we suggest that the expression of cortactin, which is associated with reduced expression of E-cadherin, may be a marker for poor prognosis of SCCs in the head and neck region.
Acknowledgments
This work was supported by a National Research Foundation of Korea Grant funded by the Korean Government (No. 2011-0028223).
Notes
No potential conflict of interest relevant to this article was reported.