Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(5); 2013 > Article
-
Case Study
Abdominal Fibromatosis in a Young Child: A Case Study and Review of the Literature - Hyun Hee Chu1, Pyoung Han Hwang2, Yeon Jun Jeong3, Myoung Ja Chung1,4
-
Korean Journal of Pathology 2013;47(5):472-476.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.5.472
Published online: October 25, 2013
1Department of Pathology, Chonbuk National University Medical School, Jeonju, Korea.
2Department of Pediatrics, Chonbuk National University Medical School, Jeonju, Korea.
3Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea.
4Research Institute for Endocrine Sciences, Jeonju, Korea.
- Corresponding Author: Myoung Ja Chung, M.D. Department of Pathology, Chonbuk National University Medical School, 567 Baekje-daero, Deokjin-gu, Jeonju 561-756, Korea. Tel: +82-63-270-3072, Fax: +82-63-270-3135, 'mjchung@jbnu.ac.kr'
• Received: October 23, 2012 • Revised: December 17, 2012 • Accepted: December 18, 2012
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Fibromatoses comprise many different entities of well-differentiated fibroblastic proliferation with variable collagen production and form a firm nodular mass. Abdominal fibromatosis is distinguishable from other forms of fibromatosis because of its location and its tendency to occur in women of childbearing age during or following pregnancy. Abdominal fibromatosis in children is an extremely rare condition. A 15-month-old boy presented with an abdominal wall mass that had recently increased in size. Mass excision was perfomed. The tumor was 4.3×4.1 cm and partly circumscribed. Histologically, the tumor was composed of parallel long fascicles of spindle-cells with a uniform appearance. The edges of the resected mass were infiltrative, and the surgical margins were positive. Mitotic figures were <1/10 high power fields. No cellular atypia or necrosis was present. The tumor cells were positive for vimentin and nuclear β-catenin staining.
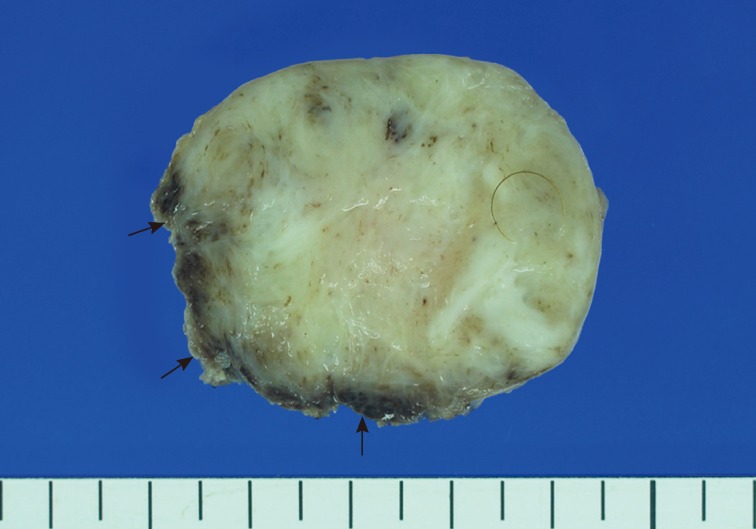
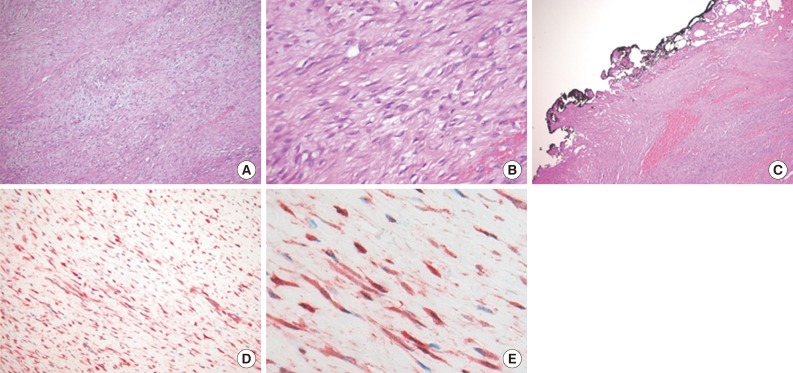
- A 15-month-old boy was referred to our surgical department because of a growing mass in the epigastric area. He had no history of trauma prior to the time that the mass was noticed by the parents. The pregnancy, birth, and neonatal history were uneventful, and there was no history of hospitalization or surgery. The child's developmental milestones were within normal limits. Ultrasonography demonstrated a solid and relatively well-circumscribed mass with mild heterogeneous echogenicity, measuring 1.9×0.9 cm. A fine needle biopsy showed proliferation of bland-looking spindle-shaped cells with collagen. A histological diagnosis of benign mesenchymal tumor, such as a neurofibroma, was made. During follow-up, the mass increased rapidly in size, and was excised one year after it was first detected. On gross examination, the tumor was 4.3×4.1 cm and partly circumscribed. The cut surface of the mass was solid and grayish-white without hemorrhage or necrosis (Fig. 1). The tumor was microscopically composed of parallel long fascicles of spindle-cells with a uniform appearance (Fig. 2A). The tumor cells had a pale eosinophilic cytoplasm and small, elongated nuclei that were embedded in a variable collagen network, consistent with abdominal fibromatosis (Fig. 2B). The edges of the resected mass were infiltrative, and the surgical margins were positive (Fig. 2C). Mitotic figures were <1/10 high power fields. No cellular atypia or necrosis was present. Most of the tumor cells were positive for nuclear β-catenin staining (Fig. 2D, E). S-100 protein, estrogen receptor, and progesterone receptor expressions were negative. The patient could not undergo reoperation to remove the remnant tumor because of a difficult abdominal wall reconstruction. One month after the operation, the patient underwent chemotherapy including vinblastine plus methotrexate (MTX) by intravenous bolus infusion. Postoperative ultrasonography at three months showed a suspected recurrence at the abdominal wall, but this was not pathologically demonstrated. Additional chemotherapy and a rework-up were planned. The patient is currently alive and healthy at seven months after the operation.
CASE REPORT
- Herein, we report an unusual occurrence of abdominal fibromatosis in a young child. The abdominal wall is a very unusual site for childhood fibromatosis. To date, the exact incidence of abdominal fibromatosis in children is unknown. We carefully searched the Internet for childhood fibromatosis and reviewed 285 cases of fibromatoses in patients younger than 21 years reported in the English literature (Table 1). Based on our review of childhood fibromatosis, the limbs (45.1%) were the most common site followed by the head and neck (28.5%) and trunk (24.7%). Among cases of trunk fibromatosis, only seven (2.4%) occurred in the abdominal wall when we excluded cases with no record of the detailed location in the trunk. Clinicopathologic and follow-up information were available in seven of eight cases including our present case, and are summarized in Table 2. Faulkner et al.6 showed a similar site distribution to our present review for childhood fibromatosis (n=63; 61% extremities, 18% head and neck, 13% trunk, 8% multicentric). However, the head and neck area was the most common site of fibromatosis followed by the extremities and the trunk in general population.1
- The biological behavior of fibromatosis in children is considered to be the same as that of adult fibromatosis. The behavior is intermediate between that of benign fibroblastic tumors and fibrosarcoma. Local recurrence after surgical resection is the major problem and depends on the completeness of the resection. Age, gender, site, size, and number of previous recurrences have no significant impact on the likelihood of recurrence.6 However, Spear et al.17 showed an age-dependent difference in outcome with a disadvantage for younger age patients. Recurrence rates varies from 10% to 80%, according to the status of resection margins. Meazza et al.8 analyzed the clinical outcomes of 94 cases of childhood fibromatosis, and patients who underwent resection with microscopic residual disease also showed a high recurrence rate (76%). However, the five year survival rate was 100% and local recurrence does not affect patient survival.
- A limited number of reports are available regarding treatment for childhood fibromatosis. According to the adult experience, surgical excision with a clear resection margin has been accepted as the most successful treatment. Chemotherapy and/or radiotherapy have been suggested as adjuvant therapy in patients with residual tumors, as the potential for morbidity is high after the second operation. Traditional cytotoxic chemotherapy may be the treatment of choice for cases of an unresectable, rapidly growing, and/or symptomatic and/or life-threatening desmoid tumor.18 Adjuvant radiotherapy in adults appears to significantly improve local control in 60-90% of patients.19 However, the control rate of fibromatosis following adjuvant radiation treatment in children is low. Radiotherapy appears to be a less effective treatment in children compared to that in adults.9 Those authors speculated that childhood fibromatosis is biologically different from the adult counterpart. Chemotherapy may be a reasonable alternative to radiotherapy for children. Our patient had a microscopic residual tumor and received chemotherapy with vinblastine plus MTX one month after the operation. Careful long-term follow-up was indicated for our patient based on previous reports about the high recurrence rate of fibromatosis with remnant tumors and the poor control rate of adjuvant treatment. A recurrence was suspected by ultrasonography three months after the operation, but it was difficult to differentiate from post-operative scars. Therefore, additional chemotherapy and a rework-up were planned.
- Similar to extra-abdominal fibromatosis, the pathogenesis of abdominal fibromatosis is most likely multifactorial, and genetic predisposition, endocrine factors, and trauma seem to play important roles.1 Nuclear β-catenin expression has been suggested as a tumor-specific marker for fibromatosis. Deep fibromatoses have somatic β-catenin gene mutations leading to intranuclear accumulation of β-catenin, which may explain the proliferative advantage of tumor cells. β-Catenin is normally detected in the cytoplasm; therefore, nuclear β-catenin expression appears to be helpful in the differential diagnosis of fibromatosis. Bhattacharya et al.20 showed that 100% of deep fibromatoses expressed nuclear β-catenin and that no other lesions (10 different types of lesions, n=67) expressed nuclear β-catenin. However, nuclear β-catenin immunostaining does not mean that the tumor has a somatic mutation in the β-catenin gene. Superficial fibromatoses lack a somatic mutation of the β-catenin gene; however, the majority of lesions show nuclear β-catenin staining.
- The differential diagnosis of fibromatosis include low-grade sarcoma and various benign conditions including infantile fibrosarcoma, low-grade myofibrosarcoma, neurofibroma, myofibroma, nodular fascitis. Infantile fibrosarcoma usually shows moderate to high cellularity with a herringbone growth pattern, often necrosis, and considerable mitotic activity. Low-grade myofibrosarcoma is also included as a differential diagnosis. Although low-grade myofibrosarcoma shows similar morphologic features, it shows at least focal nuclear atypia and the mean mitotic rate is 2/10 high power fields. Myofibroma shows characteristic zonation with hypercellular central area with primitive cells and a hemangiopericytoma-like vascular pattern with paucicellular zones in the periphery. Nodular fascitis displays a loose storiform pattern, extravasated erythrocytes, and myxoid foci. These findings were not found in our case. Furthermore, β-catenin immunohistochemistry distinguishes deep fibromatosis from those diseases.
- In conclusion, we report a rare case of abdominal fibromatosis in a young child. Although abdominal fibromatoses in children are rare, awareness about childhood abdominal fibromatosis and proper immunohistochemical staining such as β-catenin are important for an accurate diagnosis and proper tumor management.
DISCUSSION
- 1. Weiss SW, Goldblum JR. Enzinger and Weiss's soft tissue tumors. Philadelphia: Mosby Elsevier, 2008; 277–302.
- 2. Ademuyiwa AO, Bode CO, Elebute OA. Anterior abdominal wall desmoids tumor in a five year old girl: a pre operative diagnostic challenge in resource-poor setting. Ann Pediatr Surg 2010; 6: 41–43.
- 3. Atahan IL, Akyol F, Zorlu F, Gürkaynak M. Radiotherapy in the management of aggressive fibromatosis. Br J Radiol 1989; 62: 854–856. PMID: 2790427. ArticlePubMed
- 4. Ayala AG, Ro JY, Goepfert H, Cangir A, Khorsand J, Flake G. Desmoid fibromatosis: a clinicopathologic study of 25 children. Semin Diagn Pathol 1986; 3: 138–150. PMID: 3616218. PubMed
- 5. Buitendijk S, van de Ven CP, Dumans TG, et al. Pediatric aggressive fibromatosis: a retrospective analysis of 13 patients and review of literature. Cancer 2005; 104: 1090–1099. PMID: 16015632. ArticlePubMed
- 6. Faulkner LB, Hajdu SI, Kher U, et al. Pediatric desmoid tumor: retrospective analysis of 63 cases. J Clin Oncol 1995; 13: 2813–2818. PMID: 7595743. ArticlePubMed
- 7. Jain M, Shubha , Agarwal K. Infantile fibromatosis: diagnosis by fine needle aspiration cytology. Cytopathology 2001; 12: 406–409. PMID: 11843943. ArticlePubMed
- 8. Meazza C, Bisogno G, Gronchi A, et al. Aggressive fibromatosis in children and adolescents: the Italian experience. Cancer 2010; 116: 233–240. PMID: 19950127. ArticlePubMed
- 9. Merchant TE, Nguyen D, Walter AW, Pappo AS, Kun LE, Rao BN. Long-term results with radiation therapy for pediatric desmoid tumors. Int J Radiat Oncol Biol Phys 2000; 47: 1267–1271. PMID: 10889380. ArticlePubMed
- 10. Rao BN, Horowitz ME, Parham DM, et al. Challenges in the treatment of childhood fibromatosis. Arch Surg 1987; 122: 1296–1298. PMID: 3675193. ArticlePubMed
- 11. Refsum S Jr, Schistad G, Serck-Hansen A. Two cases of aggressive fibromatosis in children presenting as abdominal tumor. Pediatr Surg Int 1988; 4: 59–62. Article
- 12. Reich S, Overberg-Schmidt US, Bührer C, Henze G. Low-dose chemotherapy with vinblastine and methotrexate in childhood desmoid tumors. J Clin Oncol 1999; 17: 1086PMID: 10071306. ArticlePubMed
- 13. Scougall P, Staheli LT, Chew DE, Taylor TK, Almquist EE. Desmoid tumors in childhood. Orthop Rev 1987; 16: 481–488. PMID: 3331192. ArticlePubMed
- 14. Sharma A, Ngan BY, Sándor GK, Campisi P, Forte V. Pediatric aggressive fibromatosis of the head and neck: a 20-year retrospective review. J Pediatr Surg 2008; 43: 1596–1604. PMID: 18778992. ArticlePubMed
- 15. Skapek SX, Hawk BJ, Hoffer FA, et al. Combination chemotherapy using vinblastine and methotrexate for the treatment of progressive desmoid tumor in children. J Clin Oncol 1998; 16: 3021–3027. PMID: 9738571. ArticlePubMed
- 16. Spiegel DA, Dormans JP, Meyer JS, et al. Aggressive fibromatosis from infancy to adolescence. J Pediatr Orthop 1999; 19: 776–784. PMID: 10573349. ArticlePubMed
- 17. Spear MA, Jennings LC, Mankin HJ, et al. Individualizing management of aggressive fibromatoses. Int J Radiat Oncol Biol Phys 1998; 40: 637–645. PMID: 9486614. ArticlePubMed
- 18. Kasper B, Ströbel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist 2011; 16: 682–693. PMID: 21478276. ArticlePubMedPMC
- 19. Goy BW, Lee SP, Eilber F, et al. The role of adjuvant radiotherapy in the treatment of resectable desmoid tumors. Int J Radiat Oncol Biol Phys 1997; 39: 659–665. PMID: 9336146. ArticlePubMed
- 20. Bhattacharya B, Dilworth HP, Iacobuzio-Donahue C, et al. Nuclear beta-catenin expression distinguishes deep fibromatosis from other benign and malignant fibroblastic and myofibroblastic lesions. Am J Surg Pathol 2005; 29: 653–659. PMID: 15832090. ArticlePubMed
References
Fig. 1The cut section of the abdominal tumor reveals a grayish-white solid mass with partially irregular edges (arrows).
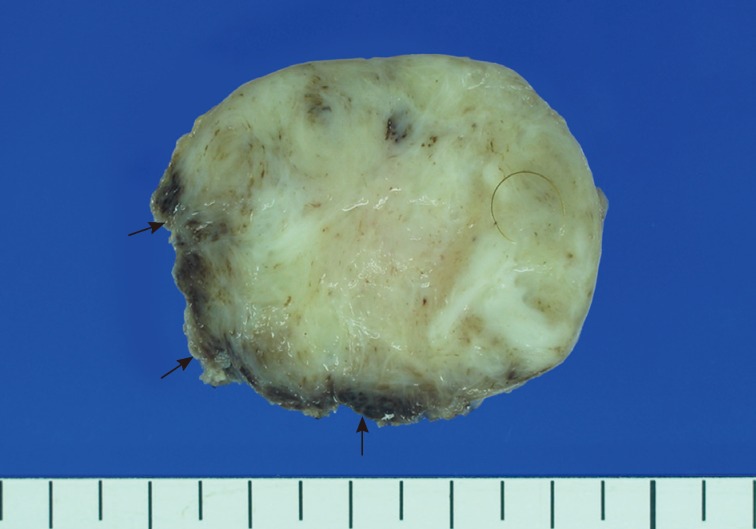

Fig. 2Histologic findings. (A, B) The tumor reveals long fascicles of bland spindle-shaped fibroblasts with collagen deposition. (C) The tumor shows infiltrative growth and the surgical margins are positive. (D, E) The tumor cells show nuclear and cytoplasmic β-catenin immunostaining.
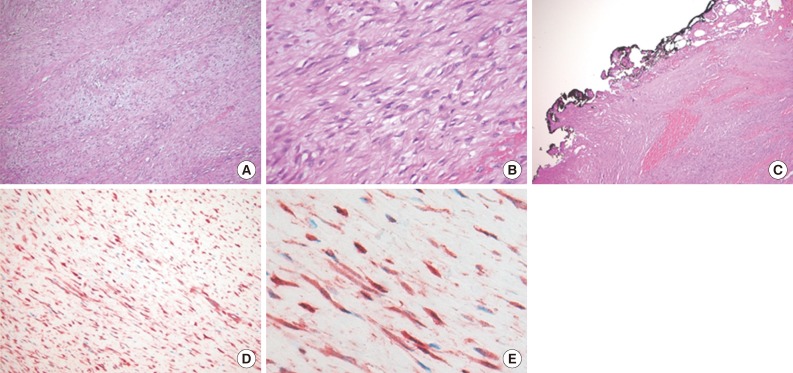

Table 1.Published series of childhood fibromatosis cases
| Reference | No. of patients |
Site of involvement |
Detailed site in trunk | ||
|---|---|---|---|---|---|
| H/N | Limb | Trunk | |||
| Ayala et al. [4] | 25 | 8 | 13 | 4 | Abdominal wall (1), NA (3) |
| Scougall et al. [13] | 8 | 0 | 4 | 4 | Pelvis (4) |
| Rao et al. [10] | 20 | 7 | 7 | 6 | Back (1), NA (5) |
| Atahan et al. [3] | 4 | 0 | 1 | 3 | Shoulder (1), gluteal region (2) |
| Faulkner et al. [6] | 63a | 11 | 39 | 8 | Abdominal wall (1), intra-abdominal type (3), NA (4) |
| Skapek et al. [15] | 10 | 1 | 6 | 3 | Chest wall (3) |
| Refsum et al. [11] | 2 | 0 | 0 | 2 | Lower abdominal wall (2) |
| Reich et al. [12] | 5 | 0 | 4 | 1 | Chest wall (1) |
| Spiegel et al. [16] | 18 | 5 | 12 | 1 | Buttock (1) |
| Merchant et al. [9] | 13 | 4 | 2 | 7 | Abdominal wall (1), paraspinal or chest wall (6) |
| Jain et al. [7] | 1 | 0 | 0 | 1 | Abdominal wall (1) |
| Buitendijk et al. [5] | 13 | 5 | 4 | 4 | Back (2), NA (2) |
| Sharma et al. [14] | 10 | 10 | 0 | 0 | - |
| Ademuyiwa et al. [2] | 1 | 0 | 0 | 1 | Abdominal wall (1) |
| Meazza et al. [8] | 94 | 31 | 38 | 25 | Intra-abdominal type (7), NA (18) |
| Present case | 1 | 0 | 0 | 1 | Abdominal wall (1) |
| Total no. (%) | 288 | 82 | 130 | 71 | Abdominal wall (8, 2.8%) |
Table 2.Characteristics of childhood abdominal fibromatosis
| Reference | Age/Sex | Tumor size (cm) | Tumor margin | R margin | Histology | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|
| Jain et al. [7] | 18 mo/M | 4 × 3 | Unencapsulated | NA | Typical | OP | 3 yr, NR |
| Refsum et al. [11] | 5 yr/M | 6 × 5 | Unencapsulated | Positive | Typical | OP | 7 yr, NR |
| 4.5 yr/M | 6.5 × 5.5 | Unencapsulated | Positive | Typical | OP | 15 mo, NR | |
| Ademuyiwa et al. [2] | 5 yr/F | 6 | Unencapsulated | Negative | NA | OP | 12 wk, NR |
| Merchant et al. [9] | 15 yr/F | NA | NA | NA | NA | OP, CTx, RTx | 53 mo, dead |
| Faulkner et al. [6] | 18 yr/NA | NA | NA | Positive | NA | OP, CTx | Recurrence |
| Present case | 15 mo/M | 4.3 × 4.1 | Unencapsulated | Positive | Typical | OP, CTx | 7 mo, suspicious of recurrence |
Figure & Data
References
Citations
Citations to this article as recorded by 

- A rare tumor of the large bowel in a young boy
Shyam Srinivasan, Soumitra Saha
Cancer Research, Statistics, and Treatment.2021; 4(4): 752. CrossRef - Uncommon abdominal wall mass in a young boy: Desmoid tumor
Levent Cankorkmaz, Mehmet H. Atalar, H. Reyhan Eğilmez
Cumhuriyet Medical Journal.2018; : 811. CrossRef - Lesiones ocupantes de espacio en pared abdominal (no herniaria). La visión del patólogo
Isidro Machado, Julia Cruz, Javier Lavernia, Fernando Carbonell
Revista Hispanoamericana de Hernia.2015; 3(3): 85. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission