Morphologic Alteration of Metastatic Neuroblastic Tumor in Bone Marrow after Chemotherapy
Article information
Abstract
Background
The aim of this study is to evaluate the histologic features of metastatic neuroblastic tumors (NTs) in bone marrow (BM) before and after chemotherapy in comparison with those of primary NTs.
Methods
A total of 294 biopsies from 48 children diagnosed with NTs with BM metastasis were examined. There were 48 primary neoplasm biopsies, 48 BM biopsies before chemotherapy, 36 primary neoplasm excisional biopsies after chemotherapy, and 162 BM biopsies after chemotherapy.
Results
Metastatic NTs in BM before chemotherapy were composed of undifferentiated and/or differentiating neuroblasts, but had neither ganglion cells nor Schwannian stroma. Metastatic foci of BM after chemotherapy were found to have differentiated into ganglion cells or Schwannian stroma, which became more prominent after further cycles of chemotherapy. Persistence of NTs or tumor cell types in BM after treatment did not show statistically significant correlation to patients' outcome. However, three out of five patients who newly developed poorly differentiated neuroblasts in BM after treatment expired due to disease progression.
Conclusions
Metastatic NTs in BM initially consist of undifferentiated or differentiating neuroblasts regardless of the primary tumor subtype, and become differentiated after chemotherapy. Newly appearing poorly differentiated neuroblasts after treatment might be an indicator for poor prognosis.
Neuroblastic tumors (NTs) are the most common extracranial solid tumors in children with highly variable biologic features and clinical outcomes.1,2 The natural history of NTs range from spontaneous regression to rapid and potentially fatal progression. Therefore, treatment plans may vary from close observation to multimodal interventions, including surgery, chemotherapy, and radiotherapy, according to individual clinical course.3,4 Due to the heterogeneous clinical courses in one disease entity, it is important to identify poor prognostic features of NTs and treat such cases aggressively. Among many prognostic models, histopathologic classifications are one of the most important factors.5 The International Neuroblastoma Pathology Classification (INPC) is a systematic age-linked histopathological indicator which links morphology to clinical presentation and tumor genetics.6 INPC classifies NTs into six different histological subtypes: 1) neuroblastoma (NB), undifferentiated (Schwannian stroma-poor); 2) NB, poorly differentiated (Schwannian stroma-poor); 3) NB, differentiating; 4) ganglioneuroblastoma (GNB), intermixed (Schwannian stroma-rich); 5) ganglioneuroma (GN) (Schwannian stroma-dominant); and 6) GNB, nodular (composite Schwannian stroma-rich/stroma-dominant and stroma poor).7,8
Although primary sites of NTs have been the target of extensive pathological review and analyses, there is a paucity of literature characterizing metastatic sites, especially bone marrow. Indeed, bone marrow metastasis is an important poor prognostic factor, and marrow biopsy is incorporated as a part of routine diagnosis and staging work-up of NTs.9 The aims of this study are to evaluate the histological features of NTs metastasized to bone marrow metastasis in comparison to the primary tumor and to compare histological characteristics of metastatic NTs in bone marrow before and after chemotherapy.
MATERIALS AND METHODS
A total of 48 patients diagnosed with NTs with metastasis to bone marrow from January 2004 to December 2011 at Samsung Medical Center were included in this study. After diagnostic biopsies of the primary tumors and bone marrow were obtained, all patients underwent nine cycles of alternating chemotherapy with cisplatin, etoposide, doxorubicin and cyclophosphamide (CEDC) and ifosfamide, carboplatin and etoposide (ICE) regimens. Of the 48 total patients, 45 patients additionally received autologous stem cell transplantation. Second-look bone marrow biopsies were performed every three cycles after starting chemotherapy to assess treatment response. We retrospectively reviewed a total of 294 biopsies from 48 patients, which consisted of 48 biopsies from primary tumors before chemotherapy, 48 bone marrow biopsies before chemotherapy, 36 primary neoplasm excisional biopsies after chemotherapy, and 162 bone marrow biopsies after chemotherapy.
Hematoxylin and eosin (H&E) sections were prepared after paraffin embedding biopsy specimens fixed in 10% buffered formalin. We evaluated neoplastic cells according to the degree of differentiation (undifferentiated neuroblasts, differentiating neuroblasts, and ganglion cells), composition of stroma (neuropil and Schwannian stroma), mitotic karyorrhexis index (MKI), and amount of necrosis.
We subdivided NTs into NB, GNB, and GN according to the INPC.7,8 By definition, NB undifferentiated subtype consists of neuroblastic cells without evidence of differentiation. NB poorly differentiated subtype consists of tumor cells with varying amounts of neuropil. Most tumor cells in this subtype are undifferentiated and make up less than 5% of the population with morphological evidence of differentiation. NB differentiating subtype has abundant neuropil, and 5% or more of the tumor cells are differentiating neuroblasts. GNB, intermixed subtype has ganglioneuromatous components that occupy more than 50% of the tumor, with scattered microscopic foci of neuroblastic cells. GN is predominantly composed of Schwannian stroma and individually distributed ganglion cells with either a small proportion (<5%) of differentiating neuroblasts (maturing type) or without any neuroblasts (mature type). GNB nodular subtype is a unique group with grossly distinct neuroblastomatous nodule(s) in the background of a GNB intermixed subtype or GN.6
Neoplastic cells in the bone marrow biopsy were confirmed by immunohistochemical staining (IHC) with antibodies against neuron-specific enolase (1:100, polyclonal, CAT 18-0042, Invitrogen, Carlsbad, CA, USA) and CD56 (1:200, CD564, NCL-56-5041, Novocastra, Newcastle upon Tyne, UK).10,11
For statistical analysis, the Mann-Whitney U and chi-square tests were used for comparison of metastatic NTs in bone marrow before and after chemotherapy. Survival rate was estimated using Cox proportional hazards model with a 95% confidence interval. Overall survival (OS) was defined as time from diagnosis to death. Disease-specific survival (DSS) was defined as the time from diagnosis to death due to disease progression. The SPSS for Windows TM release 11.0 (SPSS Inc., Chicago, IL, USA) was used for the analysis.
RESULTS
Clinical characteristics of patients
There were a total of 48 patients included in this analysis. The median follow-up time was 1.84 years (range, 0.7 to 8.9 years). Median patient age at the time of initial diagnosis was 19 months (range, 1 day to 96 months), and eight patients were younger than 12 months (16.7%). In terms of disease stage, 46 patients were stage IV (95.8%), and two patients were stage IVs (4.2%). During follow-up visits, 16 patients (33.2%) had disease relapse or progression, and seven patients died from causes other than relapse or progression. The clinical characteristics of all patients are summarized in Table 1.
Primary neuroblastic tumors at the time of diagnosis
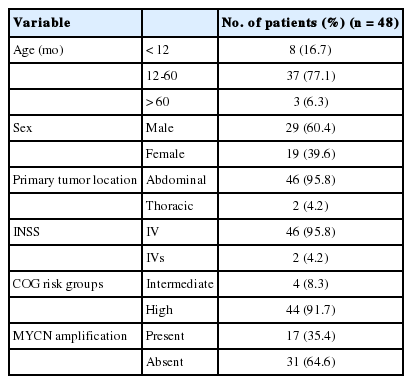
The majority of primary NTs were located in the abdominal cavity (95.8%, n=46). Extra-abdominal primaries were found in the thoracic cavity in two cases (4.2%). Either needle biopsies or incisional biopsies were performed to confirm histological diagnosis. Based on the INPC, primary neoplasms were classified into NB undifferentiated (4.1%, n=2), NB poorly differentiated (45.8%, n=22), NB differentiating (29.2%, n=14), GNB intermixed (2.1%, n=1), GNB nodular (8.3%, n=4), or GN maturing (2.1%, n=1) (Figs. 1, 2A). Initial biopsy specimens of four cases were too limited for histological subtyping, but these cases were eventually confirmed to be NTs by excisional biopsy after chemotherapy
The histological subtypes of metastatic tumor subtypes in bone marrow according to the subtypes of the primary tumors. Regardless of the primary tumor subtype, poorly differentiated or differentiating subtypes predominated at the time of diagnosis. NBU, neuroblastoma undifferentiated subtype; NBP, neuroblastoma poorly differentiated subtype; NBD, neuroblastoma differentiating subtype; GNBN, ganglioneuroblastoma nodular; GNBI, ganglioneuroblastoma intermixed; GNMI, ganglioneuroma maturing.
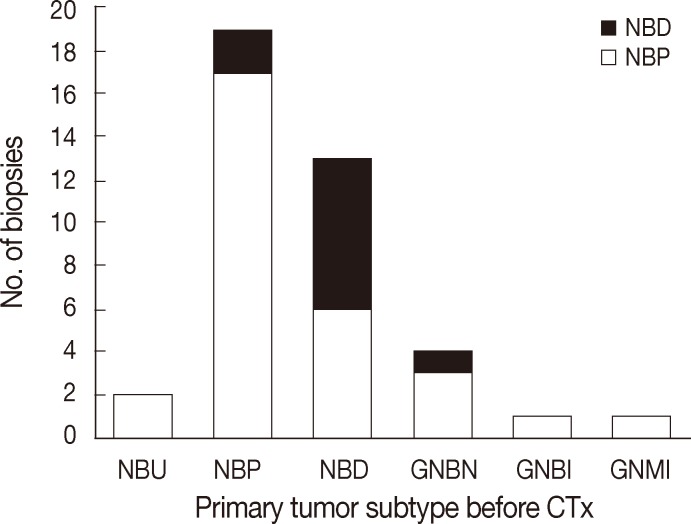
The histological features of primary and metastatic neuroblastic tumors in a representative case. (A) Primary tumor initially diagnosed as neuroblastoma (Schwannian stroma-poor), poorly differentiated subtype. (B) Primary tumor after multiple cycles of chemotherapy showing maturation evidenced by scattered differentiating neuroblasts in Schwannian stroma and fibrosis. (C) Metastatic tumor in bone marrow at initial diagnosis composed of undifferentiated neuroblasts in neuropil. (D) Metastatic tumors in bone marrow after chemotherapy showing differentiating neuroblasts or ganglion cells in Schwannian stroma.
Metastatic neuroblastic tumors in bone marrow before chemotherapy
All patients underwent two bone marrow biopsies at the time of diagnosis, taken from both the right and left pelvic bones. Metastatic NTs in bone marrow were evaluated by histological review of H&E and IHC sections. Metastatic tumors consisted mainly of undifferentiated and/or differentiating neuroblasts. Neuropil was observed in 43 patients (89.6%). Neither ganglion cells nor Schwannian stroma was detected (Table 2, Fig. 2C). We categorized histological subtypes of metastatic NTs in bone marrow and compared these findings to their original primary tumor subtype (Fig. 1). Regardless of the primary tumor subtype, the most common subtype of metastatic NT in bone marrow was the poorly differentiated subtype (n=38, 79%). However, cases with more differentiated primary tumors tended to have more differentiated metastatic lesions as well. The differentiated subtype in bone marrow was found in 0% of patients classified with the NB undifferentiated subtype by the primary tumor (0/2), 9.1% of poorly differentiated NB cases (2/22), and 50% of NB differentiating cases (7/14). There were two cases with GNB intermixed and GN maturing as the primary tumor biopsies had metastatic tumors in bone marrow identified as NB poorly differentiated. Other detailed pathologic characteristics are summarized in Table 2.

Histological characteristics of metastatic tumors in bone marrow (BM) before and after chemotherapy (CTx)
A total of 33 pairs of bone marrow and primary tumor biopsies were available for comparison of MKI. The primary tumors of 21 cases had a high MKI (63.6%), four cases had an intermediate MKI (12.9%), and eight cases had a low MKI (24.2%). Metastatic tumors in the bone marrow of 15 cases had a high MKI (48.3%), two cases had an intermediate MKI (6.4%), and 16 cases had a low MKI (51.6%), suggesting low active tumor proliferation and karyorrhexis in bone marrow with metastatic tumors.
Primary neuroblastic tumor excision after multiple cycles of chemotherapy
Excisional biopsies of primary neoplasms were performed in 36 patients after multiple cycles of chemotherapy. Histology before and after chemotherapy was compared in 33 cases excluding three cases in which histologic subtyping before chemotherapy was not feasible due to limited tissue. After chemotherapy, 17 cases showed histological differentiation (52.4%) (Fig. 2B). Poorly differentiated and undifferentiated NB subtypes at initial diagnosis showed frequent differentiation (82.4%). Fourteen cases were found to have the same histological grade regardless of chemotherapy (43.4%). Only one case was found to have a more immature subtype after chemotherapy, which morphed from differentiating NB into poorly differentiated NB. In one case, the excisional biopsy of the primary tumor after chemotherapy was confirmed to have no viable tumor cells (2.1%).
We also observed a shift from undifferentiated or poorly differentiated subtypes with a high MKI to a more differentiated subtype with a low or intermediate MKI after chemotherapy. Among 40 primary biopsies that were available for the assessment of MKI before chemotherapy, 27 cases had a high MKI (67.5%) and 13 cases showed an intermediate or low MKI (32.5%). After chemotherapy, only two of 26 excised tumors were found to have a persistently high MKI (7.7%).
Metastatic neuroblastic tumors in bone marrow after chemotherapy
After multiple cycles of chemotherapy, repeat bone marrow biopsies were performed, and 23 of 48 cases had persistent metastatic NTs in the bone marrow (47.9%). These metastatic sites showed a variable degree of differentiation after chemotherapy. Ganglion cells and Schwannian stroma were found in addition to undifferentiated and differentiating neuroblasts (Figs. 2D, 3C). Other histological features in the bone marrow included decreased neuropil and increased necrosis following chemotherapy. Persistent metastatic NTs in subsequent bone marrow biopsies after further cycles of chemotherapy were found in ten patients, but the degree of differentiation in metastatic foci became more prominent (Table 3, Fig. 3). Among the ten cases of persistent bone marrow metastasis, four patients died during the study period (40.0%).
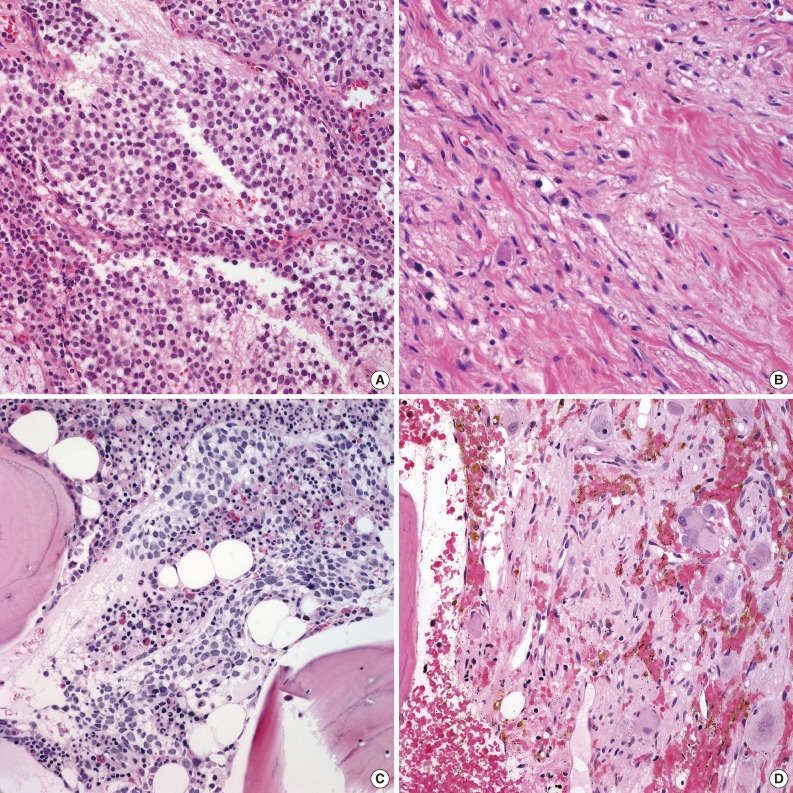
Time course of histological maturation of metastatic foci in bone marrow in a single case. (A) Metastatic neuroblastic tumor (NT) in bone marrow at initial diagnosis comprised of undifferentiated neuroblasts with neuropil. (B) Metastatic NT in bone marrow after nine cycles of induction chemotherapy two months after diagnosis. Differentiating neuroblasts are seen in a background of Schwannian stroma. (C) Ganglion cells appear in a background of Schwannian stroma ten months after the initial diagnosis without additional chemotherapy.
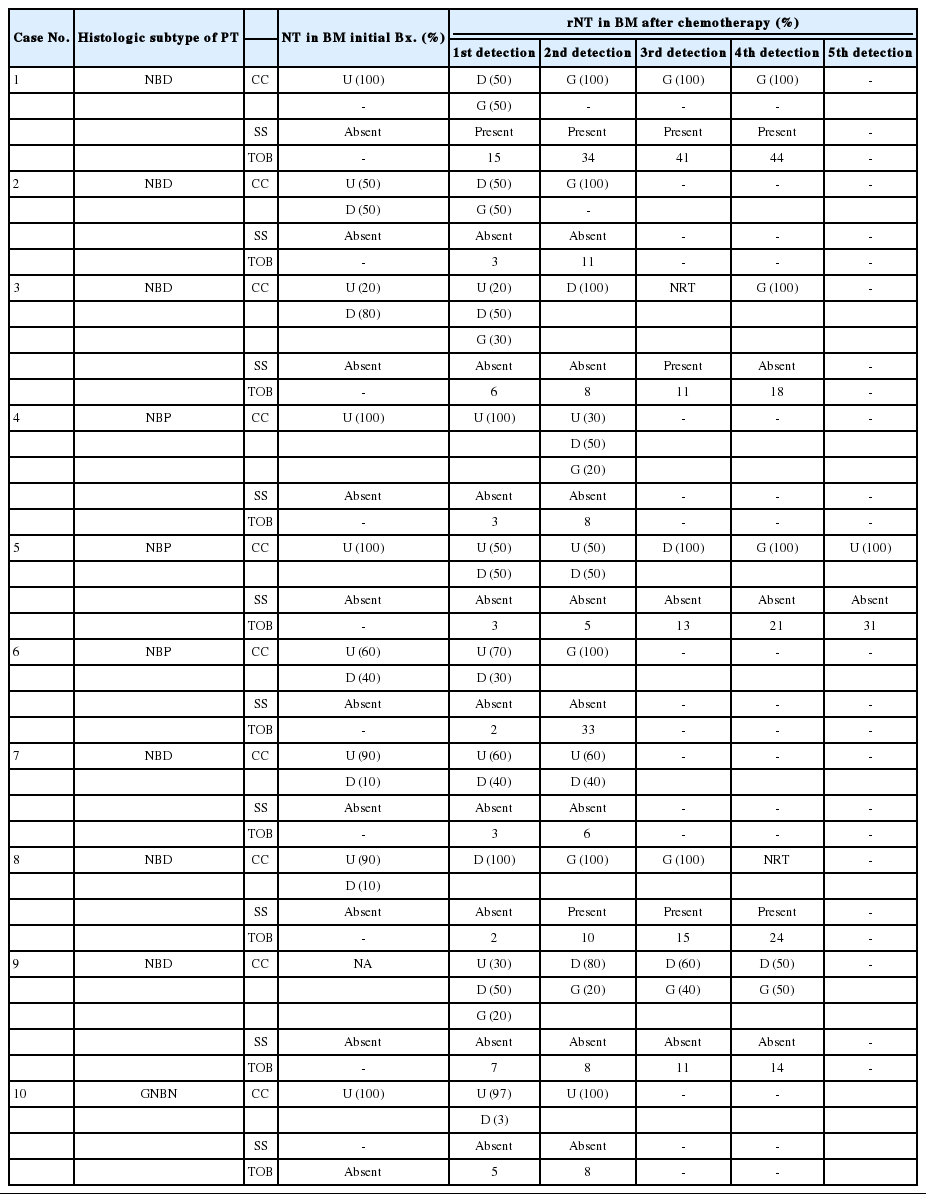
Histological changes of neuroblastic tumors (NTs) in a series of bone marrow (BM) biopsies after multi-cycle chemotherapy
We reviewed an additional five cases with newly developed metastatic lesions in bone marrow among children with stable disease after multiple cycles of chemotherapy. New metastatic foci in bone marrow after chemotherapy revealed poorly differentiated neuroblasts regardless of previous histology (Fig. 4), and three of these patients died despite additional treatments.

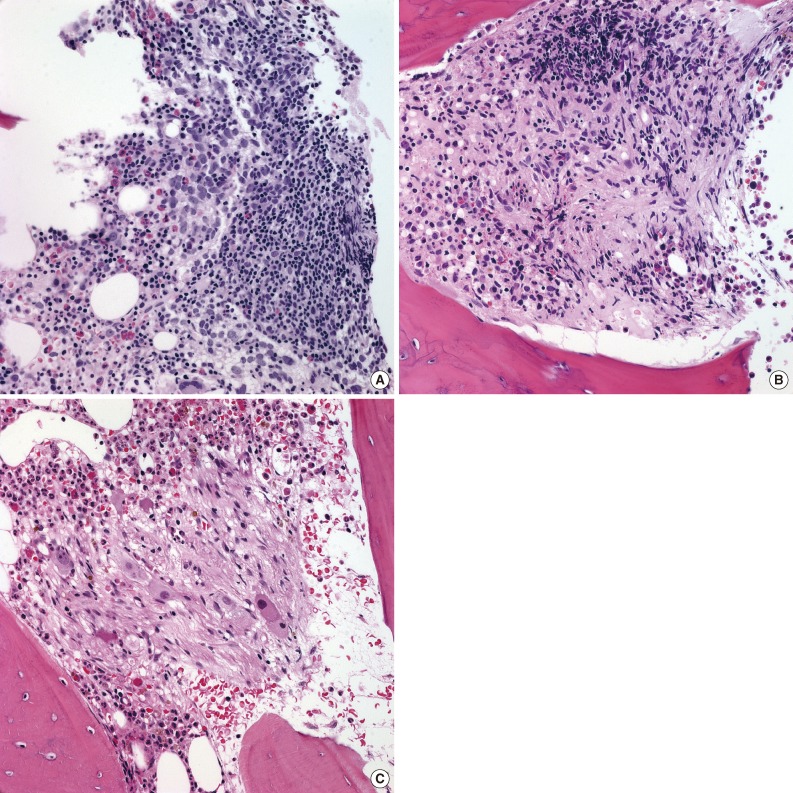
Newly developed poorly differentiated neuroblastic tumor (NT) in bone marrow after multi-cycle chemotherapy in a single case. (A) Poorly differentiated neuroblasts in the background of neuropil in bone marrow at the initial diagnosis. (B, C) Metastatic NT in bone marrow after multiple cycles of chemotherapy reveal progressive differentiation of tumor cells with Schwannian stroma at three months (B) and 19 months (C) after the initial diagnosis. (D) Newly developed metastatic NTs in bone marrow composed of poorly differentiated neuroblasts approximately five years after the initial diagnosis. This patient eventually died 2.5 months after the diagnosis of recurrence.
Clinical significance of histological findings in metastatic neuroblastic tumors in bone marrow
We analyzed the relationship between histological findings in metastatic NTs in bone marrow and clinical outcomes by focusing on the histologic findings known to have significant correlation to the primary tumor. Neither the degree of differentiation (the presence of undifferentiated NB cells), presence of neuropil, nor Schwannian stroma of metastatic bone marrow tumors correlated with OS or DSS. Neither tumor necrosis nor high MKI in the metastatic tumors had a statistically significant correlation with survival. Persistent presence of neuroblasts in the bone marrow after chemotherapy was not a statistically significant prognostic factor dictating patient outcome. We also quantified the amount of tumor cells in bone marrow before and after chemotherapy, which was also not statistically significantly correlated with patient outcome.
DISCUSSION
The major cellular components of metastatic neuroblastic tumors found in bone marrow prior to chemotherapy are undifferentiated and differentiating neuroblasts
Regardless of the categories and subtypes of primary NTs, metastatic lesions at initial diagnosis before treatment were composed of undifferentiated or differentiating neuroblasts without ganglion cells. Neuropil and pseudo-rosettes were frequently detected in metastatic foci, while Schwannian stroma was not found even in the presence of fibrosis. These findings suggest that undifferentiated and differentiating neuroblasts have a potential for metastasis, whereas mature ganglion cells do not. The INPC stated that histologic evaluation of a metastatic site could provide information equivalent to that of a primary site when biopsy of the primary site was not available.7 In our study, bone marrow from a small number of patients diagnosed with GNB or GN after biopsies of primary tumors was found to have NBs of poorly differentiated or differentiating subtype at the metastatic foci. Although the number of these cases was small, the findings suggest that it is possible to classify a metastatic tumor into a poorly differentiated category when the primary tumor is well differentiated. This hypothesis should be further validated in an investigation with a larger number of cases, which may call for modification of the INPC recommendation.
Metastatic neuroblastic tumors become more differentiated after chemotherapy
When patients in this study underwent a second bone marrow biopsy after three cycles of chemotherapy, 23 of 48 cases had persistent metastatic lesions in the bone marrow (47.6%). Subsequent biopsies of persistent metastatic tumors in the bone marrow of ten cases after further cycles of chemotherapy showed further maturation of the tumors. Metastatic tumors had more differentiating neuroblasts and ganglion cells in the background of Schwannian stroma, indicating differentiation after chemotherapy as the maturing sequence of the primary NTs.12 Chemotherapy-induced histological tumor maturation can occur in tumors showing neuronal differentiation including NTs, olfactory NB, and sinonasal teratocarcinosarcoma.13-15 Morphological maturation of advanced NTs as a result of cytotoxic anti-tumor chemotherapy is a relatively well-known phenomenon.14,16 Goldstein and Plurad17 reported that, after treatments with alkylating agents, a gradual transformation of small immature NB cells into larger non-dividing GNB cells in 40-75% of cultured cells occured. Other agents that have been reported to induce cell maturation include retinoic acid, prostaglandin E1, papaverine, and nerve growth factor.14,18 Therapeutic agents delivered in our cases include 13-cis-retinoic acid, cisplatin, cyclophosphamide, and other alkylating agents. The other possible mechanism explaining maturation of metastatic NTs is an interaction between neuroblasts and Schwann cells. Schwann cells in NTs are recruited from surrounding non-neoplastic tissue by neoplastic neuroblasts that produce mitogens and differentiation-inducing factors for Schwann cells. In return, Schwann cells produce differentiation-inducing factors essential for neuronal differentiation.19,20 In metastatic tumors of bone marrow, these recruited Schwann cells may cause the induction of neuroblast differentiation.
New appearance of undifferentiated neuroblasts indicate poor outcome
According to the International Neuroblastoma Risk Group (INRG) classification, several factors are very important for prognosis of NTs: disease stage, histologic category, grade of tumor differentiation, MYCN oncogene status, and DNA ploidy.21 Other significant prognostic factors include high MKI and high degree of tumor necrosis, which are related to poor outcome.7,22-24
Although bone marrow metastasis of NTs is a grim prognostic factor, histopathological characterization of bone marrow-infiltrating tumors has not yet been comprehensively studied.25,26 Herein, we analyzed the relationships between patient outcome and the histopathological features of metastatic NTs in bone marrow before and after chemotherapy. No histologic feature including tumor subtype had a significant correlation with OS or DSS. There are a few reports regarding the significance of differentiating neuroblasts in bone marrow metastasis after chemotherapy, but its prognostic value is still controversial.27,28 A recent study suggested the presence of differentiating neuroblasts in bone marrow was a favorable prognostic factor.27 However, our study revealed that the presence of differentiating neuroblasts was not associated with favorable prognosis. This controversy may be explained by the heterogeneity of the treatment group in the previous report, which included cases with three different treatment modalities,27 whereas our study had a more homogenous population undergoing a single treatment modality.
The presence of persistent mature NTs in bone marrow after chemotherapy was not predictive of poor outcome in this study. There were five cases of newly developed metastatic lesions with poorly differentiated neuroblasts in bone marrow, and three of those patients eventually died of disease progression (60%). The high mortality of this subgroup suggests that the emergence of poorly differentiated NTs in bone marrow may be correlated with poor outcome. This calls for regular bone marrow biopsies and careful histologic examinations to detect newly developed immature neuroblasts.
In conclusion, this is the first study reporting histological characteristics of metastatic NTs in bone marrow before and after chemotherapy. Metastatic NTs in bone marrow at the time of initial diagnosis usually have undifferentiated or differentiating neuroblasts, which can disappear or become more mature after chemotherapy. Newly appearing poorly differentiated neuroblasts after treatment might be an indicator of poor outcome. A detailed histological analysis of metastatic NTs in a series of bone marrow biopsies could be informative in determining prognosis and may be helpful for determining management.
Notes
No potential conflict of interest relevant to this article was reported.