DPC4 Expression in the Small Intestinal Adenocarcinomas
Article information
Abstract
Background
Small intestinal adenocarcinomas (SACs) are rare malignancies of the alimentary tract with uncertain carcinogenesis.
Methods
We investigated the expression of deleted in pancreatic cancer 4 (DPC4) in 188 cases of surgically resected SACs, using tissue microarray technology.
Results
Twenty-four of the 188 tumors showed complete loss of Smad4/DPC4 expression in cytoplasm (score, 0; 12.8%). Eighty-four and 31 cases were moderately and strongly positive, respectively (score, 2 and 3; 44.7% and 16.5%, respectively) and 49 cases were focally or weakly stained (score, 1; 29.1%). Immunohistochemistry analysis showed that the expression of Smad4/DPC4 was related to an increased risk of lymphatic invasion but not to other clinicopathological features of the tumors (tumor location, differentiation, growth pattern, T stage, direct invasion, vascular invasion, and nodal metastasis). There was no significant association between Smad4/DPC4 expression and patient survival.
Conclusions
The present research is the first study to evaluate Smad4/DPC4 expression in a large sample of SACs with clinicopathologic correlation. Future studies should focus on the immunohistochemical and molecular characteristics of SACs to clarify their tumorigenesis.
The small intestine is the largest part of the digestive system. At a length of nearly 3 m, it represents 75% of the length of the digestive system and 90% of the mucosal surface.1-5 Despite its length and unique location between the stomach and the colon-two organs with a high incidence of malignancies-small intestinal neoplasms are very rare; the site presents only 3.1% of digestive tract malignancies.2 There is no obvious interpretation for this puzzling discrepancy, although several hypotheses have been suggested, including that the rapid transition time and the diluted nature of the contents in the small intestine may decrease the exposure time of small intestinal epithelial cells to carcinogenic substances. In addition, a low bacterial count may reduce the synthesis of carcinogens from the bacterial decomposition of biliary materials. Alternatively, the rapid replacement of enterocytes may competitively resist the neoplastic overgrowth of mutant cells, and the local immune system in the small intestine may play a role in the suppression of carcinogenesis.2,4-6
There are nearly 40 different histological subtypes of small intestine malignancies. The most commonly developed neoplasms, in order of frequency, are adenocarcinomas, malignant neuroendocrine tumors, malignant lymphomas, sarcomas, and gastrointestinal stromal tumors.1,2 Small intestinal adenocarcinomas (SACs) are most frequently detected in the duodenum; the frequency decreases distally.1,2 SAC is usually discovered at an advanced stage because of its rareness and nonspecific signs and symptoms.1,2 Consequently, the prognosis is usually poor. Some gastrointestinal disorders, including familial adenomatous polyposis, Crohn's disease, Peutz-Jeghers syndrome, celiac disease, and cystic fibrosis, are known risk factors for small intestinal malignancies.2-5 Unlike other gastrointestinal carcinomas, little is known about the histogenesis of SACs, mostly because of the small number of applicable cases.3-6
The Smad4/deleted in pancreatic cancer 4 (DPC4) gene, which was first described as a tumor suppressor gene of pancreatic cancer by Hahn et al. in 1996,7 has been shown to control growth suppression through the transforming growth factor beta signaling pathway, resulting in downstream growth inhibition.3,5,8-15 The gene is located at chromosome 18q21.1 and contains 11 exons with a predicted 552-amino-acid coding sequence.8-13 The mutation of this gene occurs in several tumor types: pancreas (55%), lung (24-65%), ovary (27-67%), prostate (19-45%), bladder (12-35%), and proximal (10%) and distal bile duct carcinomas (55%).8-14
This encouraged us to immunohistochemically label the Smad4/DPC4 gene product in a series of sporadic SACs. This study systematically evaluated 195 specimens of surgically resected primary SACs gathered from 22 medical centers in South Korea, to provide better information on the tumorigenesis of SACs and identify relationships between the expression of the Smad4/DPC4 protein and other known prognostic factors in SACs. To this end, we used tissue microarray (TMA) technology. These data were correlated with common clinicopathological features.
MATERIALS AND METHODS
Specimen selection
Carcinomas arising from the small intestinal mucosa, including the duodenum, jejunum, and ileum, were selected in this study. Carcinomas that continued into the small intestines from the neighboring digestive system, such as those of the stomach, cecum, appendix, ampulla of Vater, or pancreas, were excluded from this study. Tumors located in the serosa or the subserosa of the intestinal wall with no mucosal involvement were considered secondary carcinomas metastasized to the small intestine, and were also excluded. A tumor with mucosal involvement, regardless of the serosal extension, was characterized as a primary small intestinal lesion.
In total, 195 specimens of surgically resected SACs were gathered from the surgical pathology departments of 22 hospitals. The histologic features of all specimens were reviewed by two pathologists (S.-M.Hong and G.S.Yoon). The patients' biological data and personal information (sex, age, diagnoses of previous or present malignancies, additional previous or present modalities of treatment such as radiation or chemotherapy, latest date of follow-up, and survival status) were collected through review of the medical records.
Histologic data were obtained from pathologic reports and microscopic review. The tumor location, size, growth pattern, and date of operation were collected from the patients' pathologic reports. Microscopic features including differentiation of tumors, invasion depth, peritoneal seeding, invasion status of the pancreas or other intestinal loop, lymph nodal metastasis, and the invasion status of nerve fibers, blood vessels, or lymphatic channels, were obtained from the microscopic review of hematoxylin and eosin (H&E)-stained slides.
This study was approved by the Institutional Review Board at Kyungpook National University Medical Center.
Tissue microarray (TMA)
Areas of invasive adenocarcinoma were selected on corresponding H&E slides. Core biopsies, 1.0 mm in diameter, were obtained from each donor block and arrayed without flipping into recipient paraffin blocks on 1.2 mm center, 3.0 mm edges; the array had a maximum of 27 rows, with four cores from each case, resulting in four histological spots on the corresponding slides: two invasive carcinomas, one metastatic lymph node, and one normal small intestinal mucosa. If there was no lymph node metastasis, three invasive carcinomas and one normal small intestinal mucosa were used. The positive controls were normal liver, kidney, spleen, placenta, and normal small intestinal mucosa.
Immunohistochemistry
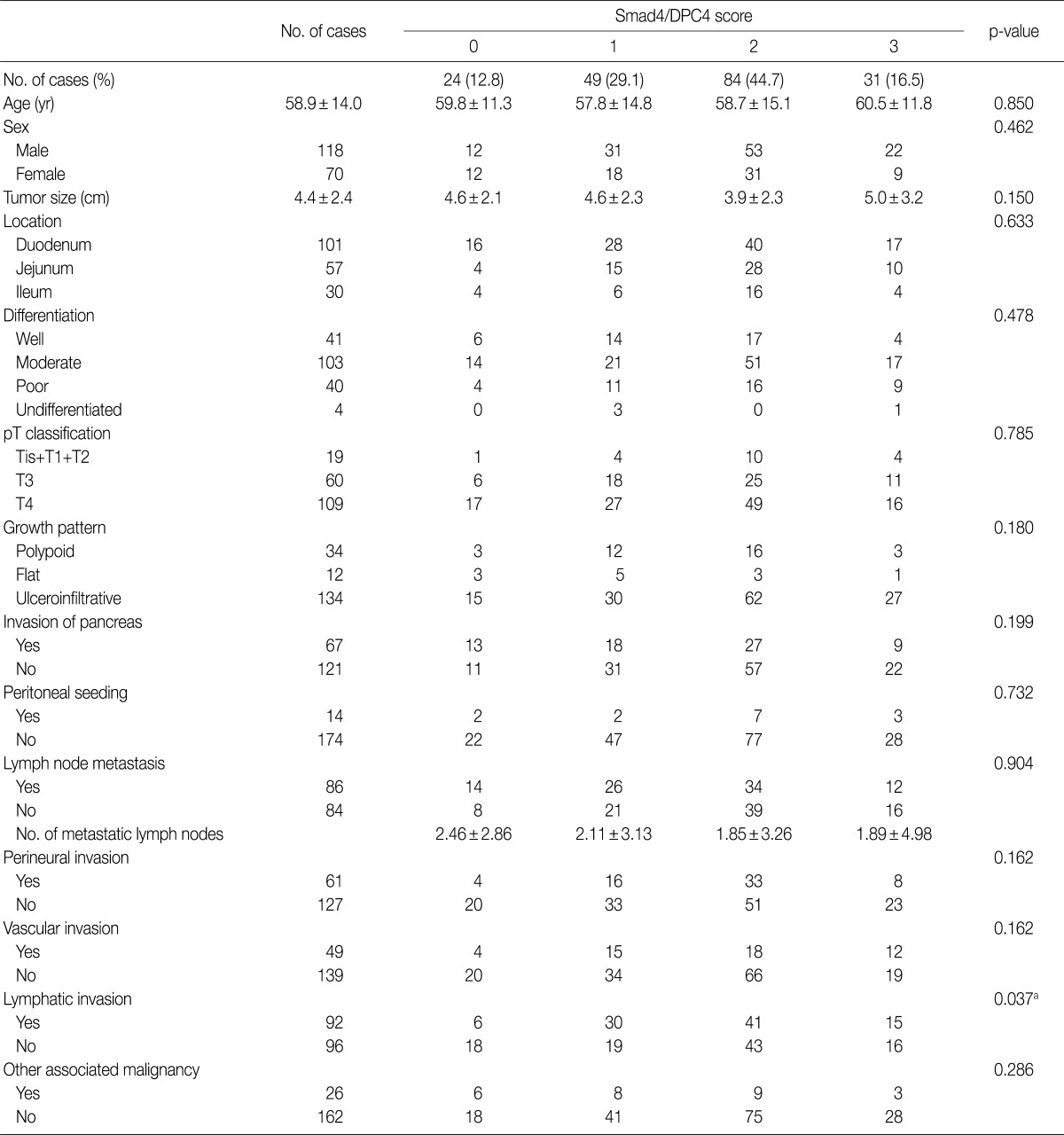
Immunohistochemical staining using the Benchmark XT slide stainer (Ventana Medical Systems, Inc., Tucson, AZ, USA) was performed in accordance with the manufacturer's instructions. Smad4 (1 : 100, clone B-8, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was applied to TMA slides. The stained sections were reviewed without any knowledge of the clinical data of the patient cohort. Cytoplasmic staining in less than 10% of tumor cells was given a score of 0, focal or weak staining (10-50% staining) were scored as 1, and diffuse moderate and diffuse strong cytoplasmic staining (more than 50%) were scored as 2 and 3, respectively. Moderate staining is similar in intensity to that of internal controls, such as fibroblasts or endothelial cells, weak staining is paler, and strong staining is darker. Negative staining in the internal controls was regarded as false negative staining.9,16,17
Statistical analysis
Statistical analyses were calculated using SAS ver. 9.2 (SAS Inc., Chicago, IL, USA). The relationship between the clinicopathological features and expression decrease of Smad4/DPC4 in immunohistochemical staining was estimated using the χ2 test and Fisher's exact test. A p-value of less than 0.05 was regarded as statistically significant. Using the multivariate logistic regression model, we evaluated the relationship of clinicopathologic features to Smad4/DPC4 expression in immunochemical staining.
Overall, patient survival was defined as the date from surgical resection of SACs to death or the last follow-up of the patient. Survival rates were analyzed by the Kaplan-Meier method. A comparison of survival rates with regard to the expression of Smad4/DPC4 was investigated using the log-rank test and the Breslow test. The regression models were adjusted for age, sex, histological type, and the pT stage as characterized by the tumor-node-metastasis staging system. Then we calculated the significance using the Cox proportional hazards model.
RESULTS
Clinicopathological characteristics of patients
A total of 188 tumors were analyzed, excluding the lost cores and false negative cores of TMA slides. The patients included 118 men (62.8%) and 70 women (37.2%) and their ages ranged from 23 to 86 years (mean±standard deviation, 58.9±14.0 years; median, 60.0 years). Of these samples, 101 tumors were located in the duodenum (53.7%), 57 in the jejunum (30.3%), and 30 in the ileum (16.0%). The tumors ranged in size from 1 to 16 cm (mean, 4.4 cm). The tumors were well differentiated in 41 cases (21.8%), moderately differentiated in 103 cases (54.8%), poorly differentiated in 40 cases (21.3%), and undifferentiated in 4 cases (2.1%). As classified by pT staging, 19 cases (10.1%) were pTis, pT1, or pT2; 60 (31.9%) were pT3; and 109 (58.0%) were pT4. The subtype growth pattern could be characterized in 180 cases, with a polypoid growth pattern in 34 cases (18.1%), a flat pattern in 12 cases (6.4%), and an ulceroinfiltrative pattern in 134 cases (70.2%). Sixty-seven cases (35.6%) revealed invasion into the pancreas and 5 cases (2.7%) into another small intestinal loop. Peritoneal tumor seeding was found in 14 cases (7.4%). Dissection of regional lymph nodes was performed in 170 of 188 cases; regional lymph nodal metastasis was observed in 86 (50.3%) of these cases. Vascular and lymphatic invasion was observed in 49 (26.1%) and 92 (48.9%) cases, respectively. Seventy-two (38.3%) and 24 (12.8%) cases were treated by chemotherapy and radiation therapy, respectively. Synchronous or metachronous malignancies of other organs appeared in 26 cases. The follow-up period after surgery ranged from 1.1 to 127.5 months (mean, 26.3 months) and the median survival time was 39.7 months (Table 1).
Expression of Smad4/DPC4
As shown in Fig. 1, 24 cases of SAC showed cytoplasmic staining in less than 10% of tumor cells (score, 0; 12.8%). Moderately and strongly positive staining was observed in 84 and 31 cases, respectively (score, 2 and 3; 44.7% and 16.5%, respectively), and 49 cases were focally or weakly stained (score, 1; 29.1%).
Association between Smad4/DPC4 expression and clinicopathological features
As reported in detail in Table 1, there was no significant association between the expression of Smad4/DPC4 as evaluated through immunohistochemistry and the clinicopathological features of the tumors (tumor location, differentiation, growth pattern, T stage, direct invasion, vascular invasion, and nodal metastasis), with the exception of lymphatic invasion (p=0.037). The odds ratio from the adjusted logistic regression analysis revealed that the intensity and positivity of Smad4/DPC4 expression was associated with increased risk of lymphatic invasion (95% confidence interval) (Table 2).
Association between Smad4/DPC4 expression and patient survival
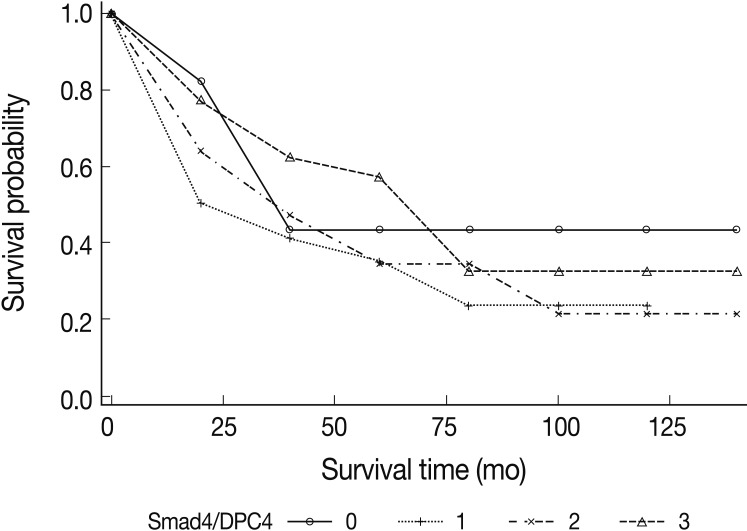
The univariate analysis showed no significant difference in survival based on the intensity of Smad4/DPC4 expression (Fig. 2). Negative Smad4/DPC4 expression produced mild survival benefits, although the results were not statistically significant (p=0.2661 in the log-rank test and p=0.3603 in the Breslow test) (Fig. 3). Using the Cox proportional hazards model, the hazard ratio for the mortality rate based on positive Smad4/DPC4 expression was 1.80, although this was not statistically significant (p=0.065) (Table 3).
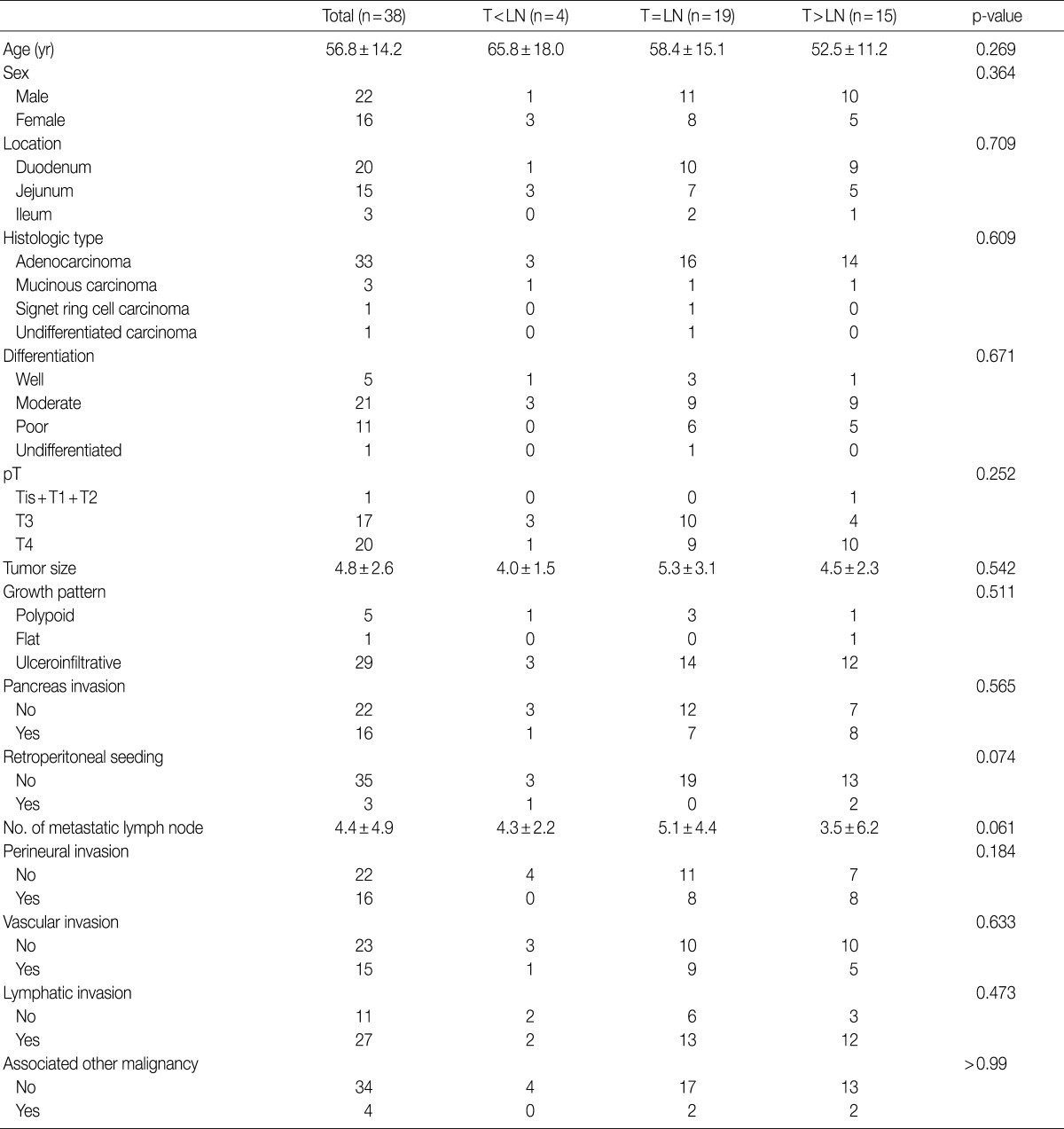
Kaplan-Meier survival analyses of small intestinal adenocarcinomas based on the intensity of Smad4/DPC4 stains. There is no significant association between the intensity of Smad4/DPC4 staining and patient survival.
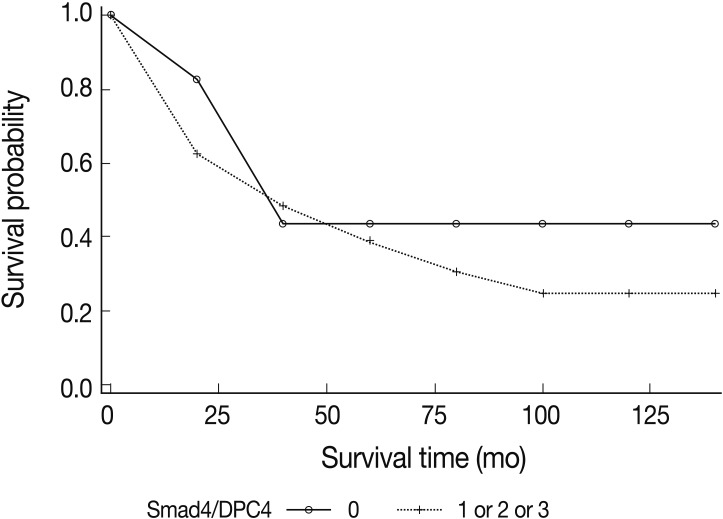
Kaplan-Meier survival analyses of small intestinal adenocarcinomas based on the positivity of Smad4/DPC4 stains. Mild survival benefits are expected in Smad4/DPC4-negative cases, but this result is not statistically significant.
Difference in Smad4/DPC4 expression between primary tumor lesions and metastatic lesions of lymph nodes
We performed Smad4/DPC4 staining in 38 of 86 cases showing lymph node metastasis. Compared to primary tumor lesions, expression of metastatic lymph nodes was increased in 4 cases and decreased in 15 cases; in 19 cases, there were no expression differences. There were no significant correlations with other clinicopathological features (Table 4).
DISCUSSION
We evaluated the clinical informations and histological characteristics of 197 cases with surgically resected SACs. Our key findings include the following: 1) SACs are usually diagnosed at an advanced stage; 2) SACs with sporadic adenomas or peritumoral dysplasia have better anticipated survival; and 3) distal location (jejunum and/or ileum) and lymph node metastasis of SACs are the most important prognostic factors.1
A few studies have attempted to define the tumorigenesis of SACs, including studies of Smad4/DPC4 expression. Blaker et al.5 studied the molecular features of 17 SAC cases using comparative genomic hybridization, microsatellite analysis, and SMAD4 mutational analysis. They found a 18q loss in 8 cases (47%) and a loss of heterogeneity (LOH) of 18q in 13 cases (76%). SMAD4 sequence alterations (24%) were found in five cases (24%); three of these cases had missense point mutations with loss of the wild-type allele and one case had a 7-bp deletion with retention of the wild-type allele. The other alteration was a silent polymorphism.
Svrcek et al.3 conducted a TMA study of 27 SAC samples using several immunohistochemical stains to evaluate the expression of Smad4/DPC4, p53, beta-catenin, and DNA mismatch repair (MMR) genes such as hMLH1, hMSH2, and hMSH6. Five cases showed an absence of Smad4/DPC4 expression and 14 cases showed p53 overexpression. Beta-catenin nuclear translocation was observed in two cases. Loss of hMLH1 was found in two cases but no depletion of hMLH1 and hMSH6 was detected.
Wheeler et al.4 studied the immunohistochemical features of 21 SACs including the expression of beta-catenin, E-cadherin, p53, adenomatous polyposis coli (APC), and MMR genes (MLH1 and MSH2). They reported increased nuclear translocation of beta-catenin in 48% of cases and overexpression of p53 in 24%, similar to Svrcek et al.3 Wheeler et al.4 observed decreased membranous expression of E-cadherin in 38% of cases. There was no APC gene mutation and no loss of MLH1 or MSH2 expression.
Zhang et al.6 published an immunohistochemical investigation of SACs compared to colorectal carcinomas (CRACs). They reported that a complete loss of APC immunoreactivity occurred in 8 of 26 (31%) SACs and 36 of 51 (71%) CRACs. Nuclear translocation of beta-catenin occurred in 5 (19%) SACs and 36 (71%) CRACs. In contrast to other studies, they found a total loss of nuclear staining for one or more of the MMR enzymes at a similar low frequency in both SACs (2 of 25 cases, 8%) and CRACs (10 of 47, 21%). The frequencies of aberrant p53 and retinoblastoma expression were also similar between SACs and CRACs.
To the best of our knowledge, the present research is the first study to evaluate Smad4/DPC4 expression in a large number of SACs with clinicopathologic correlation. Our study included 24 Smad4/DPC4-negative cases (12.8%). This is a slightly lower rate compared to previous research by Blaker et al.5 (24%) and Svrcek et al.3 (18.5%), and may be influenced by the criteria used to classify negative staining. The study by Svrcek et al.3 classified specimens into two groups, positive and negative, with only diffuse strong staining regarded as positive.3 In this study, however, we categorized positive groups based on the intensity and partiality of stains. If the criteria of Svrcek et al.3 are used, the "negative" rate increases by about 38.8% (73/188 cases).
There was no significant correlation between Smad4/DPC4 expression and clinicopathological characteristics, with the exception of lymphatic invasion. According to the odds ratio, the intensity and positivity of Smad4/DPC4 expression was related to an increased risk of lymphatic invasion (Table 2). There was no significant association between the Smad4/DPC4 expression and nodal metastasis, however, so the interpretation of this result may be controversial.
The Smad4/DPC4 expression of metastatic lymph node lesions was the same as in half cases of all the primary tumor. Fifteen cases had decreased expression in lymph nodes and four cases showed increased expression. No clinicopathologic features were significantly related to expression. This result may be correlated with the association between Smad4/DPC4 expression and lymph node metastasis, which was not statistically significant.
This research is the first to investigate the relationship between Smad4/DPC4 expression and patient survival in SACs, although there was no significant association between them. A mild survival benefit was observed with negative Smad4/DPC4 expression, but it was not significant.
These negative results have a few possible explanations. First, the loss of Smad4/DPC4 expression may occur too early in carcinogenesis to affect the prognosis of the disease. In addition, the loss of Smad4/DPC4 expression may not influence the invasion or metastasis of SACs. Finally, because most of our cases were at an advanced stage.pT3 and pT4 (89.9%).we could not determine the step at which the loss of Smad4/DPC4 expression occurs in carcinogenesis.
In conclusion, the present study provides a small foothold in the effort to establish the tumorigenesis of SACs. To clarify this process, future studies should evaluate the immunohistochemical and molecular characteristics of these tumors.
Acknowledgments
This research was supported by Kyungpook National University Research Fund, 2012.
Notes
No potential conflict of interest relevant to this article was reported.