Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(1); 2017 > Article
-
Original Article
Increased Expression of Thymosin β4 Is Independently Correlated with Hypoxia Inducible Factor-1α (HIF-1α) and Worse Clinical Outcome in Human Colorectal Cancer - Seung Yun Lee, Mee Ja Park, Hye Kyung Lee, Hyun Jin Son, Chang Nam Kim1, Joo Heon Kim,, Dong Wook Kang
-
Journal of Pathology and Translational Medicine 2016;51(1):9-16.
DOI: https://doi.org/10.4132/jptm.2016.08.23
Published online: October 16, 2016
Department of Pathology, Eulji University Hospital, Daejeon, Korea
1Department of Surgery, Eulji University Hospital, Daejeon, Korea
-
Corresponding Author: Joo Heon Kim, MD, PhD Department of Pathology, Eulji University Hospital, 95 Dunsanseo-ro, Seo-gu, Daejeon 35233, Korea Tel: +82-42-611-3454 Fax: +82-42-611-3459 E-mail: 'kjh2000@eulji.ac.kr'
Dong Wook Kang, MD, PhD Department of Pathology, Eulji University Hospital, 95 Dunsanseo-ro, Seo-gu, Daejeon 35233, Korea Tel: +82-42-611-3454 Fax: +82-42-611-3459 E-mail: 'astrias@eulji.ac.kr'
• Received: May 6, 2016 • Revised: August 9, 2016 • Accepted: August 23, 2016
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Thymosin β4 is a multi-functional hormone-like polypeptide, being involved in cell migration, angiogenesis, and tumor metastasis. This study was undertaken to clarify the clinicopathologic implications of thymosin β4 expression in human colorectal cancers (CRCs).
-
Methods
- We investigated tissue sections from 143 patients with CRC by immunohistochemistry. In addition, we evaluated the expression patterns and the clinico-pathological significance of thymosin β4 expression in association with hypoxia inducible factor-1α (HIF-1α) expression in the CRC series.
-
Results
- High expression of thymosin β4 was significantly correlated with lymphovascular invasion, invasion depth, regional lymph node metastasis, distant metastasis, and TNM stage. Patients with high expression of thymosin β4 showed poor recurrence-free survival (p = .001) and poor overall survival (p = .005) on multivariate analysis. We also found that thymosin β4 and HIF-1α were overexpressed and that thymosin β4 expression increased in parallel with HIF-1α expression in CRC.
-
Conclusions
- A high expression level of thymosin β4 indicates poor clinical outcomes and may be a useful prognostic factor in CRC. Thymosin β4 is functionally related with HIF-1α and may be a potentially valuable biomarker and possible therapeutic target for CRC.
- Case selection and immunohistochemistry
- CRC cases were selected from patients who underwent surgical treatment at Eulji University Hospital from January 2000 to June 2005. We excluded those specimens obtained from patients who underwent preoperative neoadjuvant chemoradiation. Pertinent clinical and pathological information was obtained from electronic operation records and pathology reports. All cases were histologically confirmed to be primary colorectal adenocarcinoma and hematoxylin and eosin slides were re-evaluated by two independent pathologists. The tumor grade of the adenocarcinoma was classified into low grade (≥50% of tumor glands) and high grade (<50% of tumor glands) [25]. For signet ring cell carcinoma and mucinous adenocarcinoma, less than 50% glands were defined as high grade. Tumor budding was defined as a single or group of less than five detached tumor cells and classified into two grades [26,27]. Tumor recurrence was designated as tumor occurring at the anastomosing site, in the regional lymph nodes, and the pelvic cavity diagnosed by radiology, colonoscopy, exploratory surgical, and/or histological examination. In addition, metastasis was defined as the presence of tumor cells outside the area of resection, including the liver, pancreas, lung, and other organs.
- All cases of CRC tissue with accompanying normal mucosal tissue were fixed in 10% buffered formalin for 24 hours and embedded in paraffin. Tissue sections of 3–4 μm thickness were cut and mounted on ProbeOn slides (Fisher Scientific, Pittsburgh, PA, USA). Sections that contained both tumor and adjacent uninvolved colonic mucosa were selected for immunohistochemistry (IHC) in most cases. In a few cases, sections were trimmed in order to decrease the surface area for an even distribution of antibodies, so that only the tumor portion was included in the IHC evaluation. IHC conditions for thymosin β4 and HIF-1α were optimized according to the manufacturers’ instructions. Paraffin embedded tissue sections were deparaffinized and rehydrated through a series of xylene and alcohol. Slides were then treated with 10 mM/L sodium citrate buffer (pH 6.1) for 15 minutes and autoclaved at 120°C for antigen retrieval. All slide sections for IHC were incubated in 3% H2O2 for 10 minutes to inactivate endogenous peroxidase, washed with 10 mM/L phosphate buffered saline buffer (pH 7.4), and then incubated with normal bovine serum to reduce false-positive staining. Mouse monoclonal antibodies against thymosin β4 (1:100, Biodesign Int., Saco, ME, USA) and HIF-1α (1:50, Novus Biologicals, Littleton, CO, USA) were used as primary antibodies. Slide sections were incubated with primary antibodies overnight at 4°C in a wet chamber and stained with diaminobenzidine as the substrate using an EnVision-HRP kit (Dako, Glostrup, Denmark). An irrelevant mouse IgG of the same isotype served as a negative control. Sections were counterstained with Mayer’s hematoxylin solution and then mounted.
- Assessment of IHC staining
- To evaluate the expression of thymosin β4 and HIF-1α in association with various clinico-pathological factors, the immunoreactivity of both thymosin β4 and HIF-1α were analyzed in a semi-quantitative manner by two independent pathologists who were blinded to outcomes. Immunoreactivity for thymosin β4 and HIF-1α was observed primarily in the cytoplasm and nuclei of normal mucosal epithelium and tumor cells, respectively. The intensity of immunohistochemical staining was scored as 0 to 2 (0, weaker staining than the normal mucosal epithelium; 1, staining similar to the normal mucosal epithelium; and 2, stronger staining than the normal mucosal epithelium). The percentage of positive cells was scored as 1 (< 25% of tumor cells), 2 (25%–49% of tumor cells), 3 (50%–74% of tumor cells), and 4 (≥ 75% of tumor cells). To evaluate the statistical significance between thymosin β4 and HIF-1α expression and clinico-pathological factors, the median value (25% of tumor cells showing a strong positive reaction than normal epithelium) of the series was used as the cutoff value to distinguish between tumor cells with low expression (<25% tumor cells) and high expression (≥ 25% of tumor cells). Cases with conflicting results were reevaluated and a consensus was reached.
- Statistical analysis of prognostic parameters
- We performed statistical analysis using the SPSS ver. 18 (SPSS Inc., Chicago, IL, USA). The correlation between thymosin β4 and the various clinico-pathological parameters were analyzed with the Pearson’s chi-square test or Fisher exact test. To evaluate statistical analysis, recurrence-free survival was defined as the duration from the date of surgery to the first date of recurrence or the date of last follow-up. Similarly, overall survival was defined as the duration from the date of surgical therapy to the date of death or date of last follow-up. The mean follow-up duration for all patients was 53.3 months, ranging from 0.6 to 121.9 months. Using the Kaplan-Meier method, the recurrence-free survival curve and the overall survival curve were formulated. To examine the statistical significance of the differences in survival distribution, log-rank test was utilized. Multivariate analysis for overall survival and recurrence-free survival was performed using Cox proportional hazard regression analysis. In all statistical analyses, p-values less than .05 were considered statistically significant.
- Ethical permission
- The Institutional Review Board of Eulji University Hospital approved the study protocol and provided all necessary ethical permission.
MATERIALS AND METHODS
- Association of clinico-pathological characteristics with thymosin β4 and HIF-1α expression status
- The median age of the 143 CRC patients (75 men and 68 women) at surgery was 62.2 years (range, 28 to 86 years) and the median tumor size was 5.2 cm (range, 0.8 to 12.0 cm) in maximum diameter. The majority of CRCs were moderately differentiated adenocarcinoma and 111 cases (77.6%) were classified as low grade and 32 cases (22.4%) as high grade (poorly differentiated 19, signet ring cell carcinoma 3, and mucinous carcinoma 10). One hundred and four cases (72.7%) showed lymphovascular tumor invasion and 106 cases (74.1%) exhibited high-grade tumor budding. According to the seventh edition of the AJCC TNM system [28], 26 patients (18.2%) were diagnosed with early-stage tumor invasion (five cases of pT1 and 21 cases of pT2) and 117 patients (81.8%) were diagnosed with advanced-stage tumor invasion (105 cases of pT3 and 12 cases of pT4). Seventy-six patients (53.1%) presented with regional lymph node metastasis and 22 patients (15.4%) with distant metastasis. Twenty-one patients (14.7%) were at pTNM stage I, 45 patients (31.5%) were at stage II, 55 patients (38.5%) were at stage III, and 22 patients (15.4%) were at stage IV. Twenty-seven patients (18.9%) were treated with chemoradiation after the first surgery (data not shown). Clinico-pathological characteristics of the 143 CRC patients are summarized in Table 1.
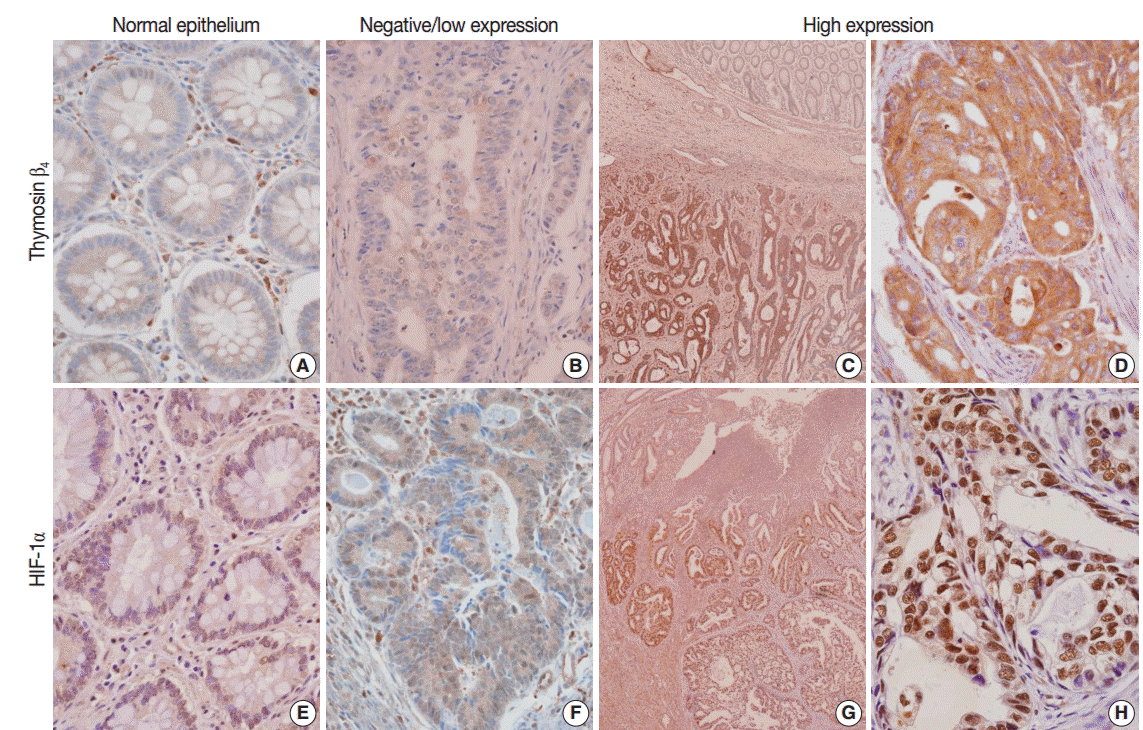
- In the normal colonic epithelium, immunoreactivity for thymosin β4 and HIF-1α was mostly none or weak. While thymosin β4 expression was found primarily in the cytoplasm of cancer cells, HIF-1α was stained predominantly in the nuclei of tumor cells (Fig. 1A–H). A high level of thymosin β4 and HIF-1α expression was observed in 66 of the 143 patients (46.2%) and in 67 of the 143 patients (46.9%), respectively. We analyzed whether thymosin β4 expression level was associated with clinicopathological factors. We found that predictive factors for prognosis, such as lymphovascular invasion, invasion depth (pT), regional lymph node metastasis (pN), distant metastasis, and TNM stage showed statistically significant correlations with thymosin β4 immunoreactivity (Table 1). Patients with high thymosin β4 expression levels showed a significantly greater presence of lymphovascular invasion, more frequent regional lymph node metastasis, deeper invasion depth, and more advanced tumor stage than in those with low thymosin β4 expression levels (p<.001). We also evaluated the association between HIF-1α expression levels and clinico-pathological variables. We found a statistically significant correlation between high HIF-1α immunohistochemical expression and clinico-pathological factors such as lymphovascular invasion (p=.006), invasion depth (p=.002), regional lymph node metastasis (p=.007), distant metastasis (p=.029), and TNM stage (p=.002) (data not shown).
- High thymosin β4 expression level correlated with tumor recurrence and overall survival
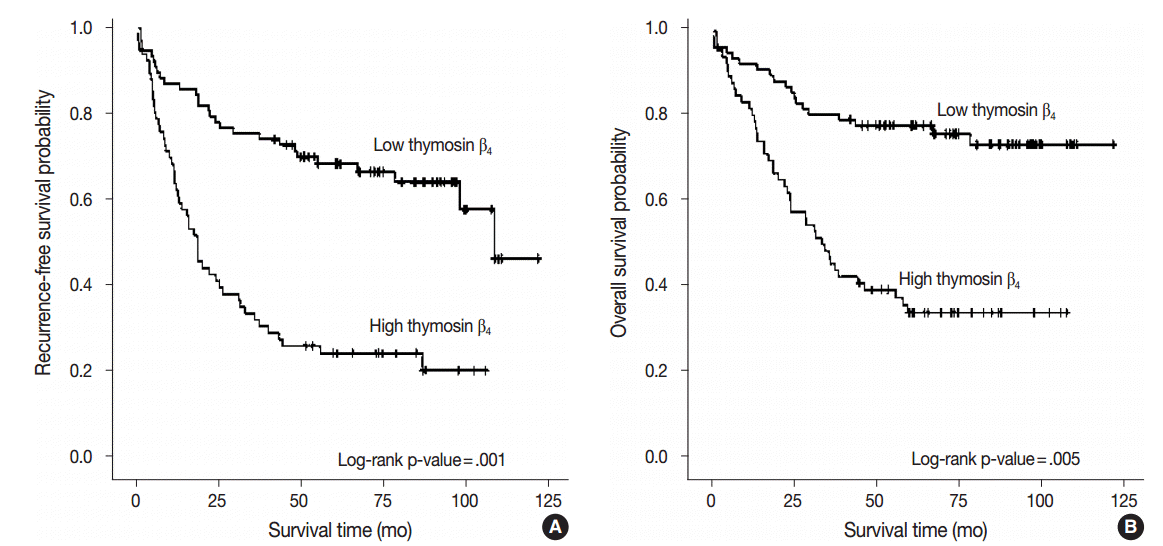
- We performed multivariate analysis to examine the correlation between thymosin β4 expression levels with recurrence-free survival and overall survival. Forty patients (28.0%) presented with cancer recurrence during follow-up and 55 patients (38.5%) died of CRC with or without metastasis. Seven patients (4.9%) died of unknown causes, 17 patients (11.9%) were alive with local recurrence and/or distant metastasis, and 64 patients (44.8%) remained alive and recurrence-free. Kaplan-Meier analysis showed that high thymosin β4 expression was significantly correlated with decreased recurrence-free survival (p<.001) (Fig. 2A).
- Recurrence-free survival was shorter in patients with high expression levels of thymosin β4, with a mean duration of 37.3 months (95% confidence interval [CI], 27.833 to 46.684), and was longer in patients with low levels of thymosin β4 expression, with a mean duration of 84.6 months (95% CI, 73.538 to 95.571). We also found that high thymosin β4 expression significantly correlated with worse overall survival (p=.001). Thymosin β4 expression status also significantly split the cumulative overall survival curves (Fig. 2B). While the overall survival of CRC patients with high thymosin β4 expression was mean duration of 51.7 months (95% CI, 41.381 to 61.939), the overall survival of CRC patients with low thymosin β4 expression was longer with mean duration of 96.8 months (95% CI, 86.952 to 106.732). Multivariate analysis was also performed to assess the prognostic value of thymosin β4 expression for recurrence-free survival and overall survival using various clinico-pathological parameters. Patients with high thymosin β4 expression were found to have worse survival outcomes. Statistically significant clinico-pathological factors that were correlated with overall survival were high thymosin β4 expression (p=.005), high tumor grade (p=.013), high tumor budding (p=.047), and presence of distant metastasis (p=.001). The relative risk (RR) of death in patients with a high expression level of thymosin β4 was more than two times greater (RR, 2.457; 95% CI, 1.315 to 4.592) than those with low thymosin β4 expression levels. High thymosin β4 expression was also an independent and relevant factor of decreased recurrence-free survival (p=.001). The RR of recurrence for patients with high thymosin β4 expression level was 2.540 (95% CI, 1.479 to 4.362). Table 2 summarizes the results from the Cox proportional hazards analysis.
- A statistically significant correlation between high HIF-1α expression and high thymosin β4 expression was also found (p<.001). Specifically, of the 66 cases exhibiting high thymosin β4 expression, 49 cases (74.2%) also showed high nuclear immunoreactivity of HIF-1α. Of the 77 cases expressing low thymosin β4 expression, 59 cases (76.6%) revealed corresponding low HIF-1α expression (Table 3).
RESULTS
- Growing tumors require oxygen and nutrient delivery through neovasculature. However, intratumoral hypoxia induced by imbalance between tumor growth and insufficient angiogenesis can lead to expression of HIF-1α, which is a transcriptional factor that activates tumor survival in an unstable hypoxic tumor microenvironment [29-32]. Recent reports have shown that thymosin β4 stabilizes HIF-1α in human cancer cells [7] and that thymosin β4 also induces migration and metastasis of colon cancer cells via the ILK/IQGAP1/Rac1 signal transduction pathway [22,33].
- Few studies have evaluated whether overexpression of thymosin β4 influences clinical prognosis and whether thymosin β4 is related to HIF-1α in CRCs. To better understand the relationship, we analyzed the clinical significance and expression status of thymosin β4 and HIF-1α in CRC patients. Our study demonstrates that high thymosin β4 expression has a significant association with lymphovascular invasion, nodal status, distant metastasis, and tumor progression in CRC patients (p<.001). This finding is consistent with our previous study showing hypoxia-induced high expression of thymosin β4, which also significantly correlated with regional lymph node metastasis in breast cancer [24]. In a previous study, we found thymosin β4 to be up-regulated under hypoxic conditions (5% O2) using an in vitro hypoxia-induced model to generate transcription profiles in human CRC. Based on these findings, for this study we examined the association between thymosin β4 expression and HIF-1α expression in CRC specimens. We then discovered that the overexpression of thymosin β4 in CRC is closely related to the restricted overexpression of HIF-1α in the CRC cells (p<.001).
- Recently, there have been reports regarding the association between thymosin β4 expression with tumor development and epithelial mesenchymal transition (EMT) [18,34]. In particular, Nemolato et al. [34] reported high expression of thymosin β4 at the invasive front in colon cancer and discussed its associated with EMT as well as invasion and metastasis of tumor cells. However, from our study, since we were unable to find an association between thymosin β4 expression and tumor budding (p=.118) and tumor border (p=.560), we found the direct association of thymosin β4 expression with EMT to be weak.
- The HIF complex, which involves various hypoxia-regulated genes, is a group of critical gene products in the tumor microenvironment of hypoxic adaptation and in angiogenesis [35]. The HIF complex is also an essential mediator in coordinating transcription of various factors in the tumor cells to survive in the hypoxic environment and its overexpression has been associated with increased mortality in various cancer types [31,35-37]. Among HIF complex proteins, HIF-1α is the best-characterized isoform. Whether HIF-2α, HIF-3α, and HIF-1β also play critical roles in the HIF pathway and regulate HIF target genes is not yet clearly known [38-41]. Hypoxic conditions induce HIF-1α expression in normal cells. HIF-1α is frequently upregulated in various cancer cells and the overexpression of HIF-1α correlates with advanced cancer progression or aggressiveness [42]. However, the clinical significance of HIF-1α in CRC has not been extensively studied. In this study, we observed a significant association between thymosin β4 expression and HIF-1α expression (p<.001). This result coincides with previous studies that found overexpression of HIF-1α to be associated with poor prognosis [36,37].
- Thymosin β4 has various functional roles in normal cell biology and its mechanism of action has recently been studied in various tumors. In this study, we found that high cytoplasmic expression of thymosin β4 is clinically important and an independent prognostic factor for CRC patients. As our results demonstrate that high thymosin β4 expression significantly correlates with tumor recurrence and worse overall survival, we suggest that high thymosin β4 expression may be a useful prognostic factor in CRC.
- Our results demonstrate that HIF-1α is correlated with overexpression of thymosin β4 in human CRC. Although further studies are necessary to further validate our findings, we suggest that thymosin β4, has potential as a prognostic biomarker and has potential as a HIF pathway target in human CRC.
DISCUSSION
-
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Notes
Fig. 1.Immunohistochemical expression of thymosin β4 (A–D) and hypoxia inducible factor-1α (HIF-1α) (E–H) in human colorectal cancer. (A) No or weak expression of thymosin β4 in normal colonic epithelium. (B) Low expression of thymosin β4 in tumor glands. (C) Tumor cells show high thymosin β4 expression, but no or weak thymosin β4 expression in normal colonic epithelium. (D) Tumor cells reveal strong thymosin β4 expression primarily in the cytoplasm of tumor cells. (E) No or weak immunoreactivity of HIF-1α in normal colonic epithelium. (F) Low expression of HIF- 1α in tumor cells. (G) Tumor cells show strong HIF-1α expression. (H) HIF-1α is highly expressed predominantly in the nucleus of tumor cells.
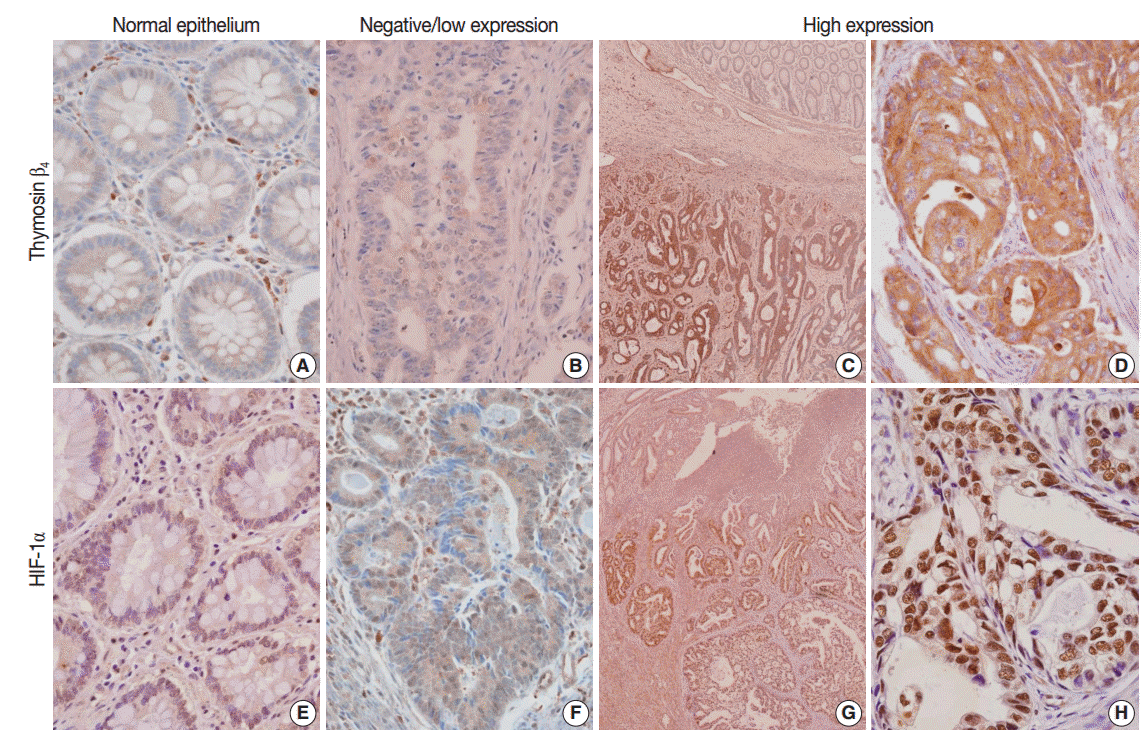

Fig. 2.Kaplan-Meier survival analysis by thymosin β4 expression status. (A) Cumulative recurrence-free survival differences between patients with high and low thymosin β4 expression. (B) Cumulative overall survival differences between patients with high and low thymosin β4 expression. p-values were obtained using the log-rank test of differences.
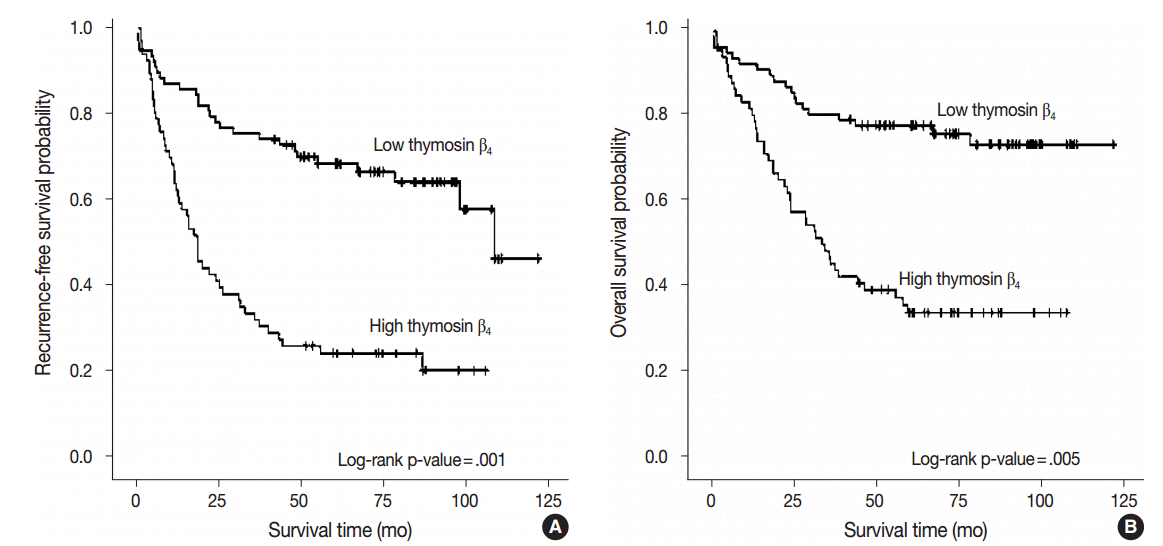

Table 1.Clinico-pathological variables and thymosin β4 expression status
| Characteristic | Total |
Thymosin β4 expression level |
p-value | |
|---|---|---|---|---|
| Negative/Low | High | |||
| Age (yr) | ||||
| < 50 | 26 | 10 (13.0) | 16 (24.2) | < .082 |
| ≥ 50 | 117 | 67 (87.0) | 50 (75.8) | |
| Gender | ||||
| Female | 68 | 35 (45.5) | 33 (50.0) | < .587 |
| Male | 75 | 42 (54.5) | 33 (50.0) | |
| Site | ||||
| Right/Transverse colon | 34 | 20 (26.0) | 14 (21.2) | < .505 |
| Left colon and rectum | 109 | 57 (74.0) | 52 (78.8) | |
| Size | < .814 | |||
| < 5 cm in diameter | 60 | 33 (42.9) | 27 (40.9) | |
| ≥ 5 cm in diameter | 83 | 44 (57.1) | 39 (59.1) | |
| Grade | < .926 | |||
| Low | 111 | 60 (54.1) | 51 (45.9) | |
| High | 32 | 17 (53.1) | 15 (46.9) | |
| LV invasion | < .001* | |||
| Not identified | 39 | 31 (40.3) | 8 (12.1) | |
| Present | 104 | 46 (59.7) | 58 (87.9) | |
| Tumor border | .560 | |||
| Pushing | 13 | 8 (10.4) | 5 (7.6) | |
| Infiltrating | 130 | 69 (90.6) | 61 (92.4) | |
| Tumor budding | < .118 | |||
| Low | 37 | 24 (31.2) | 13 (19.7) | |
| High | 106 | 53 (68.6) | 53 (80.3) | |
| Invasion depth | < .001*a | |||
| pT1 | 5 | 5 (6.5) | 0 | |
| pT2 | 21 | 18 (23.4) | 3 (4.5) | |
| pT3 | 105 | 50 (64.9) | 55 (83.3) | |
| pT4 | 12 | 4 (5.2) | 8 (12.1) | |
| LN metastasis | < .001* | |||
| pN0 | 67 | 52 (67.5) | 15 (22.7) | |
| pN1 | 23 | 8 (10.4) | 15 (22.7) | |
| pN2 | 53 | 17 (22.1) | 36 (54.5) | |
| Distant metastasis | < .001*a | |||
| M0 | 121 | 74 (96.1) | 47 (71.2) | |
| M1 | 22 | 3 (3.9) | 19 (28.8) | |
| TNM stage | < .001*a | |||
| I | 21 | 20 (26.0) | 1 (1.5) | |
| II | 45 | 32 (41.6) | 13 (19.7) | |
| III | 55 | 22 (28.6) | 33 (50.0) | |
| IV | 22 | 3 (3.9) | 19 (28.8) | |
Table 2.Multivariate Cox proportional hazard analysis for recurrence-free survival and overall survival
| Characteristic | No. |
Recurrence-free survival |
Overall survival |
||
|---|---|---|---|---|---|
| Relative risk (95% CI) | p-value | Relative risk (95% CI) | p-value | ||
| Thymosin β4 | .001* | .005* | |||
| Low/negative | 77 | 1.000 | 1.000 | ||
| High | 66 | 2.540 (1.479–4.362) | 2.457 (1.315–4.592) | ||
| Size (diameter, cm) | .230 | .094 | |||
| < 5 | 60 | 1.000 | 1.000 | ||
| ≥ 5 | 83 | 1.349 (0.828–2.197) | 1.636 (0.919–2.913) | ||
| Grade | .048* | .013* | |||
| Low | 111 | 1.000 | 1.000 | ||
| High | 32 | 1.785 (1.005–3.171) | 2.241 (1.186–4.236) | ||
| LV invasion | .938 | .974 | |||
| Not identified | 39 | 1.000 | 1.000 | ||
| Present | 104 | 1.025 (0.547–1.922) | 1.012 (0.485–2.111) | ||
| Budding | .022* | .047* | |||
| Low | 37 | 1.000 | 1.000 | ||
| High | 106 | 2.094 (1.112–3.942) | 2.068 (1.010–4.235) | ||
| Invasion depth | .022* | .074 | |||
| pT1 + pT2 | 26 | 1.000 | 1.000 | ||
| pT3 + pT4 | 117 | 3.491 (1.194–10.206) | 3.849 (0.878–16.868) | ||
| LN metastasis | .833 | .437 | |||
| Not identified | 67 | 1.000 | 1.000 | ||
| Present | 76 | 1.060 (0.617–1.821) | 1.290 (0.678–2.455) | ||
| Distant metastasis | .005* | .001* | |||
| M0 | 121 | 1.000 | 1.000 | ||
| M1 | 22 | 2.368 (1.301–4.311) | 2.997 (1.586–5.664) | ||
Table 3.Correlation between thymosin β4 and HIF-1α expression status
| Frequency | Total |
HIF-1α |
p-value | ||
|---|---|---|---|---|---|
| High | Low/negative | ||||
| Thymosin β4 | High | 66 | 49 (74.2) | 17 (25.8) | < .001 |
| Low/negative | 77 | 18 (23.4) | 59 (76.6) | ||
- 1. Cannito S, Novo E, Compagnone A, et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis 2008; 29: 2267–78. ArticlePubMed
- 2. Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-κB via c-SRC and oxidant-dependent cell death. Cancer Res 2007; 67: 7368–77. ArticlePubMed
- 3. Sansone P, Piazzi G, Paterini P, et al. Cyclooxygenase-2/carbonic anhydrase-IX up-regulation promotes invasive potential and hypoxia survival in colorectal cancer cells. J Cell Mol Med 2009; 13: 3876–87. ArticlePubMed
- 4. To KK, Koshiji M, Hammer S, Huang LE. Genetic instability: the dark side of the hypoxic response. Cell Cycle 2005; 4: 881–2. ArticlePubMed
- 5. Huang LE, Bindra RS, Glazer PM, Harris AL. Hypoxia-induced genetic instability: a calculated mechanism underlying tumor progression. J Mol Med (Berl) 2007; 85: 139–48. ArticlePubMed
- 6. Bristow RG, Hill RP. Hypoxia and metabolism: hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008; 8: 180–92. ArticlePubMed
- 7. Oh JM, Ryoo IJ, Yang Y, Kim HS, Yang KH, Moon EY. Hypoxia-inducible transcription factor (HIF)-1 alpha stabilization by actin-sequestering protein, thymosin beta-4 (TB4) in Hela cervical tumor cells. Cancer Lett 2008; 264: 29–35. ArticlePubMed
- 8. Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis 2005; 22: 25–30. ArticlePubMed
- 9. Sivridis E, Giatromanolaki A, Koukourakis MI. Proliferating fibroblasts at the invading tumour edge of colorectal adenocarcinomas are associated with endogenous markers of hypoxia, acidity, and oxidative stress. J Clin Pathol 2005; 58: 1033–8. ArticlePubMedPMC
- 10. Giles RH, Lolkema MP, Snijckers CM, et al. Interplay between VHL/HIF1alpha and Wnt/beta-catenin pathways during colorectal tumorigenesis. Oncogene 2006; 25: 3065–70. ArticlePubMed
- 11. Koukourakis MI, Giatromanolaki A, Polychronidis A, et al. Endogenous markers of hypoxia/anaerobic metabolism and anemia in primary colorectal cancer. Cancer Sci 2006; 97: 582–8. ArticlePubMed
- 12. Huff T, Müller CS, Otto AM, Netzker R, Hannappel E. Beta-thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol 2001; 33: 205–20. ArticlePubMed
- 13. Kobayashi T, Okada F, Fujii N, et al. Thymosin-beta4 regulates motility and metastasis of malignant mouse fibrosarcoma cells. Am J Pathol 2002; 160: 869–82. ArticlePubMedPMC
- 14. Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000; 406: 532–5. ArticlePubMed
- 15. Xie D, Jauch A, Miller CW, Bartram CR, Koeffler HP. Discovery of over-expressed genes and genetic alterations in breast cancer cells using a combination of suppression subtractive hybridization, multiplex FISH and comparative genomic hybridization. Int J Oncol 2002; 21: 499–507. ArticlePubMed
- 16. Kim L, Kim YJ, Choi SJ, et al. Prognostic significance of thymosin- 4 in gastric adenocarcinoma patients. Korean J Pathol 2007; 41: 176–82.
- 17. Hsiao HL, Wang WS, Chen PM, Su Y. Overexpression of thymosin beta-4 renders SW480 colon carcinoma cells more resistant to apoptosis triggered by FasL and two topoisomerase II inhibitors via downregulating Fas and upregulating Survivin expression, respectively. Carcinogenesis 2006; 27: 936–44. ArticlePubMed
- 18. Huang HC, Hu CH, Tang MC, Wang WS, Chen PM, Su Y. Thymosin beta4 triggers an epithelial-mesenchymal transition in colorectal carcinoma by upregulating integrin-linked kinase. Oncogene 2007; 26: 2781–90. ArticlePubMed
- 19. Wang WS, Chen PM, Hsiao HL, Ju SY, Su Y. Overexpression of the thymosin beta-4 gene is associated with malignant progression of SW480 colon cancer cells. Oncogene 2003; 22: 3297–306. ArticlePubMed
- 20. Wang WS, Chen PM, Hsiao HL, Wang HS, Liang WY, Su Y. Overexpression of the thymosin beta-4 gene is associated with increased invasion of SW480 colon carcinoma cells and the distant metastasis of human colorectal carcinoma. Oncogene 2004; 23: 6666–71. ArticlePubMed
- 21. Wang WS, Chen PM, Su Y. Colorectal carcinoma: from tumorigenesis to treatment. Cell Mol Life Sci 2006; 63: 663–71. ArticlePubMed
- 22. Piao Z, Hong CS, Jung MR, Choi C, Park YK. Thymosin beta4 induces invasion and migration of human colorectal cancer cells through the ILK/AKT/beta-catenin signaling pathway. Biochem Biophys Res Commun 2014; 452: 858–64. ArticlePubMed
- 23. Kang YJ, Jo JO, Ock MS, et al. Thymosin beta4 was upregulated in recurred colorectal cancers. J Clin Pathol 2014; 67: 188–90. ArticlePubMed
- 24. Yoon SY, Lee HR, Park Y, et al. Thymosin beta4 expression correlates with lymph node metastasis through hypoxia inducible factor-alpha induction in breast cancer. Oncol Rep 2011; 25: 23–31. PubMed
- 25. Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med 2009; 133: 1539–51. ArticlePubMedPMCPDF
- 26. Prall F. Tumour budding in colorectal carcinoma. Histopathology 2007; 50: 151–62. ArticlePubMed
- 27. Wang LM, Kevans D, Mulcahy H, et al. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol 2009; 33: 134–41. ArticlePubMed
- 28. Puppa G. TNM staging system of colorectal carcinoma: surgical pathology of the seventh edition. Diagn Histopathol 2011; 17: 243–62. Article
- 29. Höckel M, Vaupel P. Biological consequences of tumor hypoxia. Semin Oncol 2001; 28(2 Suppl 8):36–41. Article
- 30. Foo SS, Abbott DF, Lawrentschuk N, Scott AM. Functional imaging of intratumoral hypoxia. Mol Imaging Biol 2004; 6: 291–305. ArticlePubMed
- 31. Mabjeesh NJ, Amir S. Hypoxia-inducible factor (HIF) in human tumorigenesis. Histol Histopathol 2007; 22: 559–72. PubMed
- 32. Lee JW, Ryu YK, Ji YH, Kang JH, Moon EY. Hypoxia/reoxygenation-experienced cancer cell migration and metastasis are regulated by Rap1- and Rac1-GTPase activation via the expression of thymosin beta-4. Oncotarget 2015; 6: 9820–33. ArticlePubMedPMC
- 33. Tang MC, Chan LC, Yeh YC, et al. Thymosin beta 4 induces colon cancer cell migration and clinical metastasis via enhancing ILK/IQGAP1/Rac1 signal transduction pathway. Cancer Lett 2011; 308: 162–71. ArticlePubMed
- 34. Nemolato S, Restivo A, Cabras T, et al. Thymosin beta 4 in colorectal cancer is localized predominantly at the invasion front in tumor cells undergoing epithelial mesenchymal transition. Cancer Biol Ther 2012; 13: 191–7. ArticlePubMed
- 35. Jo JO, Kim SR, Bae MK, et al. Thymosin beta4 induces the expression of vascular endothelial growth factor (VEGF) in a hypoxia-inducible factor (HIF)-1alpha-dependent manner. Biochim Biophys Acta 2010; 1803: 1244–51. ArticlePubMed
- 36. Brahimi-Horn MC, Pouysségur J. HIF at a glance. J Cell Sci 2009; 122(Pt 8):1055–7. ArticlePubMed
- 37. Ryan HE, Poloni M, McNulty W, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res 2000; 60: 4010–5. PubMed
- 38. Swami M. Hypoxia: the HIF2alpha puzzle. Nat Rev Cancer 2010; 10: 603.ArticlePubMed
- 39. Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr 1998; 7: 205–13. PubMed
- 40. Kietzmann T, Cornesse Y, Brechtel K, Modaressi S, Jungermann K. Perivenous expression of the mRNA of the three hypoxia-inducible factor alpha-subunits, HIF1alpha, HIF2alpha and HIF3alpha, in rat liver. Biochem J 2001; 354(Pt 3):531–7. ArticlePubMedPMC
- 41. Clottes E. Hypoxia-inducible factor 1: regulation, involvement in carcinogenesis and target for anticancer therapy. Bull Cancer 2005; 92: 119–27. PubMed
- 42. Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 1999; 59: 5830–5. PubMed
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Thymosin β4 Is an Endogenous Iron Chelator and Molecular Switcher of Ferroptosis
Joanna I. Lachowicz, Giusi Pichiri, Marco Piludu, Sara Fais, Germano Orrù, Terenzio Congiu, Monica Piras, Gavino Faa, Daniela Fanni, Gabriele Dalla Torre, Xabier Lopez, Kousik Chandra, Kacper Szczepski, Lukasz Jaremko, Mitra Ghosh, Abdul-Hamid Emwas, Mass
International Journal of Molecular Sciences.2022; 23(1): 551. CrossRef - Metal coordination of thymosin β4: Chemistry and possible implications
Joanna Izabela Lachowicz, Mariusz Jaremko, Lukasz Jaremko, Giuseppina Pichiri, Pierpaolo Coni, Marco Piludu
Coordination Chemistry Reviews.2019; 396: 117. CrossRef - Adipose-Derived Mesenchymal Stem Cells Enhance Ovarian Cancer Growth and Metastasis by Increasing Thymosin Beta 4X-Linked Expression
Yijing Chu, Min You, Jingjing Zhang, Guoqiang Gao, Rendong Han, Wenqiang Luo, Tingting Liu, Jianxin Zuo, Fuling Wang
Stem Cells International.2019; 2019: 1. CrossRef - An Investigation on the Therapeutic Effect of Thymosinβ4 and Its Expression Levels in Streptozotocin-Induced Diabetic Mice
Kyung Sook Cho, Dong-Jin Kim, Bomee Shim, Jung Yeon Kim, Jun Mo Kang, Seon Hwa Park, Sang-Ho Lee, Hyung-In Yang, Kyoung Soo Kim
BioMed Research International.2018; 2018: 1. CrossRef - Hypoxia-inducible factor-1α expression in colorectal carcinoma
Ahmed M. Abd ElAziz, Hanan S. Abd ElHamid, Rasha R. Mostafa, Yousra R.A. Shalaby
Egyptian Journal of Pathology.2018; 38(1): 18. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission