Cystic Brunner’s Gland Hamartoma in the Gastric Body: A Case Report
Article information
Cystic Brunner’s gland hamartoma has been described under the various names including cystic hamartoma of Brunner’s glands, cystic Brunner’s gland heterotopia, Brunner’s gland cysts, Brunner’s gland cystadenoma, Brunner’s cyst, mucocele of Brunner’s glands, and cyst of Brunner’s glands[1-4].
Here, we report a rare case where a patient presented with an incidentally found, and long-standing pedunculated submucosal mass in the stomach body, which was diagnosed as a cystic Brunner’s gland hamartoma. To the best of our knowledge, no previous occurrence of cystic Brunner’s gland hamartoma in the gastric fundic glands has been reported[1-7].
CASE REPORT
A previously healthy 72-year-old female presented with a gastric submucosal tumor at the gastric angle. She had been under evaluation for ten years and over this follow-up period, the tumor size increased from 10 mm to 20 mm (Fig. 1A). Endoscopic ultrasound (EUS) showed a heterogeneous mixed hypoechoic lesion in the third layer of the gastric wall, measuring 20 mm (Fig. 1B). Multiple small compartments were separated by thin-walled septae and a regular border. Because we expected that the patient had a gastric cystic submucosal tumor, including a gastrointestinal stromal tumor with cystic change or a heterotopic pancreas, endoscopic submucosal dissection was performed using a dual knife and hemostatic forceps. There were no acute complications during the procedure.
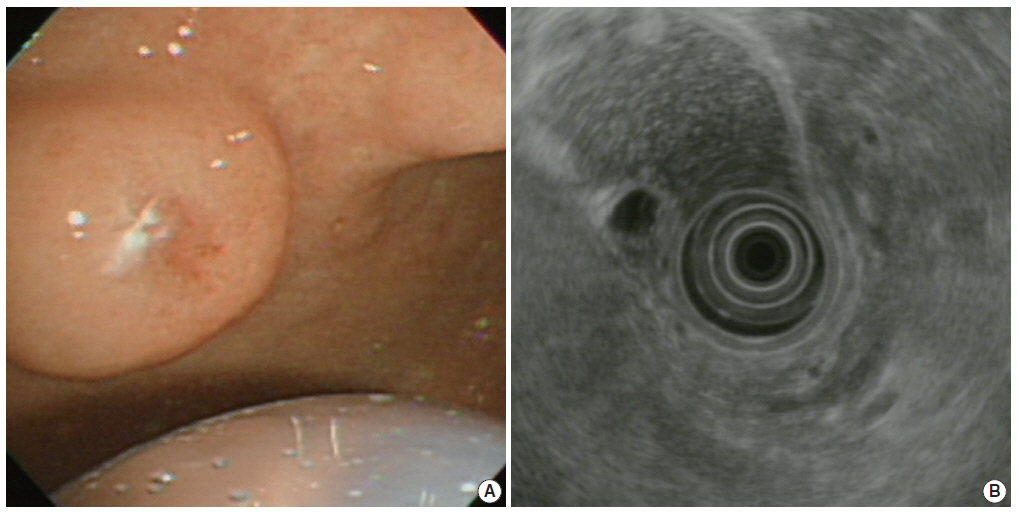
(A) Upper gastroesophageal endoscopy reveals a pedunculated polyp in the anterior wall of the gastric angle. Note the polyp is covered by normal-appearing mucosa. (B) Endoscopic ultrasound reveals a heterogeneous mixed echoic lesion in the submucosal layer.
On gross examination, the resected specimen was polypoid in appearance and had an intact surface. The cut surface showed a multiloculated cyst containing clear fluid (Fig. 2A). On microscopic examination, the polypoid cyst was covered by intact fundic mucosa, and the underlying multilocular cysts consisted of dilated ducts and intermixed fibromuscular bundles in the submucosa (Fig. 2B). The cysts deeply infiltrated mature lymphoid cells with germinal centers. A few dilated lymphatics and capillaries were observed. The cysts were lined by clear columnar epithelia, similar to that found in Brunner’s gland ducts (Fig. 2C). The epithelial lining was observable after staining with periodic-acid Schiff stain, but not with mucicarmine. The overlying fundic mucosa epithelial cells were positive for MUC5AC (1:500, CLH5, Novocastra Laboratories, Newcastle, UK) and negative for MUC6 (1:500, Ccp58, Novocastra Laboratories). These findings are all consistent with having cystic Brunner’s gland hamartoma in the stomach.
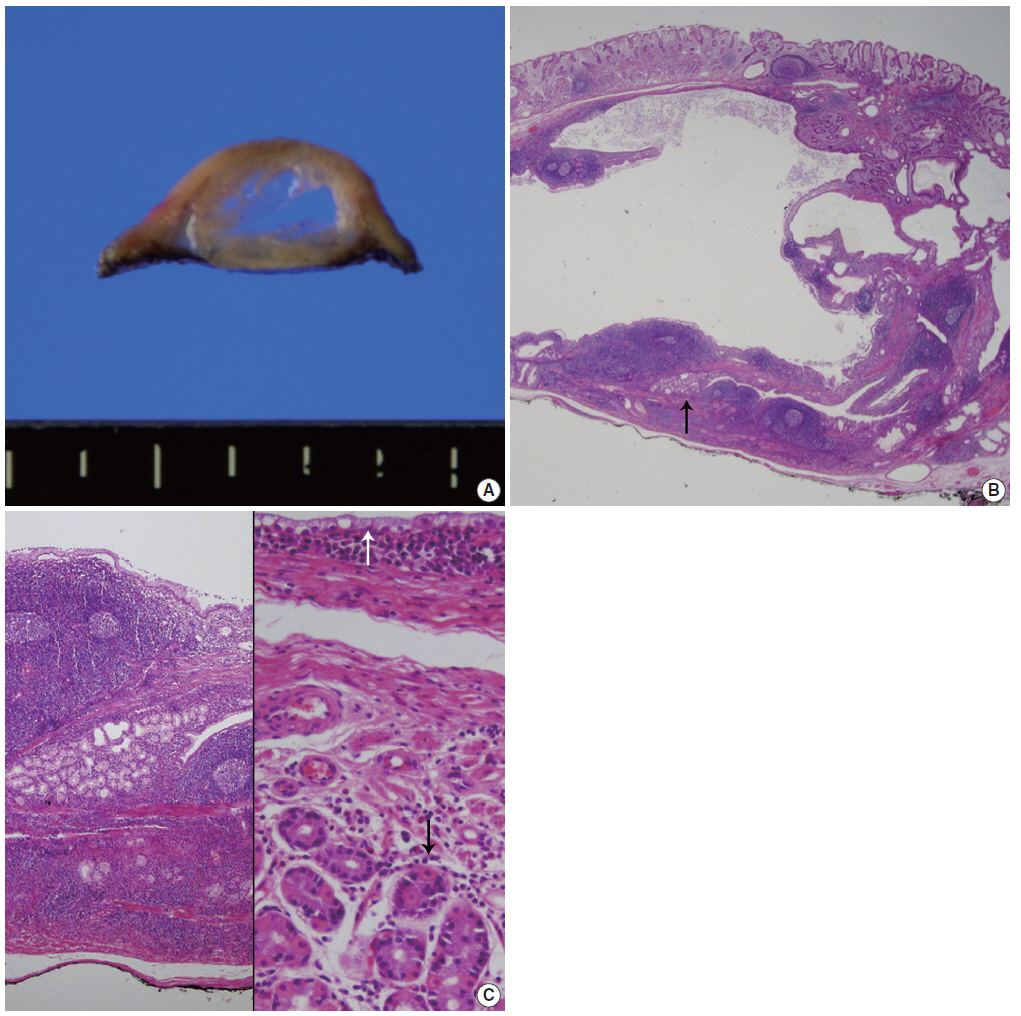
(A) Cut surface of an endoscopic submucosal dissected specimen shows dilated cysts with septation. (B) Low-power view shows a multilocular cyst containing amorphous fluid. Note the wall containing marked lymphoid cells with germinal center formation and some Brunner’s glands (arrow). (C) High magnification of the normal-appearing portion of Brunner’s glands (left). The cysts are lined by a single layer of mucinous columnar cells (white arrow) as well as the intervening smooth muscle bundle and overlying fundic glands (black arrow) (right).
During the ten-month follow-up period, the patient remained asymptomatic and her follow-up endoscopic biopsy was unremarkable.
DISCUSSION
Brunner’s glands are mucus-secreting acinar glands, which are normally found in the deep portion of the duodenal mucosa and the submucosal layer[4]. Histologically, normal-appearing Brunner’s glands have a heterotopic presence and are prolific. By definition, cystic Brunner’s gland hamartoma is characterized by a cystic space in the submucosa immediately underneath the muscularis mucosae and hyperplastic Brunner’s glands are not characteristically found. The hamartoma can be distinguished from classic Brunner’s gland adenoma in that the latter leads to predominantly solid aggregates of Brunner’s glands[7]. The most important differential diagnoses include gastritis cystica polyposa (GCP) and fundic-gland polyps with metaplasia. GCP is characterized by elongated gastric foveolae and cystic dilated glands that extend into the submucosa, as occurs during ischemia, chronic inflammation, and the presence of foreign bodies, especially at a prior surgical site. Findings such as submucosal cysts within disorganized smooth muscle and infiltration of mixed inflammatory cells in the lamina propria are also consistent with cystic Brunner’s gland hamartoma. However, hyperplastic Brunner’s glands do not include GCP. Fundic gland polyps are characterized by cystic dilatations of the oxyntic glands, which are lined by flattened parietal and chief cells, with or without mucous gastric foveolar epithelial cells.
Irregular proliferation of deep fundic glands and the smooth muscle portion were found, but stromal inflammation was minimal and Brunner’s glands were not present in the fundic gland polyp. As with the present case, overlying fundic glands that are MUC5AC-positive and MUC6-negative indicate that the cells possess no gastric antral or cardiac mucous glandular phenotypes except the foveolar cellular phenotypes[8]. Cystic Brunner’s gland hamartoma also differs from pancreatic heterotopia, which includes pancreatic acini, ducts, islets of Langerhans, and intervening connective tissue. Other differential diagnoses of cystic structures of the stomach include gastrointestinal stromal tumor (GIST) with cystic change, duplication cyst, heterotopic pancreatic tissue, and Brunner’s gland cysts. Rupture or flattening of polypoid lesions after biopsy offers a diagnostic clue but Brunner’s gland hamartoma is not always diagnosed preoperatively because it often yields nonspecific radiological findings and is not always adequately biopsied. Additionally, Brunner’s gland hamartoma is generally unfamiliar to pathologists and gastroenterologists[9]. For preoperative evaluation of submucosal tumors or proper muscle layers, EUS provides information that is useful for characterizing the gastrointestinal layers of origin by measuring the echogenicity of the tumor and its internal features, including echogenic foci or septae, and the smoothness of the border and the adjacent organs. Brunner’s gland cysts, being located in the submucosa, appear in the third layer on EUS. EUS can differentiate intramural tumors from extraluminal compressions. When cystic changes are observed, Brunner’s gland hamartoma has variable echogenicity and a more heterogeneous pattern[10].
GIST is typically observed in the fourth hypoechoic mass and appears as echogenic foci with an irregular border and anechoic spaces. Polypoid lipomas appear in the third layer of hyperechogenicity. Anechoic cysts in the third or fifth layer with a compressible nature are suggestive of a duplication cyst. Pancreatic heterotopia can also be submucosally located and are often found in the gastric antrum, which may appear as hypoechoic or mixed echogenicity lesions in the second, third, or fourth layer. Cystic Brunner’s gland hamartoma takes a completely benign course and is typically discovered incidentally. Endoscopic or surgical excision is performed for diagnostic confirmation and treatment, depending on size and location.
The pathogenesis of cystic Brunner’s gland hamartoma is unknown[9]. It has long been considered a hamartomatous lesion, believed to be the result of migration of embryonic remnants during embryogenesis. Another theory is that a cystic hamartoma may form as a hyperplastic reaction to an obstruction or inflammatory cell infiltration, mainly by lymphocytes. The pathology of the current case, which included multilocular cysts lined by columnar epithelium, dilated ducts, and lymphoid cells heavily infiltrated by germinal centers, also included a secondary retention cyst that was caused by an obstruction of the major Brunner’s gland duct as well as hyperplasia of Brunner’s glands. Cystic Brunner’s gland hamartomas are exceedingly rare, and only 13 cases have been described, all of which were reported in the first or second portion of the duodenum or, most rarely, in the gastric antrum[1-7]. No case occurring in the gastric angle that contains the overlying fundic glands has been reported.
Here, we report on a gastric cystic Brunner’s gland hamartoma based on EUS findings and an endoscopic submucosal dissection. The current case is unique in the following aspects: 1) it is a rare cystic Brunner’s gland hamartoma, 2) it showed multilocular cystic changes, 3) the hamartoma presented in a rare location—the gastric angle—and was composed of fundic glands, as seen under light microscopy.
Notes
No potential conflict of interest relevant to this article was reported.