Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(4); 2022 > Article
-
Case Study
Hepatic carcinoma expressing inhibin: case report of a proposed novel entity and review of the literature -
Antonia Syrnioti1
 , Evangelia Athanasiou2
, Evangelia Athanasiou2 , Prodromos Hytiroglou1
, Prodromos Hytiroglou1
-
Journal of Pathology and Translational Medicine 2022;56(4):225-230.
DOI: https://doi.org/10.4132/jptm.2022.04.07
Published online: June 15, 2022
1Department of Pathology, Aristotle University School of Medicine, Thessaloniki, Greece
2“Microdiagnostiki” Laboratory, Thessaloniki, Greece
- Corresponding Author: Prodromos Hytiroglou, MD, Department of Pathology, Aristotle University School of Medicine, 54006 Thessaloniki, Greece, Tel: +30-2310-999-218, Fax: +30-2310-244-604, E-mail: 'pchytiro@auth.gr'
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,573 Views
- 115 Download
Abstract
- Hepatic carcinoma expressing inhibin is a recently described neoplasm with varied architecture, including trabecular, pseudoglandular, follicular/microcystic, organoid, solid and tubular patterns of growth. We report a case of hepatic carcinoma expressing inhibin that occurred in a 47-year-old woman presenting with epigastric and back pain. The tumor was located in the left hepatic lobe and measured 12 cm in diameter. On immunohistochemical stains, the neoplastic cells were positive for inhibin, as well as cytokeratins 7, 8/18 and 19. There was mild focal expression of synaptophysin, and lack of expression of hepatocytic markers. The histogenesis of hepatic carcinoma expressing inhibin is presently uncertain. From a practical point of view, this neoplasm can potentially cause diagnostic pitfalls by simulating other primary or metastatic tumors, such as hepatocellular carcinoma, cholangiocarcinoma, neuroendocrine tumors, and follicular carcinoma of thyroid gland. Performing inhibin immunostain could assist in the differential diagnosis of liver tumors with unusual histologic features.
- Clinical summary
- We have recently examined in consultation a left hepatic lobe tumor from a 47-year-old woman, who presented with epigastric and back pain of several months’ duration. Physical examination revealed a palpable mass in the left upper abdominal quadrant. Her past medical history was unremarkable. The patient did not smoke or drink alcohol, had no history of liver disease, and was not on any medication. There was no family history of liver disease. On magnetic resonance imaging (MRI) of the upper abdomen, a lobulated mass was seen, which occupied almost the entire left hepatic lobe. The tumor appeared inhomogeneously enhanced after intravenous contrast administration. The remaining liver parenchyma appeared normal. There were no other abnormal findings in the pancreas, spleen, adrenal glands and kidneys. Chest X-ray was normal. Liver function tests, hematology work-up, and serum levels of tumor markers (carcinoembryonic antigen, CA19-9, CA125, and α-fetoprotein) were within normal limits. The patient underwent a left hepatic lobectomy. On postoperative chest computed tomography scan, as well as upper and lower abdominal MRI, there were no additional lesions or residual tumor.
- Pathologic findings
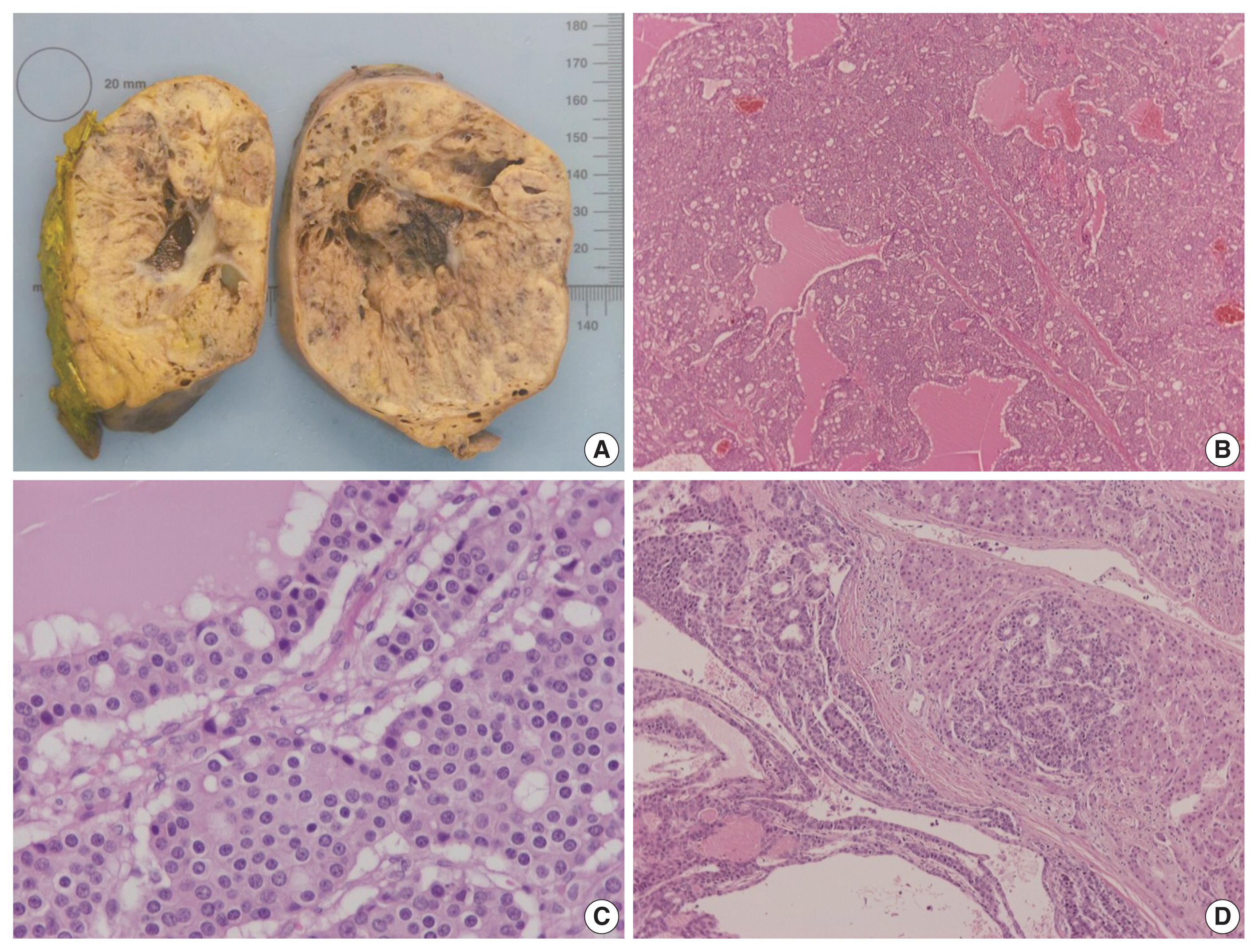
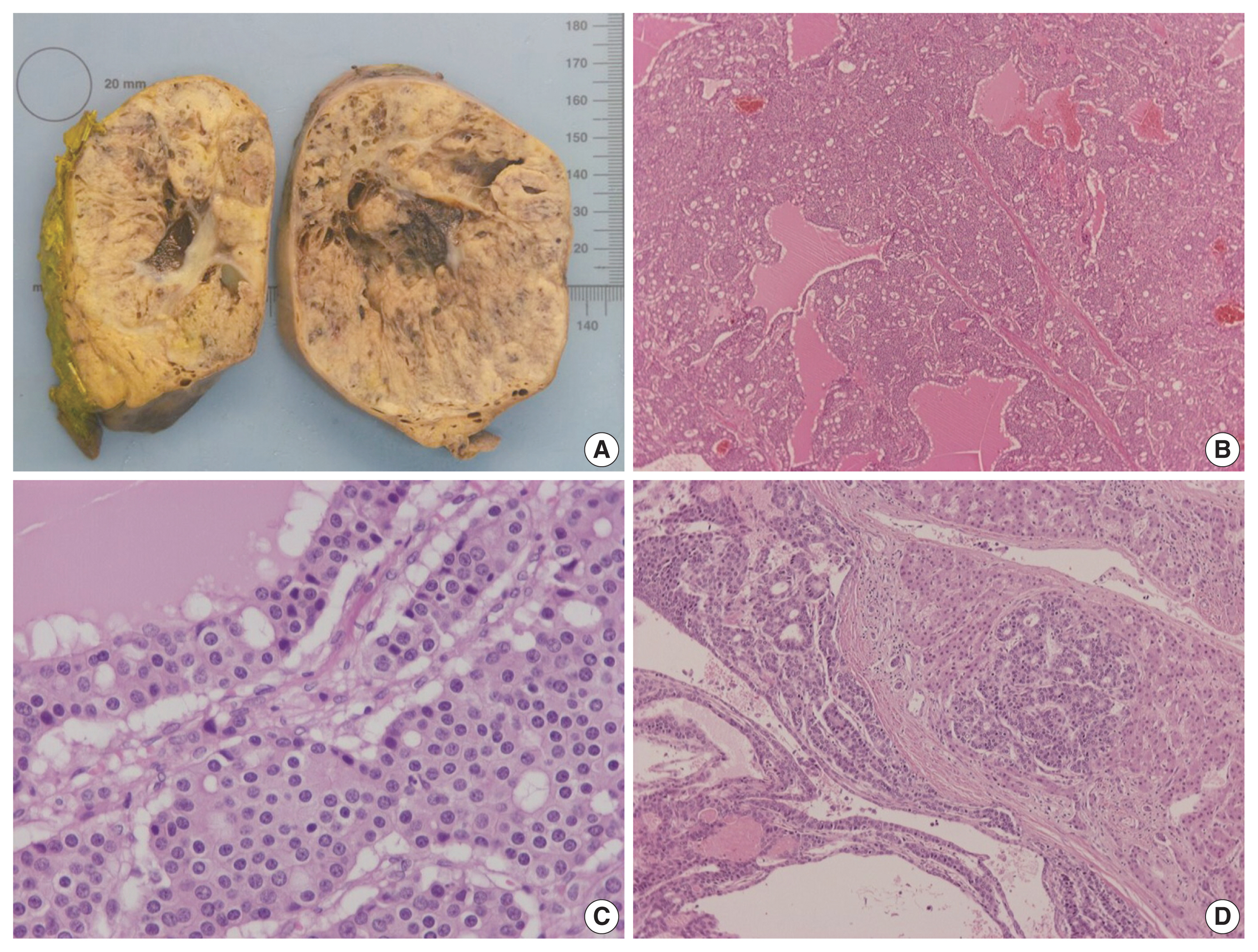
- On gross examination, the tumor was relatively well-circumscribed, displayed a tan-yellow cut surface, and measured 12 cm in greatest dimension (Fig. 1A). On microscopic examination, it exhibited trabecular, follicular and pseudoglandular architecture (Fig. 1B). The tumor cells were columnar or ovoid in shape, with eosinophilic cytoplasm and roundish nuclei with inconspicuous nucleoli (Fig. 1C). Mild nuclear pleomorphism and rare mitotic figures were found. The follicular structures contained amorphous eosinophilic material with peripheral vacuolization, reminiscent of thyroid follicles (Fig. 1B). Occasional fibrous septa were observed (Fig. 1B). Regions of hemorrhage and cystic degeneration were present. No necrosis was found. At the tumor border, there was invasive growth, with tongues and groups of neoplastic cells extending into the adjacent hepatic parenchyma (Fig. 1D). The hepatic parenchyma was otherwise unremarkable. No evidence of biliary intraepithelial neoplasia was present. The surgical margins of resection were free of tumor.
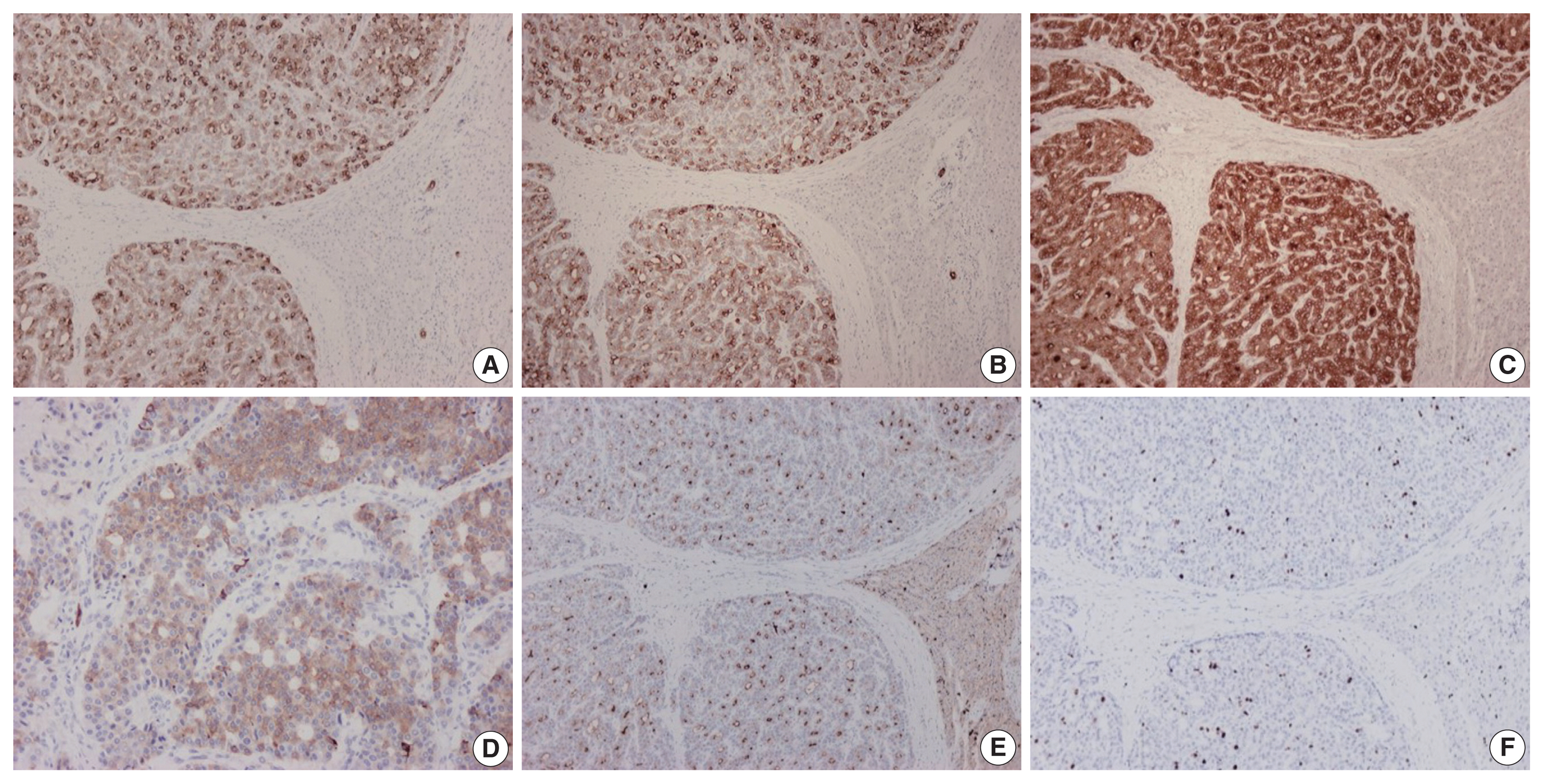
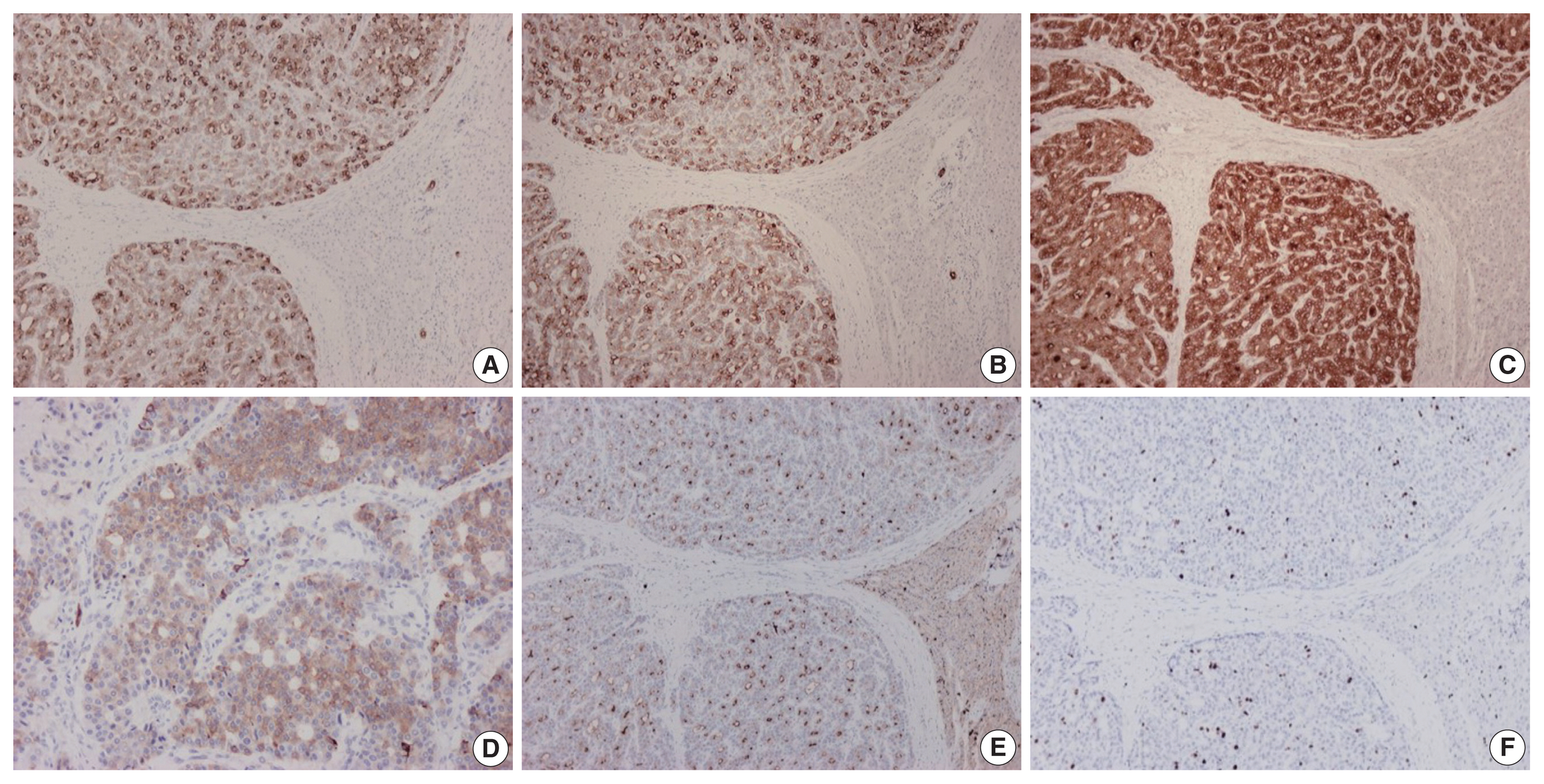
- On immunohistochemical evaluation, the neoplastic cells were positive for cytokeratin (CK) 7 (Fig. 2A), CK8/18, and CK19 (Fig. 2B), as well as inhibin (Fig. 2C). There also was patchy mild positivity of tumor cells for synaptophysin (Fig. 2D). Membranous positivity with polyclonal carcinoembryonic antigen was present around pseudoglandular structures (Fig. 2E). The neoplastic cells were negative for HepPar-1, arginase-1, glypican-3, α-fetoprotein, glutamine synthetase, β-catenin (nuclear), CK20, epithelial membrane antigen, chromogranin, INSM1, thyroid transcription factor-1, thyroglobulin, human chorionic gonadotrophin, CA19-9, Melan A, CD10, CD56, CD99, vimentin, calretinin, estrogen and progesterone receptor proteins, PAX8, GATA3, CDX2, and trypsin. On immunohistochemical stains for Ki67 antigen, there was positivity in 10% of tumor cells (Fig. 2F).
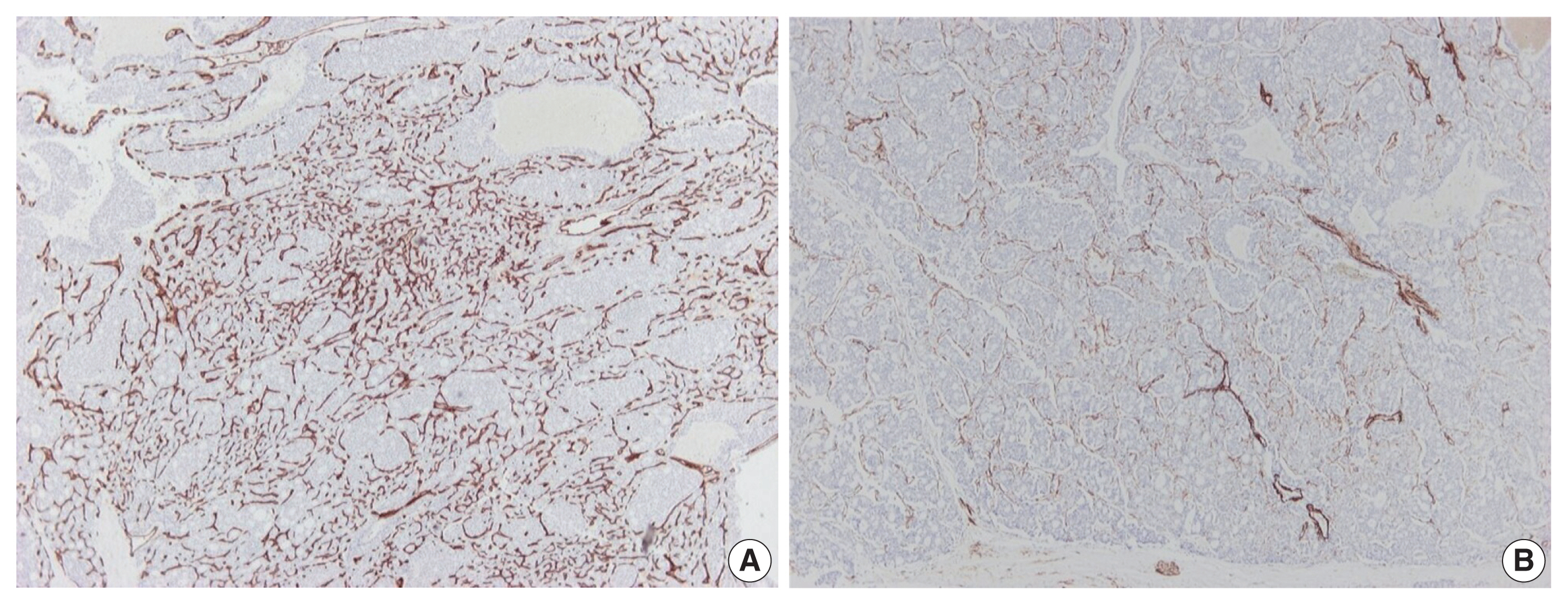
- Further immunohistochemical stains for CD34 and α-caldesmon were performed to explore the vascular architecture of the tumor. A rich, thin-walled vascular bed was evident on CD34 stains (Fig. 3A), while sparse larger vessels with venous features were seen on stains for α-caldesmon (Fig. 3B).
CASE REPORT
- HCEI is an enigmatic neoplasm that was originally recognized because of the combination of its unusual histologic features with the immunohistochemical expression of inhibin [1]. Sixteen cases of HCEI have been thus far reported, including the present one (Table 1) [1–6]. Twelve cases occurred in women and four in men; the age range of the patients was 17 to 54 years. HCEI has been found to exhibit varied architecture, with trabecular, pseudoglandular, follicular/microcystic, organoid, solid and tubular patterns of growth. The neoplastic cells diffusely express inhibin. Prognosis is variable. The patient of our original report [1] received no further treatment following surgical resection, and is well, without evidence of disease, 20 years after presentation. The patient of our current report is also alive and without evidence of disease 9 months after presentation. However, three of the other 14 patients reported to date have died of the disease, 5 had disease progression, one had stable disease, 3 were disease-free at last follow-up, and two were lost to follow-up (Table 1).
- Despite the small number of reported cases, there is sufficient evidence that HCEI is a distinct clinicopathologic entity [1–6]: (1) These tumors are relatively large (size range, 6.9 to 36 cm) when they become symptomatic, suggesting an indolent initial growth phase. (2) These tumors have characteristic histologic features, including trabecular, pseudoglandular, follicular/microcystic, organoid, solid and tubular patterns of growth. 3) In addition to inhibin positivity, HCEI characteristically shows positivity for ‘biliary’ markers (CK7 and CK19), mild or patchy positivity for neuroendocrine markers (synaptophysin, chromogranin, CD56), and absence of staining for hepatocellular markers (HepPar-1, arginase-1, glypican-3, α-fetoprotein). Albumin mRNA has also been detected in 10 cases by in situ hybridization [3–5]. (4) Molecular studies recently performed in three cases [5] have detected a novel NIPBL-NACC1 gene fusion in HCEI.
- The histogenesis of HCEI remains elusive. Inhibin expression characterizes a limited range of normal cell types, including granulosa cells, luteinized thecal cells and hilus cells of the ovary, syncytiotrophoblastic cells and adrenocortical cells [1]. Theoretically, any of these cell types could be present in a heterotopic location, such as the liver, and give rise to tumors. However, tumors with the features of HCEI have not been described in the ovaries or adrenals. On the other hand, inhibin expression has occasionally been reported in tumor types without obvious connection to the normal cells mentioned above, such as mucinous cystic neoplasm of the liver and pancreas, as well as neuroendocrine tumors of clear cell type occurring in patients with von Hippel-Lindau disease [7]. It might be worth exploring whether there is any connection between these neoplasms and HCEI. Nevertheless, the possibility that inhibin expression may be triggered in cells with a biliary phenotype by NIPBL-NACC1 gene fusion cannot be ruled out.
- From a practical point of view, it is possible that HCEI has been underreported, due to its histologic and immunohistochemical similarities with hepatocellular carcinoma, cholangiocarcinoma, primary or metastatic neuroendocrine tumors and metastatic follicular carcinoma of the thyroid gland. The diagnosis of HCEI can be easily missed if pathologists do not think of performing inhibin immunostain. Reports of additional cases, including further immunohistochemical and molecular studies, will be useful to define this proposed new entity with certainty. In this context, it would be of interest to assess inhibin expression in neoplasms that have histologic similarities with HCEI, such as primary hepatic neuroendocrine tumors (including a recently described “primary hepatic neuroendocrine tumor with unusual thyroid follicular-like morphologic characteristics”, which occurred in a 57-year old woman [8]), as well as tumors recently described as “thyroid-like intrahepatic cholangiocarcinoma”, which occurred in three women and one man, between the ages of 26 and 59 years [9–12].
- In conclusion, HCEI is an emerging entity that pathologists should be aware of. This neoplasm can potentially cause diagnostic pitfalls by simulating histologically other primary or metastatic hepatic tumors. Performing inhibin immunostain could be of great help in the differential diagnosis of liver tumors with unusual histologic features.
DISCUSSION
-
Ethics Statement
Informed consent was obtained from the individual participant included in this study. In our institution, case studies are exempted from Institutional Review Board submission.
-
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
-
Code Availability
Not applicable.
-
Author Contributions
Conceptualization: AS, EA, PH. Data curation: AS, EA, PH. Formal analysis: AS, EA, PH. Methodology: AS, EA, PH. Supervision: PH. Writing—original draft: AS, EA, PH. Writing—review & editing: AS, EA, PH. Approval of final manuscript: all authors.
-
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
-
Funding Statement
No funding to declare.
Notes
| No. | Study | Age (yr) | Sex | Tumor location | Tumor size (cm) | Immunohistochemistry | Albumin ISH | NIPBL/ACC1 fusion | Outcome | Non-tumorous liver status | OCP use | Serum AFP | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| CK7 | CK19 | Synaptophysin | Chromogranin | ||||||||||||
| 1 | Vrettou et al. (2005) [1] | 24 | F | Left lobe | 14 | + | + | − | − | N/A | N/A | Alive, NOD (> 19 yr) | Normal | Yes (10 yr) | N/A |
| 2 | Braxton et al. (2017) [2] | 17 | F | Right lobe | 23 | + | + | + (weak) | + (weak) | N/A | N/A | DOD (41 mo) | Normal | None | Normal |
| 3 | Braxton et al. (2017) [2] | 44 | F | Left lobe | 16 | + (weak) | + | + | + (weak) | N/A | N/A | Unknown | Normal | None | N/A |
| 4 | Braxton et al. (2017) [2] | 25 | F | Caudate lobe | 7.5 | + | + | + (weak) | + (weak) | N/A | N/A | DOD (30 mo) | Normal | None | Normal |
| 5 | Wen et al. (2021) [3] | 37 | F | N/A | 6.9 | + | + (patchy) | − (focal) | − | + | N/A | NOD (2 yr) | N/A | N/A | N/A |
| 6 | Wen et al. (2021) [3] | 28 | F | N/A | 15 | + | N/A | + (patchy) | − | + | N/A | NOD (5 mo) | N/A | N/A | N/A |
| 7 | Wen et al. (2021) [3] | 19 | F | N/A | 14 | + | + | N/A | − (focal) | + | N/A | DOD (2 yr) | N/A | N/A | N/A |
| 8 | Wen et al. (2021) [3] | 34 | F | N/A | 20 | + | N/A | − | − | + | N/A | NOD (1 yr) | N/A | N/A | N/A |
| 9 | Wen et al. (2021) [3] | 44 | F | N/A | 23.5 | + | + (patchy) | + (patchy variable) | + (patchy) | + | N/A | Intrahepatic recurrence (1.5 yr) | N/A | N/A | N/A |
| 10 | Wen et al. (2021) [3] | 33 | F | N/A | 15.5 | + | + | + (patchy) | + (focal) | + | N/A | Metastases (at diagnosis) | N/A | N/A | N/A |
| 11 | Liang et al. (2021) [4] | 26 | M | Most of liver | 36 | + | N/A | + | − | + | N/A | Disease progression and metastases, AWD (15 mo) | N/A | N/AP | N/A |
| 12 | Argani et al. (2021) [5] | 24 | F | Right lobe | 25.4 | + | + | + (patchy) | + (focal) | + | + | Multifocal local recurrence and metastases, AWD (9 mo) | Normal | N/A | N/A |
| 13 | Argani et al. (2021) [5] | 54 | M | Right lobe | 11.5 | + | + | + (patchy) | + (focal) | + | + | Multifocal intrahepatic recurrence, AWD (10 mo) | Normal | N/AP | N/A |
| 14 | Argani et al. (2021) [5] | 52 | M | N/A | 7.5 | + | + | + (patchy) | + (focal) | + | + | Unknown | Normal | N/AP | N/A |
| 15 | Verhoeff et al. (2022) [6] | 26 | M | Right lobe | 21 | + | + (patchy) | + (patchy) | N/A | N/A | N/A | AWD (stable, by imaging, at 6 mo) | N/A | N/AP | N/A |
| 16 | Present case | 47 | F | Left lobe | 12 | + | + | + (focal, weak) | − | N/A | N/A | NOD (9 mo) | Normal | None | Normal |
Literature search was implemented using the following keywords in MEDLINE database (PubMed, English language): liver, carcinoma (or tumor), inhibin.
CK, cytokeratin; ISH, in situ hybridization; OCP, oral contraceptive; AFP, α-fetoprotein; F, female; N/A, not available; NOD, no evidence of disease; DOD, died of disease; M, male; N/AP, not applicable; AWD, alive with disease.
- 1. Vrettou E, Hytiroglou P, Sikas N, Soultoyannis I, Goodman ZD. Hepatic adenocarcinoma expressing inhibin in a young patient on oral contraceptives. Virchows Arch 2005; 446: 560–5. ArticlePubMedPDF
- 2. Braxton DR, Saxe D, Damjanov N, et al. Molecular and cytogenomic profiling of hepatic adenocarcinoma expressing inhibinA, a mimicker of neuroendocrine tumors: proposal to reclassify as “cholangioblastic variant of intrahepatic cholangiocarcinoma”. Hum Pathol 2017; 62: 232–41. ArticlePubMed
- 3. Wen KW, Joseph NM, Srivastava A, et al. Inhibin-positive hepatic carcinoma: proposal for a solid-tubulocystic variant of intrahepatic cholangiocarcinoma. Hum Pathol 2021; 116: 82–93. ArticlePubMed
- 4. Liang TZ, Whang G, Chopra S. Primary hepatic carcinoma with inhibin positivity in a young male patient: a rare tumor previously only reported in females: case report and review of literature. Virchows Arch 2021; 478: 605–10. ArticlePubMedPDF
- 5. Argani P, Palsgrove DN, Anders RA, et al. A novel NIPBL-NACC1 gene fusion is characteristic of the cholangioblastic variant of intrahepatic cholangiocarcinoma. Am J Surg Pathol 2021; 45: 1550–60. ArticlePubMedPMC
- 6. Verhoeff K, Bacani J, Fung C, Canterbury LA. A Cholangioblastic variant of cholangiocarcinoma. ACG Case Rep J 2022; 9: e00746.ArticlePubMedPMC
- 7. Sinkre PA, Murakata L, Rabin L, Hoang MP, Albores-Saavedra J. Clear cell carcinoid tumor of the gallbladder: another distinctive manifestation of von Hippel-Lindau disease. Am J Surg Pathol 2001; 25: 1334–9. PubMed
- 8. Ibrahim ME, Abadeer K, Zhai QJ, Nassar A. Primary hepatic neuroendocrine tumor with unusual thyroid follicular-like morphologic characteristics. Case Rep Pathol 2017; 2017: 7931975.ArticlePubMedPMCPDF
- 9. Chable-Montero F, Shah BS, Montante-Montes de Oca D, Angeles-Angeles A, Henson DE, Albores-Saavedra J. Thyroid-like cholangiocarcinoma of the liver: an unusual morphologic variant with follicular, trabecular and insular patterns. Ann Hepatol 2012; 11: 961–5. ArticlePubMed
- 10. Shah A, Chandibhamar BS, Gami A, Trivedi P. Case report of intrahepatic cholangiocarcinoma showing thyroid like follicular pattern: a rare morphological variant. Eur J Med Case Rep 2020; 4: 280–4. Article
- 11. Fornelli A, Bondi A, Jovine E, Eusebi V. Intrahepatic cholangiocarcinoma resembling a thyroid follicular neoplasm. Virchows Arch 2010; 456: 339–42. ArticlePubMedPDF
- 12. Chen SH, Zheng ZY, Wang HL, et al. Thyroid-like intrahepatic cholangiocarcinoma: report of a case and review of the literature. Int J Surg Pathol 2018; 26: 649–54. ArticlePubMedPDF
References
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission