Low-Grade Adenosquamous Carcinoma of the Breast with Diverse Expression Patterns of Myoepithelial Cell Markers on Immunohistochemistry: A Case Study
Article information
Abstract
This paper reports a case of low-grade adenosquamous carcinoma (LGASC) arising in a 69-year-old woman, who presented with a 1-cm palpable mass on her right breast. Core needle biopsy diagnosed the mass as a fibroadenoma. After six months, the mass increased in size, and the patient received subsequent mammotome excision. On microscopic examination, bland-looking small glands were infiltrating into the fibrotic stroma with lymphocytic infiltrates at the periphery. Hematoxylin and eosin staining revealed relatively easily detectable myoepithelial cells along the outside in each of the glandular structures with variable degrees of squamous metaplasia. Based on histologic features, the patient was diagnosed with LGASC. LGASC is a rare variant of metaplastic carcinoma, which is characterized by a favorable prognosis. Due to the bland cytology and presence of myoepithelial cells, LGASC can be misdiagnosed as benign lesion. Additionally, inconsistent expression of myoepithelial markers could aid the diagnosis of LGASC.
Low-grade adenosquamous carcinoma (LGASC) of the breast is a rare variant of metaplastic carcinoma, distinguished from other metaplastic carcinoma or high-grade adenosquamous carcinoma due to its benign-mimicking histology and relatively favorable prognosis.1 Microscopically, infiltration of bland angulated or rounded glandular structures with retained myoepithelial cells and periductal lamellar stroma are observed. In addition, a diverse degree of squamous metaplasia is commonly found in bland-looking angulated or rounded glandular structures with peripheral lymphocytic infiltration. Differential diagnosis includes tubular carcinoma and syringomatous tumor (SyT) of the nipple. Careful scrutiny of histologic features along with immunohistochemical markers for myoepithelial cells can assist in diagnosis.
CASE REPORT
A 69-year-old woman presented with a palpable mass lesion in the lower central area of her right breast. This mass had been present for several months. She underwent mammographic examination, and a 1-cm mass with microcalcification and microlobulated margins was found at the 6 o'clock position on the right breast, 1 cm from the nipple (Fig. 1A). There were no remarkable findings in the left breast parenchyma and both axillary areas. Core needle biopsy was done to the right breast mass, and pathologic diagnosis was "fibroadenoma with squamous metaplasia." Six months after the core needle biopsy, the right breast mass increased in size on mammographic examination, from 1 cm to 1.4 cm. Mammotome excision was performed.
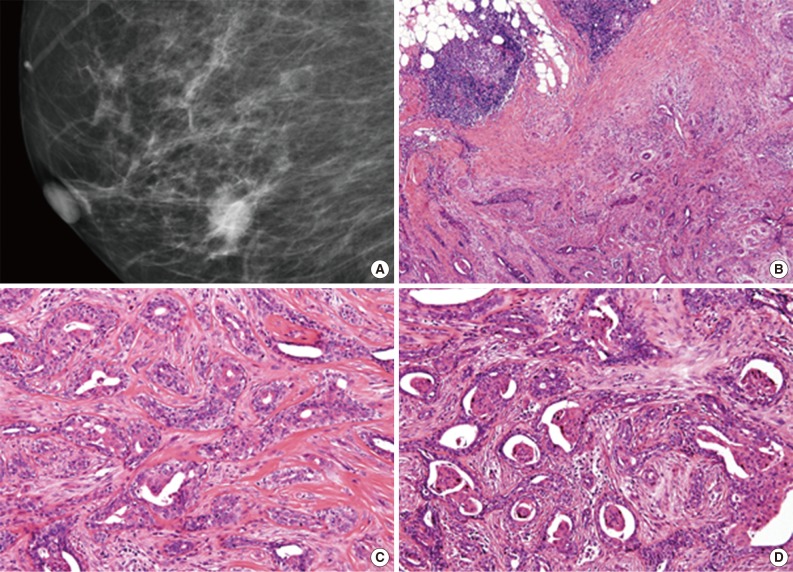
Patient's mammography and histologic features of tumor. (A) In initial mammography, nodular density on right lower central of breast parenchyma is observed. (B) At lower magnification, ill-defined lesion is surrounded by peripheral lymphoid infiltration. (C) At high power, angulated or rounded bland-looking glandular structures infiltrate into desmoplastic fibrous stroma. Myoepithelial cells are easily detected at the outside of glandular structures. (D) Variable degree of squamous metaplasia found in glandular lumens.
Microscopic examination of the mammotome excision specimen and review of the previous core needle biopsy revealed the same histologic features. At a low-power view, an ill-defined glandular proliferative lesion was identified (Fig. 1B). Bland-looking glandular structures were embedded in collagenous stroma. Periductal lamellar stroma was notable. Myoepithelial cells were easily delineated as in the adenosis condition. At the periphery of the lesion, multifocal lymphocytic infiltration was present (Fig. 1B). At higher magnification, bland-looking glands were irregularly fused, and some angulated edges infiltrated into the collagenous stroma resulting in desmoplastic fibrosis (Fig. 1C). Within glands, varying degrees and amounts of squamous metaplasia were observed, from attenuated eosinophilic lining of glandular lumens to sizable squamous pearls (Fig. 1D).
Based on histologic findings, LGASC of the breast was diagnosed. In order to better understand the myoepithelial components and invasiveness of the tumor, immunohistochemical stainings were performed for myoepithelial markers such as p63, CD10, smooth muscle actin (SMA), S-100 protein, smooth muscle myosin heavy chain (SMMHC), and caldesmon. Variable degrees and distribution patterns of myoepithelial markers were identified. p63 and SMA exhibited the most consistent and substantial expression in myoepithelial components (Fig. 2A-D). Other markers, CD10, S-100 protein, SMMHC and caldesmon, were inconsistently expressed in variable allocation within tumor glands, compared to the intense, completely continuous expression of these same markers in normal mammary myoepithelial cell layers (Fig. 2E-L). In p63 immunohistochemical staining, most of the myoepithelial cells in the tumor were positive (Fig. 2A), but some of them were negative, and some of the tumor epithelial cells were positive (Fig. 2B).
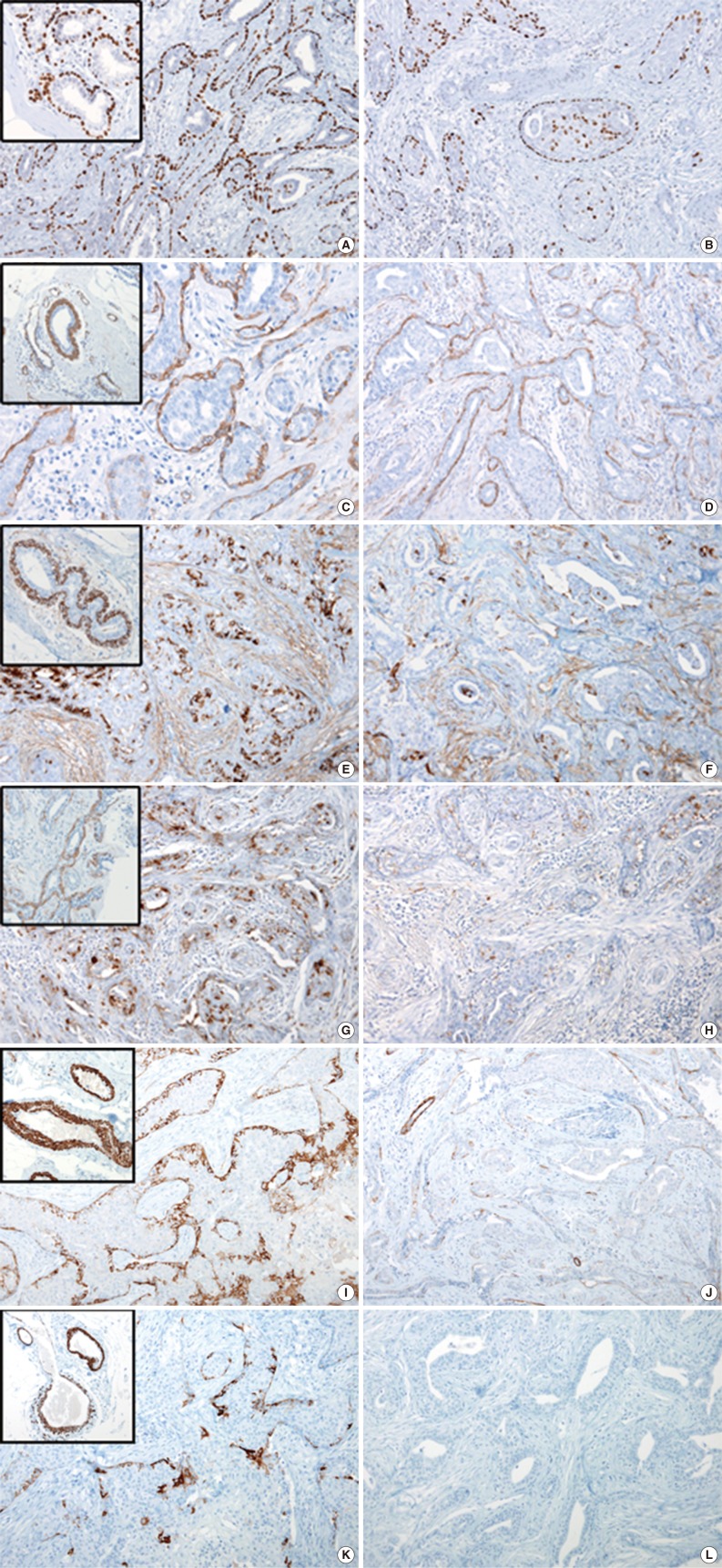
p63 (A) and actin (smooth muscle actin) (C, D) are expressed in over 90% of myoepithelial cells. (B) Some tumor epithelial cells are expressing p63, whereas some myoepithelial cells are not expressing p63. Some areas of the tumor myoepithelial cells show relatively consistent expression of myoepithelial cell markers (E, CD10; G, S-100 protein; I, smooth muscle myosin heavy chain [SMMHC]; K, caldesmon), whereas other areas show less or no expression of myoepithelial cell markers (F, CD10; H, S-100 protein; J, SMMHC; L, caldesmon) compared to normal mammary glands (insets).
DISCUSSION
Breast carcinomas originate from the mammary glands, and are typically a type of adenocarcinoma. Less than 5% of adenocarcinomas have a nonglandular growth pattern, so called metaplasia. In general, there are two types of metaplastic patterns, squamous and pseudosarcomatous (heterologous), which can exist either together or separately.2
LGASC is a rare variant of metaplastic carcinoma, and is distinguishable from other metaplastic carcinomas because it presents with a high-grade adenosquamous carcinoma and/or pseudosarcomatous pattern. Histologically, infiltrative growth of deceptively bland-looking epithelial cells is observed in breast parenchyma. Surrounding myoepithelial components are easily found in the tumor, which can be confused as normal luminal epithelial and basal myoepithelial mammary structures and may lead to misdiagnosis as a noninvasive benign lesion such as adenosis and fibroadenoma. Collagenous tumor stroma with a periductal lamellar appearance and peripheral lymphocytic infiltration are frequently present. Tumor cells show variable amounts and degrees of squamous differentiation, which is noted by the formation of squamous pearls and eosinophilic condensed cytoplasm. In a previous report by Van Hoeven et al.,3 37.5% (12/32) of cases with LGASC were accompanied by epithelial proliferative lesions, intraductal papilloma and adenomyoepithelioma. Prognosis of these patients was favorable, particularly compared to other metaplastic carcinomas, but local recurrence and rare axillary lymph node metastasis were reported.
Various myoepithelial markers are expressed in variable patterns when immunohistochemistry is performed. Kawaguchi and Shin4 studied the immunohistochemical staining pattern of LGASC, and concluded that the inconsistent staining pattern of variable myoepithelial markers is a unique finding of LGASC. They applied myoepithelial cell markers (p63, SMMHC, SMA, CD10, and calponin) in 30 cases of LGASC. None of cases exhibited a complete absence of myoepithelial cell markers. If absence of staining for a myoepithelial cell marker was observed, discontinuous circumferential staining was observed with other myoepithelial cell markers. Malignant lesions such as tubular carcinoma show a complete absence of myoepithelial markers. On the other hand, benign mimickers of LGASC such as sclerosing adenosis and benign papillary neoplasm have complete and continuous expression of myoepithelial markers.
Differential diagnosis for LGASC includes tubular carcinoma and SyT of the nipple. Sclerosing adenosis and superimposed papillary neoplasm are even less likely than LGASC but should still be considered.
Tubular carcinoma, which has a favorable prognosis over invasive ductal carcinoma of no special type, can mimic LGASC. Tubular carcinoma is characterized by well-differentiated tubular structures lined by a single layer of bland-looking epithelial cells.5 Small nuclear pleomorphisms and angulated glands in fibrotic stroma are also found in LGASC and complex sclerosing adenosis. When immunohistochemical staining of myoepithelial markers is performed in cases of tubular carcinoma, the absence of myoepithelial cells is striking. In contrast, immunohistochemical staining reveals highlighted myoepithelial cells in cases of sclerosing adenosis and LGASC, although myoepithelial cells of these two lesion are different in continuity and reactivities for myoepithelial cell markers.4 Sclerosing adenosis is a benign lesion and is often mistaken for a malignancy due to the attenuating myoepithelial cell layer. However, all myoepithelial cell markers are present in sclerosing adenosis. Myoepithelial cell layers are also observed in LGASC, although not all of the myoepithelial cell markers are expressed. At least one marker is positive, and an inconsistent pattern of expression is characteristic.4
A SyT of the nipple is a benign tumor arising in the nipple. Even though it is categorized as a nonmetastasizing benign lesion, it infiltrates into the stroma and can locally recur.5 Cyst formation with squamous cell lining and keratinous material are overlapping features with LGASC. SyT must be distinguished from LGASC and tubular carcinoma, as proper treatment for each lesion is different. One distinguishing feature is that syringomatous tumors are often located in the dermis and subcutis of the nipple or areola, unlike LGASC or tubular carcinoma. Myoepithelial cells surrounding the tubules are usually present.6 It is unclear whether myoepithelial cells are completely preserved in an SyT, as previous studies have reported both completely maintained cells and intermittent loss. Unlike SyT, myoepithelial cells of LGASC are tumor components themselves, and exhibit variable expression of myoepithelial markers along with the epithelial cells.
LGASC is a rare breast malignancy, classified as a metaplastic carcinoma of the breast. With routine hematoxylin and eosin stained slides, LGASC has several distinct features that separate it from tubular carcinoma or benign SyT of the nipple. Bland-looking glandular structures, periductal lamellar stroma, preserved myoepithelial cells, variable degrees and quantities of squamous metaplasia, and lymphocytic infiltration at the periphery of the lesion are all characteristic of LGASC. Additional immunohistochemical stainings for myoepithelial cells are helpful in excluding benign mimickers, as well as other malignancies such as tubular carcinoma.
Notes
No potential conflict of interest relevant to this article was reported.