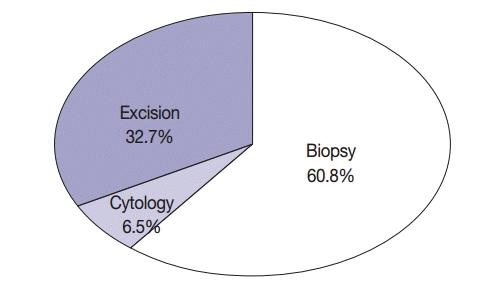Analysis of Mutations in Epidermal Growth Factor Receptor Gene in Korean Patients with Non-small Cell Lung Cancer: Summary of a Nationwide Survey
Article information
Abstract
Background:
Analysis of mutations in the epidermal growth factor receptor gene (EGFR) is important for predicting response to EGFR tyrosine kinase inhibitors. The overall rate of EGFR mutations in Korean patients is variable. To obtain comprehensive data on the status of EGFR mutations in Korean patients with lung cancer, the Cardiopulmonary Pathology Study Group of the Korean Society of Pathologists initiated a nationwide survey.
Methods:
We obtained 1,753 reports on EGFR mutations in patients with lung cancer from 15 hospitals between January and December 2009. We compared EGFR mutations with patient age, sex, history of smoking, histologic diagnosis, specimen type, procurement site, tumor cell dissection, and laboratory status.
Results:
The overall EGFR mutation rate was 34.3% in patients with non-small cell lung cancer (NSCLC) and 43.3% in patients with adenocarcinoma. EGFR mutation rate was significantly higher in women, never smokers, patients with adenocarcinoma, and patients who had undergone excisional biopsy. EGFR mutation rates did not differ with respect to patient age or procurement site among patients with NSCLC.
Conclusions
EGFR mutation rates and statuses were similar to those in published data from other East Asian countries.
Lung cancer is the leading cause of cancer-related death in Korea, accounting for approximately 20% of all cancer deaths [1]. Non-small cell lung cancer (NSCLC) accounts for more than 85% of all lung cancers, and the majority of patients with NSCLC present at an advanced cancer stage (stage III or IV) [2]. In the last decade, several studies have been performed on the molecular stratification of NSCLC in order to provide targeted treatment based on activating or driver mutations in these tumors. Activating mutations in the epidermal growth factor receptor gene (EGFR) can be used as therapeutic targets for treatment of NSCLC. In the Iressa Pan-Asia Study, tumors with EGFR mutations showed a 71.2% clinical response to first-line treatment with gefitinib, while tumors with wild-type EGFR showed only a 1.1% response [3]. Since then, several randomized control studies have shown an association between activating EGFR mutation and response to EGFR tyrosine kinase inhibitors (TKIs) [4-9]. Patient selection is important for using EGFR TKIs as the first-line treatment. At present, analysis of EGFR mutations is the accepted method for identifying patient response to EGFR TKIs. Direct DNA sequencing is a standard method for identifying mutations and is commonly used in the Asia-Pacific region [10]. Any routinely available pathological specimen can be used for analyzing EGFR mutations, including formalin-fixed, paraffin-embedded tissues from surgical resections; small tissue biopsies; or cell block preparations.
Several publications have reported the prevalence of EGFR mutations in patients with NSCLC [10-14]. The rates of EGFR mutations are higher in Asian countries than in Western countries. Further, rates of EGFR mutations in Korean patients range from 17.4% to 51.3% [10,15-22]. Therefore, we performed a nationwide study of EGFR mutations in Korean patients with NSCLC in order to provide reliable information on the incidence and characteristics of EGFR mutations. This study was led by the Korean Cardiopulmonary Pathology Study Group.
MATERIALS AND METHODS
In all, 1,826 reports of EGFR mutation in patients with lung cancer were collected from 15 hospitals between January and December 2009 (Fig. 1). Of these, 24 reports of patients with small cell carcinoma and 49 reports of patients with malignancies from other than lung primary tumors were excluded from the study. Finally, 1,544 reports of primary tumor and 209 reports of metastatic tumor were included in the study. EGFR mutation status was compared with patient age, sex, history of smoking, histologic diagnosis, specimen type, procurement site, tumor cell dissection, and laboratory status. Smoker status was defined as having a greater than 10 pack-year history and currently smoking cigarettes every day or most days. An ex-smoker was someone who had smoked more than 100 cigarettes in their lifetime and who does not currently smoke. A light smoker was defined as a current smoker with a less than 10 pack-year history. A never-smoker was an adult who had never smoked a cigarette or who smoked fewer than 100 cigarettes in their lifetime.
Tumor specimens were divided into three types, namely, biopsy, cytology, and excision specimens. Biopsy specimens included small biopsy specimens obtained by performing bronchoscopic biopsy, transbronchial lung biopsy, percutaneous needle biopsy, pleural biopsy, or needle biopsy of metastatic sites. Cytology included cytologic specimens such as sputum, bronchial washing/brushing, pleural fluids, and aspiration biopsy cytology of primary or metastatic sites. Excision specimens included specimens obtained by performing excisional surgical biopsy such as segmentectomy, lobectomy, pneumonectomy, and metastasectomy. Procurement sites were divided into two types, namely, metastasis and primary sites. Tumor dissection indicated whether or not to perform microdissection of tumor cells. Laboratory status was classified into two types: in-house mutation testing, indicating that EGFR mutation analysis was performed in the hospital’s laboratory facility, and out-sourced mutation testing, indicating that EGFR mutation analysis was not performed in the hospital’s laboratory facility. All participants in the study were active members of the Korean Cardiopulmonary Pathology Study Group. This study was approved by the Institutional Review Board of Konkuk University Medical Center (No. KUH 1210011).
Statistical analysis
All statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). Chi-square test and Fisher exact test were used to determine the correlations between EGFR mutation status and clinicopathological parameters. A p-value of <.05 was considered statistically significant.
RESULTS
Patient characteristics
The average age of 1,753 patients with NSCLC was 62.74±11.31 years (range, 16 to 89 years); of these, 875 patients (49.9%) were aged ≥65 years. Of the 1,753 patients, 1,000 (57%) were men and 753 (43%) were women. Of the 1,753 patients, 555 (31.7%) were smokers, 39 (2.2%) were light smokers, 170 (9.7%) were ex-smokers, 849 (48.4%) were never-smokers, and 140 (8.0%) patients had an unknown smoking history. Of the specimens used for EGFR mutation testing, 114 (6.5%) were cytology specimens, 1,066 (60.8%) were biopsy specimens, and 573 (32.7%) were excision specimens (Fig. 2). With respect to procurement sites, specimens from 1,544 patients (88.1%) were procured from primary tumor sites, while those from 209 patients (11.9%) were procured from metastatic sites. In all, 1,012 patients (57.7%) underwent tumor microdissection, while the remaining 741 patients (42.3%) did not. The histological types of the tumor specimens were as follows: adenocarcinoma in 1,292 (73.7%), squamous cell carcinoma in 347 (19.8%), NSCLC type undetermined in 69 (3.9%), pleomorphic carcinoma in 13 (0.7%), large cell neuroendocrine cell carcinoma in 12 (0.7%), large cell carcinoma in nine (0.5%), sarcomatoid carcinoma in five (0.3%), carcinoid tumor in two (0.1%), mucoepidermoid carcinoma in two (0.1%), carcinosarcoma in one (0.05%), and lymphoepithelioma-like carcinoma in one (0.05%). Among the 1,753 EGFR tests, 1,299 (7 institutions, 74.1%) were performed within the same pathology laboratory, and 454 (8 institutions, 25.9%) were performed in outside laboratories. EGFR mutations in specimens obtained from 14 hospitals were identified by direct sequencing, while those in specimens obtained from the remaining hospital were identified by pyrosequencing. Characteristics of patients included in the study are summarized in Table 1.
Frequency of EGFR mutations
In all, 601 cases of EGFR mutation (34.3%) were detected in NSCLC. Of these 601 patients, 560 (43.3%) had adenocarcinoma, 30 (8.6%) had squamous cell carcinoma, eight (11.6%) had NSCLC type undetermined, two (15.4%) had pleomorphic carcinoma, and one (8.3%) had large cell neuroendocrine carcinoma. In all, 389 never-smokers with adenocarcinoma (52.4%) had EGFR mutations. Of the 601 patients with NSCLC who had EGFR mutations, 30 (5%), 313 (52.1%), 31 (5.2%), and 205 (34.1%) had mutations in exons 18, 19, 20, and 21, respectively; in addition, 22 patients (3.7%) had double mutations (Fig. 3). Further, 13 patients (2.2%) had T790M mutation; of these, four patients had only the T790M mutation.
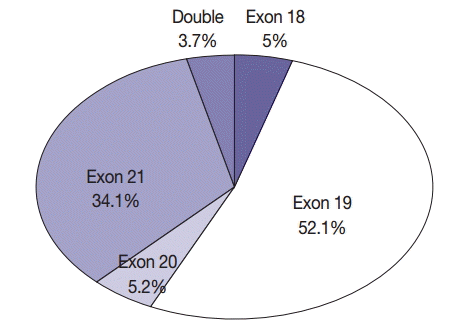
Frequency of mutations according to exons: 601 mutations in 1,753 specimens from patients with non-small cell lung cancer.
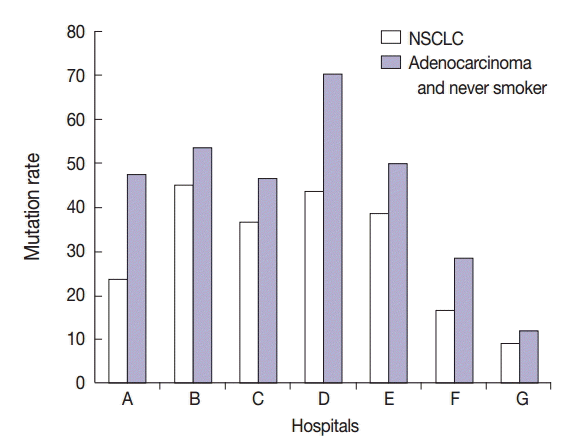
Interestingly, specimens obtained from two in-house laboratories showed low EGFR mutation rates of 16.7% (2/12 specimens) and 9.3% (4/43 specimens) in patients with NSCLC, respectively, and 28.6% (2/7 specimens) and 11.5% (3/26 specimens) in patients with adenocarcinoma and never-smokers (Fig. 4). In brief, one laboratory that detected a 16.7% mutation rate in patients with NSCLC analyzed tumor specimens from 12 patients. Of these 12 patients, 10 had adenocarcinoma, one had large cell carcinoma, and one had NSCLC. Of the 10 patients with adenocarcinoma, seven, two, and one were never-smokers, ex-smokers, and smoker, respectively. All the specimens were obtained by surgical excision and were analyzed by tumor microdissection. The laboratory that detected a 9.3% mutation rate in patients with NSCLC analyzed tumor specimens from 43 patients. Of these 43 patients, 41 had adenocarcinoma, one had NSCLC, and one had squamous cell carcinoma. Of the 41 patients with adenocarcinoma, 25, 6, and 10 were never-smokers, ex-smokers, and smokers, respectively. Of the 43 specimens analyzed in this laboratory, six were surgical excision specimens, 29 were biopsy specimens, and eight were cytology specimens. All 43 specimens were analyzed without tumor microdissection. A total of four EGFR mutations were found, and all of them were adenocarcinoma. They included three never-smokers and one ex-smoker. Moreover, of the four mutations, two were detected in excision specimens and two were detected in biopsy specimens.
A total of 125 EGFR mutations were detected in a total of 430 male patients with adenocarcinoma who had a smoking history.
Differences in EGFR mutation status
Differences in EGFR mutation status according to clinicopathological variables are summarized in Table 2. Among patients with NSCLC, female (p<.001), age <65 years (p=.007), light or no smoking (p<.001), excision specimen (p=.002), and in-house EGFR mutation testing (p<.001) were correlated with significantly higher EGFR mutation rate. Among patients with adenocarcinoma, female (p<.001), light or no smoking (p<.001), excision specimen (p<.001), tumor microdissection (p=.001), and in-house EGFR mutation testing (p=.046) were correlated with significantly higher EGFR mutation rate. According to specimen status, the EGFR mutation rate was 48.1%, 47.2%, and 63.4% in cytology, biopsy, and excision specimen, respectively, from a total of 742 patients with adenocarcinoma and never-smoker status. According to laboratory status, the EGFR mutation rate in patients with NSCLC (38.2% vs 23.1%, p<.001) and adenocarcinoma (44.7% vs 37.6%, p=.046) was significantly higher in in-house tested specimens. Among patients with squamous cell carcinoma, female (p=.001) and light to no smoking (p=.017) were correlated with significantly higher EGFR mutation rate.
DISCUSSION
The present study identified the frequencies of EGFR mutations in Korean patients with NSCLC. The EGFR mutation rate was 34.3% and 43.3% among patients with NSCLC and adenocarcinoma, respectively, and 52.4% among never-smokers with adenocarcinoma. The frequencies were in the range of those previously reported in Korean studies, with 17.4%–40.8% in NSCLC, 21.5%–54.4% in adenocarcinoma, and 47%–64.9% in adenocarcinoma with never-smokers [10,15-21]. The results of the present study were also in the range of those from other Asian countries (30%–61.1% in NSCLC and 44.1%–67.4% in adenocarcinoma) and showed a high mutation rate compared with those reported in Western countries (4.5%–13.3% in NSCLC and 16% in adenocarcinoma) [3,11,12,23]. Recently, a large retrospective database study was performed on EGFR mutation testing practices in the Asia-Pacific region [10]. EGFR mutation rates among patients with NSCLC reported in the present study were very similar to those reported by Yatabe et al. [10] (39.6% and 35.8% EGFR mutation rate among newly diagnosed patients with NSCLC) in the Asia-Pacific region and Korea, respectively, in 2011. The overall distribution pattern of EGFR mutations (i.e., high mutation rates in female patients, never-smokers, and patients with adenocarcinoma) was similar to that reported in previous studies.
The most frequent mutation was an exon 19 deletion, and the most frequent drug resistance-associated mutation was T790M. Chan et al. [24] identified EGFR mutation hot spots in exons 19 (48%) and 21 (41%) in 3,023 specimens. The highest incidence of mutations in EGFR was observed for L858R, del(E746-A750), and del(E749-T751), in that order. Shi et al. [25] reported 43% and 42.6% mutation rates in exons 19 and 21, respectively, among 1,450 specimens, with the most common drug resistance-associated mutation being S768I. The present study reported 52.1% and 34.1% mutation rates in exons 19 and 21, respectively. However, the sum of mutation rates in exons 19 and 21 was 86.2%, which was similar to that reported in studies performed in other countries.
Two laboratories in the present study detected low EGFR mutation rates. Commonalities between these two institutions included a period less than 1 year after starting the EGFR mutation analysis and use of the direct sequencing method. Well-equipped laboratories and technicians skilled at performing EGFR mutation analysis, active engagement of pathologists in molecular testing, and quality assurance were important for obtaining accurate results.
A high incidence of EGFR mutations (29.7%) has been reported in Korean male smokers with adenocarcinoma [20]. In the present study, EGFR mutation rate was 29.1% in male patients with adenocarcinoma who had a smoking history (125 out 430 patients). This result supports the recommendation of a previous study that EGFR mutation tests should be performed in all patients with adenocarcinoma regardless of sex or smoking history [20].
Because minimally invasive diagnostic procedures are often used in the diagnostic workup of lung cancer, small tissue specimens and, more importantly, cytology specimens might be the only specimens available for EGFR mutation analysis. In the present study, a total of 73.3% of specimens were cytology or biopsy samples. However, these specimens showed a significantly low mutation rate compared to excision specimens in patients with NSCLC even in patients with adenocarcinoma and never-smokers. Although our results showed significantly higher mutation rates in surgically resected specimens, many studies have reported that small biopsy and cytology specimens are more suitable for performing mutation testing [26-29].
A quick and accurate test for detecting EGFR mutations is very important for proper selection of patients for EGFR TKI therapy. This highlights the need for standard guidelines specific to medical conditions in Korea for EGFR mutation testing. Many methods are available for detecting EGFR mutations, and these methods have different advantages and disadvantages. However, there is no consensus on the best method for detecting EGFR mutations [30]. In Korea, most pathology laboratories use direct DNA sequencing, pyrosequencing, or the peptide nucleic acid (PNA) clamp method for detecting EGFR mutations in formalin-fixed, paraffin-embedded tissue specimens. In the present study, specimens obtained from 14 hospitals were analyzed using direct DNA sequencing, a classic method for detecting mutations. However, this technique is associated with low sensitivity and requires >25% mutant DNA for analysis [31]. Pyrosequencing is a more sensitive method that needs more than 1% to 20% mutant DNA for analysis [32,33]. PNA clamping is a simple, rapid, and sensitive method that can detect mutations in as few as 1% mutant alleles in a mixture of mutant and wild-type DNA [34]. These three methods show a good concordance of 82%–87.5% [35,36]. We recommend that the use of the available methods for EGFR mutation analysis in each institution and laboratory should be under strict quality control. The quality and quantity of DNA are important for avoiding false-negative results [29]. In the present study, tumor microdissection specimens from patients with adenocarcinoma showed higher mutation rates than non-dissection specimens. We recommend that pathologists verify the adequacy of specimens and reanalyze EGFR mutations to prevent false-negative results.
According to laboratory status, the EGFR mutation rate in patients with NSCLC (38.2% vs 23.1%, p<.001) and adenocarcinoma (44.7% vs 37.6%, p=.046) was significantly higher in the in-house test in the present study. However, the proportion of never-smoker patients with adenocarcinoma was 48.3% (628 out 1,299 patients) in the in-house test and 25.1% (114 out 454 patients) in the out-sourced test. Moreover, EGFR mutation rate in never-smokers with adenocarcinoma was 52.7% based on inhouse mutation testing and 50.9% based on out-sourced mutation testing. These results indicate significant differences between in-house and out-sourced mutation testing, which might be because of a bias in patient selection.
In the future, we will aim to develop recommendations for more standardized application and interpretation of results of EGFR mutation tests in patients with NSCLC. These recommendations will discuss patients, turnaround time, specimen type, minimum specimen size, specimen collection and storage, tumor cell content, methodology such as DNA extraction, and reporting form. In addition, we aim to design a QA program for use during EGFR mutation analysis.
The present study had limitations. Pathological diagnosis of patients included in the study was not confirmed. In addition, immunohistochemical staining to classify the histologic type of NSCLC was not performed for all patient samples. Further, our data were collected from hospitals where diagnosis was performed by pulmonary pathologists. Therefore, our results might represent the current status of NSCLC subtypes in Korea.
In conclusion, EGFR mutation rate showed significant differences with respect to sex, smoking history, histologic diagnosis, specimen type, tumor cell dissection, and institution. However, it did not show differences with respect to age, procurement site, or laboratory status. The relative frequency of EGFR mutations in Korea was not similar to those reported in other Asian countries.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We would like to sincerely thank Seo-Young Oh, MS; NohYoung Lee, MS; and Yoo-Min Hong, MRA for their devoted efforts toward data set mining and cleaning.