Undifferentiated Embryonal Sarcoma in Adult Liver
Article information
Undifferentiated embryonal sarcoma (UES) is the third most common primary malignant liver tumor in children, following hepatoblastoma and hepatocellular carcinoma. UES of the liver was first named by Stocker and Ishak in 1978,1 and the majority of patients at the time ranged from 6 to 10 years of age.
A recent review of the literature showed only 70 cases of UES in adults reported worldwide in 2008.2 This rare presentation is especially true for adults aged >60 years with only 14 cases of UES reported in this population until now.3,4,5,6,7 In Korea, eight cases of UES in adults have been reported: five cases were in women and four cases were in people older than 60 years.5,6,7,8 We present a new case of adult UES of the liver arising in a 67-year-old man. Like pediatric UES, the adult tumor has an unclear pathogenesis and poor prognosis.
CASE REPORT
A 67-year-old man was admitted to our hospital with abdominal pain of 10-day duration. Two days before admission, he experienced abrupt onset of an intermittent sharp pain in the right upper quadrant. His past medical history included underlying hypertension and diabetes mellitus, for which he had been taking medication for 10 years. He had no specific family history. A physical examination on admission demonstrated temperature of 36.4℃, heart rate of 84 beats/minutes, respiratory rate 16/minutes, and blood pressure 130/80 mm Hg. His abdomen was soft and flat, and he had tenderness in the right upper quadrant. Abnormal findings such as jaundice or spider naevi were not present. A chest radiograph showed no pulmonary abnormality. Routine laboratory findings included the following: total bilirubin 1.0 mg/dL (normal range [NR], 0.2 to 1.2 mg/dL), conjugated bilirubin 0.3 mg/dL (NR, 0.0 to 0.4 mg/dL), alkaline phosphatase 121 U/L (NR, 40 to 122 U/L), γ-glutamyl transferase 99 U/L (NR, 11 to 50 U/L), alanine-aminotransferase 32 U/L (NR, 5 to 44 U/L), aspartate-aminotransferase 46 U/L (NR, 13 to 36 U/L), carcinoembryonic antigen 1.64 ng/mL (NR, <5 ng/mL), cancer antigen 19-9 20.51 U/mL (NR, <39 U/mL), and alpha-fetoprotein (AFP) 9.7 ng/mL (NR, <9.6 ng/mL). Anti-hepatitis B surface, anti-hepatitis B core, and anti-hepatitis C virus antibodies, as well as hepatitis B antigen, were not detected. A dynamic computed tomography study found a 13-cm-sized hypoattenuating mass with lobulated contour in the right hepatic lobe, in segments 5 and 6 (Fig. 1). Under the diagnosis of mass-forming intrahepatic cholangiocarcinoma, the patient underwent a segmentectomy (segments 5 and 6), along with cholecystectomy and resection of the omentum.
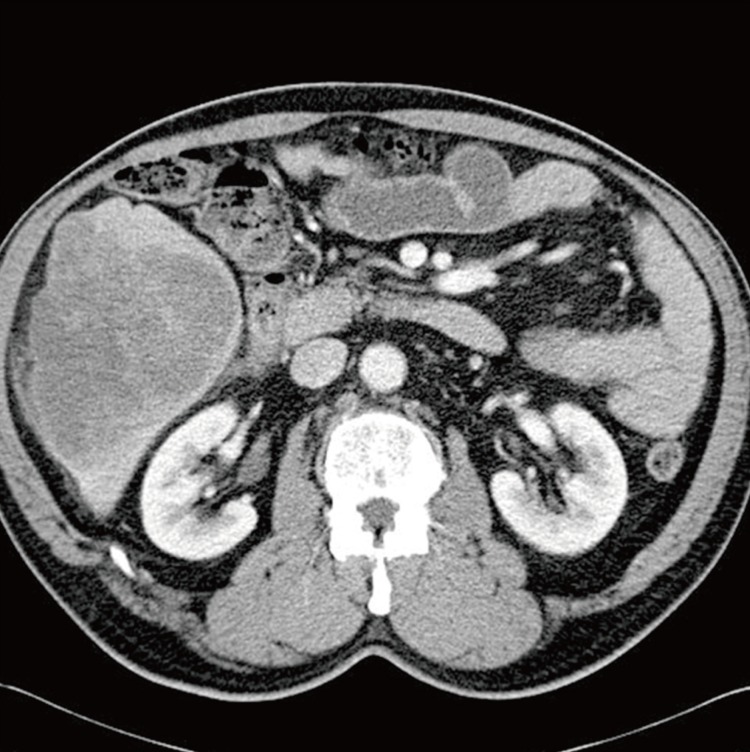
A dynamic computed tomography study shows a 13-cm-sized hypoattenuating mass with lobulated contour in the right hepatic lobe, in segments 5 and 6.
Grossly, a large mass in segments 5 and 6 of the right hepatic lobe was detected, measuring 14.5×11.2×9.0 cm. The cut section showed a large, variegated, solid, and firm mass with a grey-to-yellow surface. The mass, though mostly solid, also comprised a dark hemorrhagic and necrotic area and a gelatinous cystic area (Fig. 2). Microscopically, the mass contained compactly- or loosely-arranged tumor cells with 20% tumor necrosis, and was surrounded by an abundant myxoid matrix or fibrous stroma. Histologic findings revealed sheets of undifferentiated oval, spindle-shaped, or stellate tumor cells (Fig. 3A). Irregular, hyperchromatic nuclei with numerous abnormal mitoses (>50/10 high power fields) and highly irregular multinucleated giant cells were observed. Multiple intracytoplasmic or extracellular eosinophilic globules were expressed with periodic acid-Schiff (PAS) and diastase-resistant PAS staining (D-PAS) (Fig. 3B, C). Tumor cells had invaded into liver parenchyma, and several entrapped bile ducts were seen in the tumor mass periphery. Cytoplasm from the tumor cells showed diffusely positive expression of vimentin, alpha 1-antichymotrypsin and alpha 1-antitrypsin (Fig. 3D-F). CD68 and smooth muscle actin were positive only focally. Hepatocyte, AFP, cytokeratin (CK) 7, CK19, CD117, CD99, human melanoma black-45 (HMB-45), leukocyte common antigen (LCA), epithelial membrane antigen (EMA), myoglobin, and S-100 were all negative. The Ki-67 proliferation index was 10%. On the basis of these findings, a diagnosis of UES was made.

The cut section shows a large variegated solid and firm mass, composed of a grey-to-yellow solid area and a dark red hemorrhagic area.
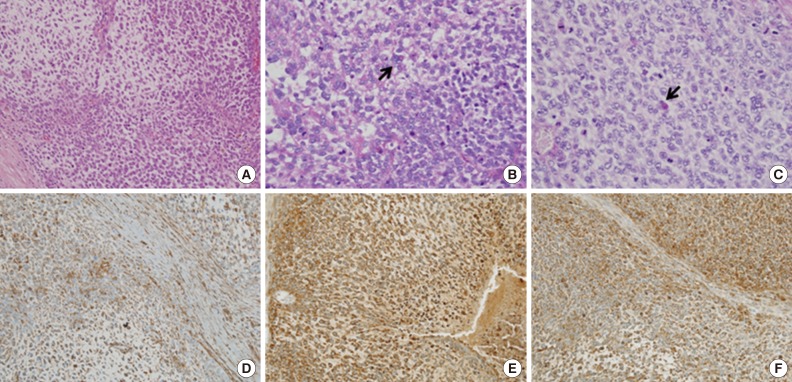
Microscopic studies of the liver mass show sheets of undifferentiated oval, spindle-shaped, or stellate tumor cells in myxoid or fibrous stroma (A). Intracytoplasmic eosinophilic globules (arrows) are seen in periodic acid-Schiff (PAS, B) and diastase-resistant PAS (C). The cytoplasms of tumor cells are diffusely stained by vimentin (D), alpha 1-antitrypsin (E), and alpha 1-antichymotrypsin (F).
One month after the operation, the patient developed a recurrent mass on segments 6 and 7 on the remnant right hepatic lobe. The mass continued to grow, so MAID (mesna, doxorubicin, ifosfamide, and dacarbazine) chemotherapy was started. During the fifth cycle of chemotherapy, the patient developed neutropenic fever and died of sepsis, eight months after his initial diagnosis and operation.
DISCUSSION
Before it was first named by Stocker and Ishak in 1978,1 UES of the liver was previously called mesenchymal sarcoma, fibromyxosarcoma, embryonal sarcoma, and malignant mesenchymoma. Although common in children between 6 and 10 years old, UES is very rare in adults over 60-years-old; for adults aged >60 years, only 14 UES cases have been reported in the world until now.3,4,5,6,7 UES was known to have no gender predominance in children, but there was female predominance (62.5%) in UES patients over the age of 30 worldwide.9 Clinical diagnosis of UES is difficult as there is no specific clinical feature. Patients complain of nonspecific symptoms such as abdominal pain, weight loss, nausea, vomiting, fatigue, or jaundice.
UES usually appears as a large, well-circumscribed solitary mass showing variable areas of necrosis, cystic degeneration, and hemorrhage. It ranges in size from 10 to 30 cm. In this case, the UES tumor contained atypical spindle or satellite cells with some giant cells set in compactly- or loosely-arranged myxoid or fibrous stroma. Some cells had nuclear pleomorphism with irregular and hyperchromatic features and numerous abnormal mitoses. Multiple intracytoplasmic or extracellular, eosinophilic, PAS and D-PAS stained, globules were characteristic. With immunohistochemistry, a diagnosis of UES could be made more easily, although immunohistological markers may not be specific. The cytoplasm of the patient's tumor cells diffusely stained positive for vimentin, alpha 1-antichymotrypsin, and alpha 1-antitrypsin, but was negative for hepatocyte marker, AFP, CK, CD117, CD99, HMB-45, LCA, EMA, myoglobin, and S-100. Ultrastructurally, his hepatic UES demonstrated dilated rough endoplasmic reticulum and electron-dense lysosomal precipitates, which correlated with the eosinophilic hyaline bodies seen microscopically. These morphological and ultrastructural findings are the same in pediatric and adult UES, suggesting that UES tumor cells are composed of fibroblastic, fibrohistioblastic, and undifferentiated cells. Other differential lineages have not been identified. UES, unlike hepatocellular carcinoma, is not associated with viral hepatitis, liver cirrhosis, alteration of hepatic function, or elevation of AFP, but the exact pathogenesis of this malignancy is not known.
In the differential diagnosis for UES, the following factors are considered: malignant fibrous histiocytoma, metastatic gastrointestinal stromal tumour, carcinosarcoma, hepatocellular carcinoma, cholangiocarcinoma (sarcomatoid differentiated), leiomyosarcoma, liposarcoma, hepatobiliary rhabdomyosarcoma (RMS), hepatoblastoma, and mesenchymal hamartoma of the liver (MHL). Both RMS and MHL arise in children and may involve myxoid mesenchymal tissue, however, MHL typically includes small bile ducts and intervening vascular channels, as well as loose mesenchymal structure. In addition, RMS can be differentiated from UES through positive immunohistochemical staining for desmin, myogenin, and myoD.
UES of the liver has been considered an aggressive type of tumor with an unfavorable prognosis. It is well known to commonly recur even after complete resection, and the majority of patients die within two years of the initial surgery. Radical resection of the tumor is recommended for the best treatment. In addition, recent studies have shown that adjuvant chemotherapy and/or radiotherapy in addition to surgery may be helpful for improving survival.10 For unresectable tumors, systemic chemotherapy and local radiotherapy can be done. The role of neo-adjuvant treatment also is being evaluated for tumors diagnosed preoperatively. Effective chemotherapy regimens include some combination of vincristine, doxorubicin or actinomycin D, cyclophosphamide or ifosfamide, and cisplatin.11 The best choice of treatment for UES of the liver is considered to be surgical resection and chemotherapy. However, the most optimal therapy is still to be proved.
Since UES of the liver is a very rare entity in adults and can seem similar to mass-forming intrahepatic cholangiocarcinoma, it is important to diagnose UES in a timely and accurate fashion-regardless of tissue quantity-in order to begin effective treatment as soon as possible.
Notes
No potential conflict of interest relevant to this article was reported.