Eosinophils in Colorectal Neoplasms Associated with Expression of CCL11 and CCL24
Article information
Abstract
Background:
A decrease in the number of tissue eosinophils is known to reflect the malignancy potential of neoplastic lesions and even prognosis. Increased levels of the chemokines CCL11 and CCL24 in serum and tissue are also known to have diagnostic value as serum tumor markers or prognostic factors. The aim of this study was to evaluate the correlation between the degree of tissue eosinophilia and the expression of these chemokines in the glandular and stromal cells of colorectal neoplastic lesions ranging from benign to malignant tumors.
Methods:
We counted the number of infiltrating eosinophils in neoplastic lesion tissue and we evaluated the expression of CCL11 and CCL24 in glandular cells and stromal cells by immunohistochemical staining.
Results:
The results showed that the number of eosinophils decreased significantly and the expression of CCL11 and CCL24 in glandular cells decreased with tumor progression, whereas the stromal expression of CCL11 and CCL24 appeared to increase.
Conclusions:
The discrepancy in CCL11 and CCL24 expression between glandular cells and stromal cells might shed light on how colorectal cancer evades the immune system, which would enable further development of immunotherapies that target these chemokines. Further research on eosinophil biology and the expression pattern of chemokines in tumor cells is needed.
Eosinophils are bone-marrow–derived granulocytes present in peripheral blood and tissue that are primarily involved as effector cells in various conditions, including inflammatory diseases, allergic diseases, and parasitic infections [1,2]. Researchers have long thought that the main role of eosinophils was to act as effector cells in allergic reactions and helminth infections, but their role as antitumor effectors has recently been revealed [3-6]. Eosinophils function as antitumor effector cells in neoplastic lesions through a variety of eosinophil-derived mediators, including tumor necrosis factor alpha and granzyme-A [3,7].
Changes in the number of tissue eosinophils have been reported in several types of cancer and premalignant lesions from a wide range of organs, including the head and neck and the gastrointestinal tract [5,8-12]. Some studies have shown that the degree of tissue eosinophilia correlates with cancer prognosis because of its significant association with response to chemoradiation therapy, operability, and even lymph node metastasis [5,11,13-16]. These findings suggest that the degree of eosinophilia could be used as a prognostic marker. Among the studies on eosinophils and neoplastic conditions, both increasing and decreasing trends in the number of eosinophils in colorectal neoplasms have been reported with regards to tumor progression. A histopathological study on tissue eosinophilia in colorectal neoplasms has revealed that the degree of eosinophilia differs according to the malignant potential of the lesion; the number of tissue-infiltrating eosinophils increases in low-grade dysplasia, decreases in high-grade lesions, and decreases even more in cancer cases, showing significant and rapid change compared to the surrounding normal tissues [17-19]. As mentioned earlier, more prominent eosinophilia in colorectal cancer is associated with a better prognosis, including significant improvement in the 5-year survival rate.
As the association between the infiltration of eosinophils and neoplasms is better understood, interest in how chemokines affect the differentiation and migration of eosinophils is increasing. Many kinds of chemokines are involved in the production, differentiation, and migration of eosinophils. Among the interleukins, interleukin (IL)-3 and IL-5 play major roles in the production and differentiation of eosinophils, and IL-4, IL-6, IL-11, and IL-12 are involved in their differentiation. A variety of C-C chemokines (named after the cysteine terminus residue near the C-terminus in the amino acid sequence), including C-C chemokine ligands-5 (CCL5, RANTES), CCL11 (eotaxin-1), CCL24 (eotaxin-2), and CCL26 (eotaxin-3), are involved in the differentiation and, in particular, the migration of eosinophils from the bone marrow to tissue stroma through enhancement and regulation of tethering, rolling, and endothelial adhesion of the eosinophils [1,2].
Expression of CCL11 and CCL24 in the human gastrointestinal tract is higher than in other tissues in the normal state, and increases during inflammatory disease, allergic reaction, and helminth infections, in which eosinophils function as effector cells [1,20-22]. The concentration of CCL24 is shown to be elevated in tumor tissue, and is also elevated in the stromal cells of colorectal tumors [23]. As mentioned earlier, a decrease in tissue eosinophils was observed in colorectal adenocarcinoma, but tissue levels of CCL11 were found to be elevated. Therefore, there is a discrepancy between decreased tissue eosinophilia and the tissue levels of chemokines such as CCL11 and CCL24, which enhance the recruitment of eosinophils. Serum chemokine levels appear to be elevated in prostate and colorectal adenocarcinoma, and they have been proposed as serum tumor markers [24-28].
In this study, we aimed to (1) evaluate the number of tissue-infiltrating eosinophils in colorectal tubular adenoma with low-grade dysplasia, tubular adenoma with high-grade dysplasia, and adenocarcinoma, and (2) evaluate the expression of CCL11 and CCL24 in colorectal neoplastic lesions by immunohistochemical staining to determine the correlation between the expression of cytokines, eosinophilia, and progression of neoplastic lesions. Even though much is known about the correlation between the prognosis and progression of colorectal neoplasms and eosinophilia, little is known about local expression of the chemokines associated with eosinophils. This study may provide insight into the relationship between tumor eosinophilia and local expression of chemokines, and thus explain the observed discrepancy between chemokine levels and tissue eosinophilia.
MATERIALS AND METHODS
Patients and tissue samples
Among the patients who underwent colonoscopic biopsy at Kyung Hee Medical Center and were diagnosed with colorectal tubular adenoma with any degree of dysplasia or colorectal cancer, a list of 50 patients was generated and their clinicopathological data were retrospectively collected. Patients were categorized into separate groups based on whether they had tubular adenoma with low-grade dysplasia, tubular adenoma with high-grade dysplasia, or adenocarcinoma. For adenocarcinoma cases, patients who had undergone resection were selected. Specimens with severe squeezing or cautery artifacts and specimens from patients with a history of other malignancies were excluded. For colorectal cancers, selection was performed among those who had undergone surgical resection and whose surgical specimens were retrieved from Kyung Hee Medical Center. Every diagnosis was reviewed and confirmed by pathologists. Each paraffin block from the specimen was cut into 4–5-µm thick sections and stained with hematoxylin and eosin for direct counting of eosinophils under microscopy. This study was approved by the Institutional Review Board (IRB) of Kyung Hee University (IRB 2015-08-039).
Evaluation of tissue eosinophilia
Tissue eosinophils were counted directly on hematoxylin and eosin-stained slides of each specimen using an Olympus BX-53 microscope (Olympus, Tokyo, Japan). Eosinophils in the mucosa and submucosa were counted in three “hotspots” near the neoplastic lesion under a high-power field (×400). Controversial results were resolved by consensus of more than two pathologists using a multiview microscope.
Immunohistochemical staining
Each specimen was prepared into 4–5-µm thick paraffin-embedded sections for immunohistochemical staining. Monoclonal mouse antibodies against CCL11 (LS-C139009, LifeSpan Biosciences, Seattle, WA, USA) and monoclonal mouse antibodies against CCL24 (LS-C104346, LifeSpan Biosciences) were used for immunohistochemical staining of the specimens. Unstained slides from each specimen were processed for 20 minutes in a pressure cooker for antigen retrieval. Immunohistochemical staining of all slides was performed using BOND-MAX (Leica Biosystems, Nusslock, Germany) with a dilution ratio of 1:200 for CCL11 and 1:800 for CCL24. Staining of CCL11 and CCL24 in glandular cells of neoplastic lesions was evaluated according to the number of glandular cells showing positive staining and the intensity of the staining over the slide. The number of positively stained glandular cells ranged between 1 and 60, and individual counts were scored as 0 (0–15 cells), 1 (16–30 cells), 2 (31–45 cells), or 3 (≥46 cells). Staining intensity was scored as 1 (faint), 2 (intermediate-strong), and 3 (strong granular). The sum of the immunohistochemical stain scores ranged from 1 to 6, and each summed score was then divided into categories of low (score 1–2), intermediate (score 3–4), and high (score 5–6). We also conducted immunohistochemical staining of CCL11 and CCL24 in stromal cells. Each specimen contained a different amount of stroma because most were endoscopically biopsied samples. Thus, expression in the stromal cells needed to be measured as a proportion rather than an absolute count. Positively stained stromal cells were counted under a microscope and scored as low, intermediate, or high according to the cutoff values of 5%, 15%, and >30% for the percentage of cells showing positive staining relative to the total number of stromal cells in the high-power field.
Statistical analyses
SPSS ver. 20.0 (IBM Co., Armonk, NY, USA) was used for the statistical analyses. Correlation between the number of eosinophils and tumor progression was analyzed with a one-way ANOVA. Correlation between the expression of CCL11 and CCL24 in glandular and stromal cells according to each different tumor progression group was analyzed via chi-square tests.
RESULTS
Tissue eosinophilia in the neoplastic lesions was strikingly different according to progression of the lesion. Tubular adenoma with low-grade dysplasia included a stunning number of infiltrating eosinophils, whereas less eosinophils were present in cases of adenoma with high-grade dysplasia, and only a few eosinophils were counted in adenocarcinoma cases. These infiltrating eosinophils were mostly found adjacent to neoplastic lesions, close to neoplastic glands in tumor stroma.
One-way ANOVA analysis was performed to evaluate the differences, and the results confirmed that the number of eosinophils differed significantly according to the malignant potential of the lesion (p<.001) (Fig. 1).
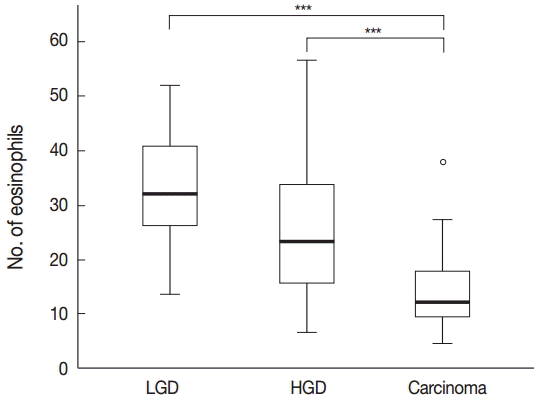
The number of eosinophils in the colorectal neoplastic lesions: the number of infiltrating eosinophils increased significantly with the progression of colorectal neoplastic lesions. Asterisk (***) indicates p < .001 in each group. LGD, low grade dysplasia; HGD, high grade dysplasia.
Immunohistochemical staining of CCL11 in the glandular cells of neoplastic lesions appeared was strong and granular in cases of low-grade dysplasia (Fig. 2A). A mix of strong and faint staining was observed in high-grade dysplasia cases (Fig. 2B). In contrast, most of the staining in adenocarcinoma cases was weak and faint (Fig. 2C).
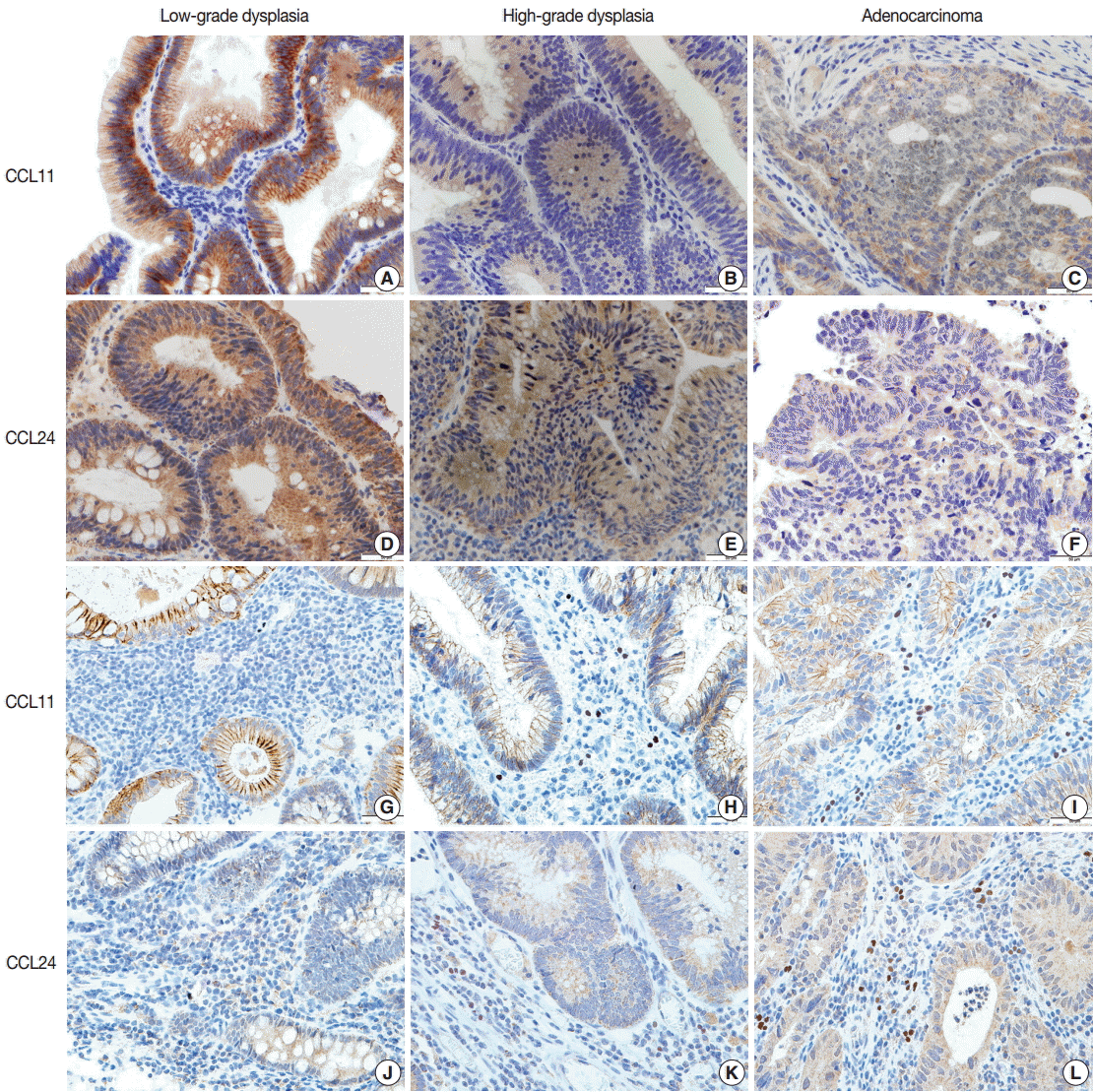
Immunohistochemical staining of CCL11 and CCL24: immunohistochemical stains of CCL11 (A) and CCL24 (D) showed, in order of the strength of the observation, positivity in the tumor cells of colorectal tubular adenomas with low-grade dysplasia, adenomas with high-grade dysplasia (B, CCL11; E, CCL24), and adenocarcinoma (C, CCL11) and CCL24 (F). Immunohistochemical stains of CCL11 and CCL24 of the stromal cells appear faint and less positive in tubular adenoma cases with low-grade dysplasia (G, CCL11; J, CCL24), high-grade dysplasia (H, CCL11; K, CCL24), and adenocarcinoma (I, CCL11; L, CCL24).
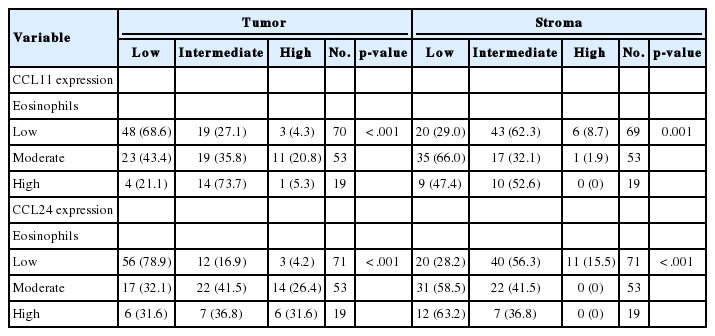
Expression of CCL24 in glandular cells of neoplastic lesions was similar to CCL11 expression. CCL24 staining revealed a mostly strong and granular pattern of expression in low-grade dysplasia cases (Fig. 2D), a mix of strong and weak staining in high-grade dysplasia cases, and an intermediate pattern in cases that are between low-grade dysplasia and adenocarcinoma (Fig. 2E). In contrast, a weak and faint pattern of staining was dominant in adenocarcinoma cases (Fig. 2F). Analysis of immunohistochemical staining showed significant differences between the groups (p<.001 in CCL11 and p<.001 in CCL24) (Table 1).
CCL24 staining in the stromal cells of patients with low-grade dysplasia was scarce and weak (Fig. 1G). Slightly more staining was observed in high-grade dysplasia cases (Fig. 2H), whereas stromal cells with CCL11 reactivity were frequently seen in adenocarcinoma cases (Fig. 2I). CCL11 expression appeared to increase with increased progression of the neoplastic lesion (p<.001) (Table 1).
CCL24 expression in stromal cells had a similar pattern to that of CCL11. CCL24-expressing stromal cells were scant and faintly stained in low-grade dysplasia cases (Fig. 2J), whereas a small number of positive stromal cells were observed in high-grade dysplasia cases (Fig. 2K). CCL24-positive stromal cells were frequently identified in adenocarcinoma cases (Fig. 2L). StromalCCL24 expression also increased significantly with tumor progression (p<.001) (Table 1).
Comparisons of tumoral and stromal expression of CCL11 and CCL24 with the degree of eosinophilia revealed that tumoral expression of CCL11 and CCL24 decreased and stromal expression of CCL11 and CCL24 increased while the number of tissue-infiltrating eosinophils decreased (both comparisons, p<.001) (Table 2).
DISCUSSION
Our study showed that the number of tissue infiltrating eosinophils in colorectal neoplasms decreased significantly in colorectal adenocarcinoma cases compared to tubular adenoma cases with low-grade dysplasia and tubular adenoma cases with high-grade dysplasia, which is consistent with the results of previous studies [17]. Colorectal cancer is well known as a non-immunogenic tumor that induces an impaired immune response to the tumor itself, thus evading the host immune response to cancer [29-31]. It is also known that an increase in tissue-infiltrating eosinophils in colorectal cancer is associated with a better prognosis. Therefore, decreased tissue eosinophilia may be an immuneevading strategy of colon cancer. Because eosinophils develop and migrate to tissues in response to chemokine signaling, chemokine expression in colorectal cancer should reveal the eosinophil-infiltration potential of colorectal cancer in individual cases.
In colorectal cancer patients, both the serum level and concentration of CCL11 in tumor tissue are elevated [24,29,30]. Since CCL11 enhances tissue recruitment of eosinophils, an increased concentration of CCL11 should attract more eosinophils and cause more severe eosinophilia in tissues where the concentration of CCL11 is high. Indeed, a study on eosinophilia in colorectal neoplastic lesions showed that tissue extracted from a tumor with a greater number of eosinophils was also highly chemotactic for eosinophils, which was thought to reflect chemokine concentrations in the tissue [19,23]. Another study involving immunohistochemical staining of colorectal cancer tissue revealed that expression of CCL11 was elevated in stromal cells, such as fibroblasts or lymphocytes [24]. The results of this study are consistent with our data showing that expression of CCL11 and CCL24 is increased in the stromal cells of adenocarcinomas compared to those that are dysplastic. The CCL11/CCL24-secreting stromal cells in our study were mostly mononuclear inflammatory cells. Increased expression of CCL11 and CCL24 in the stromal cells of tumors might explain the elevated serum chemokine levels and elevated tissue concentration of chemokines. However, previous studies on eosinophilia in colorectal neoplastic lesions reported decreased numbers of eosinophils in tissues with adenocarcinoma or high-grade dysplasia [17,19], which is consistent with our results. Thus the question remains: why is decreased eosinophilia observed in association with colorectal malignant neoplastic lesions even when the concentration of chemokines for eosinophils is elevated?
Our results showed that CCL11 and CCL24 expression was lower in glandular cells of adenocarcinomas compared to the expression levels in stromal cells. These findings might provide insight into the discrepancy between tissue eosinophilia and tissue chemokine concentrations in colorectal neoplasms. As we previously mentioned, the population of tissue-infiltrating eosinophils is lower in colorectal adenocarcinomas, as is the expression of CCL11 and CCL24 in neoplastic glandular cells of adenocarcinomas. However, one study found increased tissue concentration of CCL11 associated with colorectal adenocarcinoma [23]. We postulate that increased expression of CCL11 and CCL24 in the stromal cells of tumors might explain increased tissue CCL11 concentration. If lower expression of CCL11 and CCL24 in neoplastic glandular cells is responsible for decreased eosinophilia in adenocarcinomas, modulation of chemokine expression could contribute to the immune-evasion mechanisms of colorectal adenocarcinomas by inhibiting recruitment of eosinophils, which function as effector cells for the neoplasm.
Further studies on other chemokines involved in eosinophil physiology and studies on the status of eosinophils recruited to tumor tissues are needed for a more detailed understanding of the nature of peritumoral eosinophilia and its significance for the immunologic characteristics of colorectal cancer. CCL24 has previously been a target of anti-cancer immune therapies [23]. If reduced expression of chemokines contributes to immune evasion by colorectal cancer, modulation of chemokine expression in cancer cells could be a possible target for anticancer therapies as well as a prognostic factor for colorectal cancer [31,32]. As some studies on leukemia and CCL24 have suggested, specific kinds of chemokines might affect the migration of specific types of eosinophils in colorectal cancer [33].
In conclusion, we found a significant correlation between eosinophil numbers and immunohistochemical staining of CCL11 and CCL24 chemokines in the glandular cells of colorectal neoplasms. Lower expression of CCL11 and CCL24 was observed in tumor glandular cells, while greater expression was observed in tumor stromal cells. This differential expression of chemokines might help explain the decreased eosinophilia observed in colorectal cancer despite an apparent increased concentration of CCL11, and could provide insight into immune evasion mechanisms of colorectal cancer. Considering that eosinophils are antitumoral effector immune cells, cancer appears to induce a decrease in eosinophilia through decreased expression of chemokines in glandular cells, which is consistent with the decreased expression of CCL11 and CCL24 shown in our study, and thus, achieves immune evasion. Further study on eosinophil-associated chemokines and the nature of the eosinophils recruited by colorectal cancer cells might enhance our understanding of the immunologic characteristics and roles of eosinophils in colorectal cancer.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.