Core Needle Biopsy Is a More Conclusive Follow-up Method Than Repeat Fine Needle Aspiration for Thyroid Nodules with Initially Inconclusive Results: A Systematic Review and Meta-Analysis
Article information
Abstract
Background:
This study investigated the appropriate management of thyroid nodules with prior non-diagnostic or atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) through a systematic review and meta-analysis.
Methods:
This study included 4,235 thyroid nodules from 26 eligible studies. We investigated the conclusive rate of follow-up core needle biopsy (CNB) or repeat fine needle aspiration (rFNA) after initial fine needle aspiration (FNA) with non-diagnostic or AUS/FLUS results. A diagnostic test accuracy (DTA) review was performed to determine the diagnostic role of the follow-up CNB and to calculate the area under the curve (AUC) on the summary receiver operating characteristic (SROC) curve.
Results:
The conclusive rates of follow-up CNB and rFNA after initial FNA were 0.879 (95% confidence interval [CI], 0.801 to 0.929) and 0.684 (95% CI, 0.627 to 0.736), respectively. In comparison of the odds ratios of CNB and rFNA, CNB had more frequent conclusive results than rFNA (odds ratio, 5.707; 95% CI, 2.530 to 12.875). Upon subgroup analysis, follow-up CNB showed a higher conclusive rate than rFNA in both initial non-diagnostic and AUS/FLUS subgroups. In DTA review of followup CNB, the pooled sensitivity and specificity were 0.94 (95% CI, 0.88 to 0.97) and 0.88 (95% CI, 0.84 to 0.91), respectively. The AUC for the SROC curve was 0.981, nearing 1.
Conclusions:
Our results show that CNB has a higher conclusive rate than rFNA when the initial FNA produced inconclusive results. Further prospective studies with more detailed criteria are necessary before follow-up CNB can be applied in daily practice.
Papillary thyroid carcinoma, which has recently increased in incidence, is the most common malignant tumor in endocrine system [1]. The cause for the increased incidence of papillary thyroid carcinoma is not fully understood [1]. One possible cause is the improvement in ultrasonography and computed tomography [2,3]. In daily practice, the treatment and follow-up for thyroid nodules are based on the results of initial fine needle aspiration (FNA). According to current guidelines [4-6], FNA is recommended as the initially performed modality, and additional testing is suggested as indicated by the initial FNA results.
In daily practice, repeat FNA (rFNA) is recommended for thyroid nodules of non-diagnostic or atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS). In addition, for definite diagnosis of thyroid nodules, BRAFV600E mutation test or diagnostic surgery can also be performed. In previous studies, the rates of non-diagnostic and AUS/FLUS were 5%–17% and 3%–18%, respectively [7-9]. For thyroid nodule with non-diagnostic result, the possibility of an inconclusive reading might also be higher with rFNA. Although rFNA is recommended for non-diagnostic or AUS/FLUS thyroid nodules in the current guidelines, other modalities, such as core needle biopsy (CNB) or combination of FNA and CNB, have been introduced in recent reports [10,11]. However, the effectiveness or diagnostic role of these follow-up modalities has not been fully elucidated.
In this study, follow-up CNB was defined as CNB performed after initial non-diagnostic or AUS/FLUS findings. We investigated the conclusive rates of the follow-up procedures of CNB and rFNA in thyroid nodule with initial non-diagnostic or AUS/FLUS finding through a systematic review and meta-analysis. Indeed, the diagnostic test accuracy (DTA) review was performed to determine the diagnostic accuracy of follow-up CNB in thyroid nodules with initial inconclusive results.
MATERIALS AND METHODS
Literature search and selection criteria
Relevant articles were obtained from a search of PubMed and MEDLINE databases through December 31, 2015. The search was performed using ‘thyroid,’ ‘core needle biopsy,’ and ‘fine needle aspiration’ as search terms. The titles and abstracts of all returned articles were screened for exclusion. To find additional eligible studies, review articles were also screened. Search results were then reviewed and included if (1) initial FNA for a thyroid nodule was performed, and (2) there was information about CNB or FNA as a follow-up study for thyroid nodules with initial non-diagnostic or AUS/FLUS results. Articles were excluded if they were (1) non-original articles or case reports or (2) non-English language publications.
Data extraction
The following information was collected from the full texts of eligible studies and verified: name of first author, publication year, study location, number of patients analyzed, and method and results of initial and follow-up studies. We did not define a minimal number of patients to be included in a study. Any disagreements were resolved by consensus.
Statistical analysis
Data were analyzed using the Comprehensive Meta-Analysis software package (Biostat, Englewood, NJ, USA). We evaluated the conclusive rates of follow-up studies with follow-up CNB or rFNA after initial FNA with non-diagnostic or AUS/FLUS results. The conclusive results included benign, follicular neoplasm or suspicious for follicular neoplasm, suspicious for malignancy, and malignancy categories. The conclusive rates were measured by dividing the number of conclusive results into the total number of cases with a follow-up study. The heterogeneity between eligible studies was assessed using Q and I2 statistics and presented using p-values. A sensitivity analysis was performed to assess the impact of each study on the combined effect and the heterogeneity of eligible studies. To identify any publication bias, Egger’s test and Begg’s funnel plot were initially performed. When a significant publication bias was found, the fail-safe N and trimfill tests were additionally conducted to confirm the degree of bias. The results were considered statistically significant at p<0.05.
A DTA review was conducted using the Meta-Disc program ver. 1.4 [12]. The forest plots of pooled sensitivity and specificity and the summary receiver operating characteristic (SROC) curve were determined as described previously [13]. The diagnostic odds ratio (OR) and the value of the area under the curve (AUC) on SROC were investigated.
RESULTS
Selection and characteristics of studies
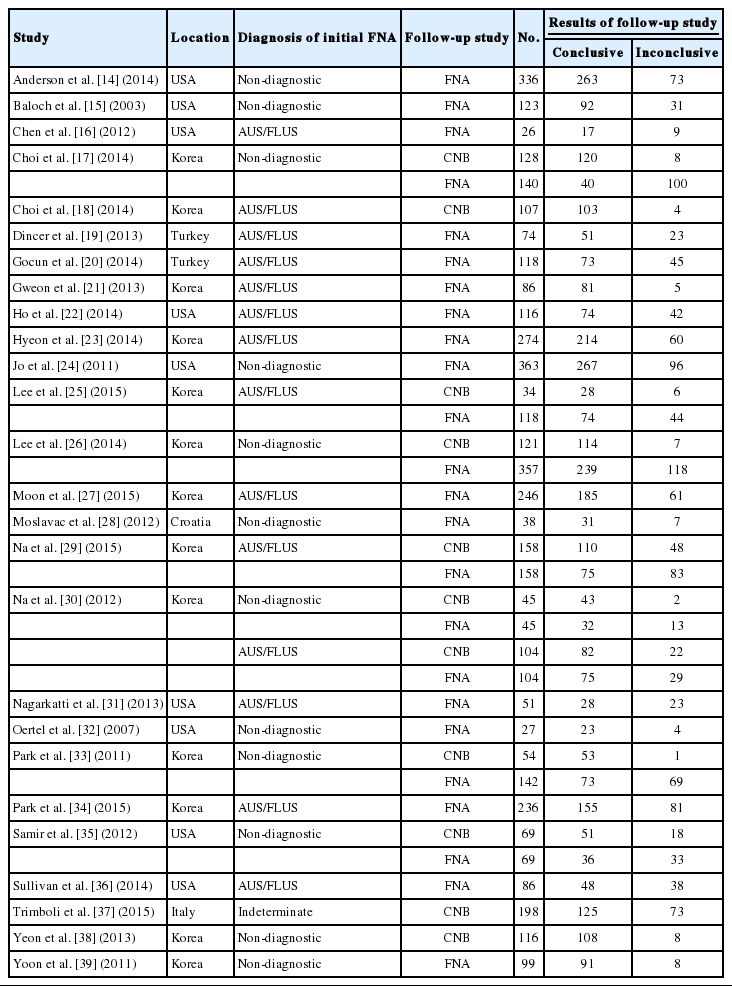
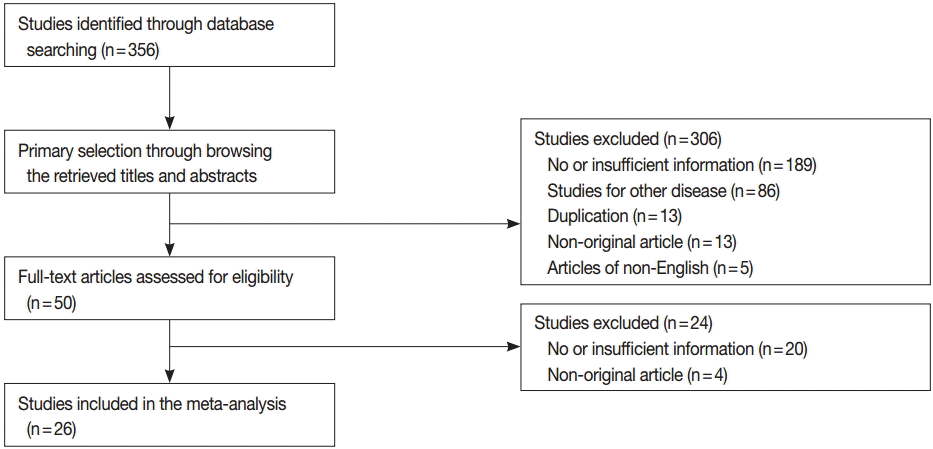
In the current study, 356 reports were identified in the database search. Among the search results, 209 reports were excluded due to insufficient information. In addition, 121 reports were excluded for the following reasons: focusing on other diseases (n=86), non-original articles (n=17), duplicate articles (n=13), and articles in a language other than English (n=5). Twenty-six eligible studies and 4,253 thyroid nodules were ultimately included in the current study (Table 1, Fig. 1) [14-39]. The characteristics of the included studies are shown in Table 1.
Higher conclusive rate in follow-up CNB than in rFNA
For initial FNA with non-diagnostic or AUS/FLUS, follow-up studies using CNB or rFNA showed an overall conclusive rate of 0.690 (95% confidence interval [CI], 0.675 to 0.704) and 0.748 (95% CI, 0.701 to 0.791) with the fixed and random effect models, respectively. For follow-up CNB, the conclusive rate was 0.775 (95% CI, 0.745 to 0.802) and 0.879 (95% CI, 0.801 to 0.929) with the fixed and random effect models, respectively. In rFNA, the conclusive rate was 0.670 (95% CI, 0.653 to 0.686) and 0.684 (95% CI, 0.627 to 0.736) with the fixed and random effect models, respectively. The ranges of conclusive rates were 0.631–0.981 and 0.286–0.942 for follow-up CNB and rFNA, respectively. Follow-up CNB showed a higher conclusive rate compared with rFNA (OR, 5.707; 95% CI, 2.530 to 12.875). The heterogeneity of eligible studies was significant in both follow-up CNB and rFNA groups (I2=90.4%, p<0.001 and I2=90.3%, p<0.001, respectively). In sensitivity analysis, no study had an effect on the concordance rates for either follow-up CNB (range, 0.864 to 0.896) or rFNA (range, 0.670 to 0.697). Subgroup analysis revealed a significant difference in conclusive rate between follow-up CNB and rFNA in both non-diagnostic (0.927; 95% CI, 0.848 to 0.966 vs 0.699; 95% CI, 0.596 to 0.785) and AUSL/FLUS cases (0.794; 95% CI, 0.675 to 0.877 vs 0.673; 95% CI, 0.608 to 0.732) with the random effect model (Table 2).
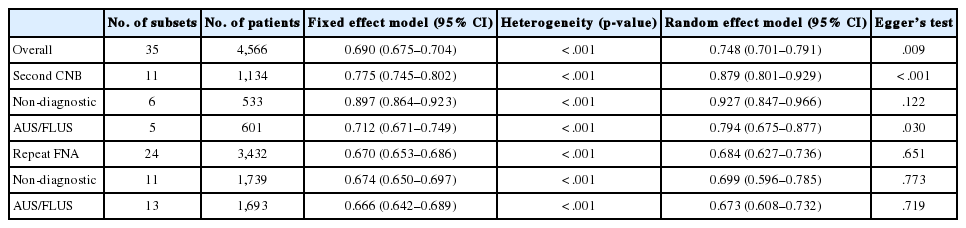
Conclusive rates of second CNB and repeat FNA after prior FNA with non-diagnostic or AUS/FLUS significance in thyroid nodules
Egger’s test revealed that the follow-up CNB group showed a significant publication bias (p=.009). Additionally, fail-safe N and trim-fill tests were performed for confirmation of the degree of publication bias in the CNB group. The number of missing studies that would produce a p-value higher than alpha was 5,401 on the fail-safe N test. Because there were 11 observed studies, the publication bias was not large. In addition, the trim and fill test showed no significant difference between the observed and adjusted values. Therefore, we concluded that the publication bias in the follow-up CNB group was not significant through interpretation of Egger’s test, Begg’s funnel plot, the fail-safe N test, and the trim-fill test. In the rFNA group, there was no significant publication bias according to Egger’s test (p=.651) or Begg’s funnel plots.
DTA review of follow-up CNB as a follow-up study
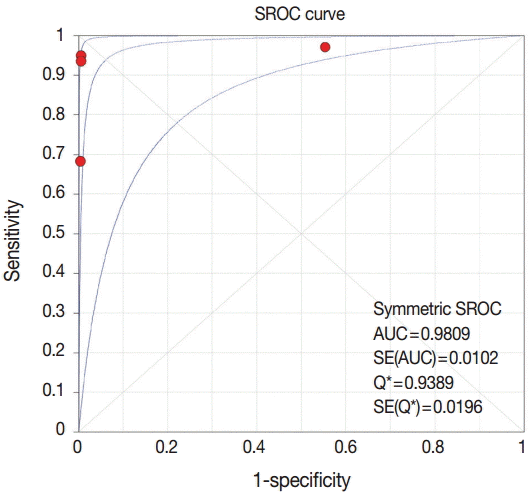
For confirmation of the diagnostic accuracy of follow-up CNB, we conducted a DTA review. The pooled sensitivity and specificity values were 0.94 (95% CI, 0.88 to 0.97) and 0.88 (95% CI, 0.84 to 0.91), respectively (Fig. 2). The sensitivity and specificity of eligible studies ranged from 0.70 to 0.97 and from 0.45 to 1.00, respectively. On the SROC curve, the value of AUC was 0.981 (Fig. 3). In addition, the diagnostic OR was 448.73 (95% CI, 36.63 to 5,497.51).

The sensitivity (A) and specificity (B) of follow-up core needle biopsy for prediction of papillary thyroid carcinoma after prior fine needle aspiration with non-diagnostic or atypia/follicular lesion of undetermined significance in thyroid nodules.
DISCUSSION
The current guidelines recommend FNA as the initial test for thyroid nodules [4-6]. Although rFNA was applied to thyroid nodules with non-diagnostic and AUS/FLUS findings [4-6], the effectiveness of rFNA has not been fully elucidated. The present study is the first meta-analysis and DTA review of published studies on the diagnostic role of follow-up CNB compared with rFNA after initial FNA with non-diagnostic or AUS/FLUS results.
In the assessment of thyroid nodule, FNA is the most cost-effective initial test. Recently, FNA using ultrasonography has produced a higher quality of specimens for more precise diagnosis [10]. However, some cases were non-diagnostic or AUS/FLUS, and the rates of inconclusive results ranged from 10%–33.6% in previous studies [6,8,9,40]. Although the current guidelines recommend rFNA as a follow-up study for thyroid nodules with initial inconclusive results [4-6], rFNA does not ensure conclusive results. The inconclusive rates of rFNA in previous studies ranged from 9.9% to 72.0% [18,29,34,36]. In the current meta-analysis, the rate of inconclusive results for rFNA was 31.6%. If the cellularity of initial FNA is low, rFNA can also show low cellularity. In a previous study, rFNA was the most significant risk factor affecting repeat non-diagnostic results [18]. This situation might be caused by the nature of the thyroid nodule, including factors such as intratumoral calcification and cystic change [41-43]. In addition, the quality of the specimen might also be affected by the experience of the operator. Consequently, diagnostic surgery is recommended for thyroid nodule with repeat inconclusive results, and the incidence of diagnostic surgery has been reported as 22.2%–94.7% [24,44-46]. Therefore, to reduce the inconclusive rate, an alternative follow-up study, such as CNB, could be considered for thyroid nodules with inconclusive readings in initial FNA.
Considering the results of a previous meta-analysis, histologic examination using CNB as an initial test might be useful for obtaining conclusive results [47]. Other previous comparative studies of FNA and CNB as an initial test have reported that CNB has a suspected higher specificity, higher positive predictive value, and lower rate of inconclusive results [11,48]. However, definitive results for the sensitivity and specificity of CNB as a follow-up study have not yet been obtained [10,11,47]. CNB showed a lower sensitivity for thyroid glands than for other head and neck lesions [49]. However, the initial test might be more important for achieving higher sensitivity and patient safety. Furthermore, CNB has some limitations, including bleeding, tumor-cell displacement, and difficulty in approaching thyroid nodules in a posterior portion or close to critical structures, including the carotid artery or trachea; these factors limit its use as an initial test [48,49]. To obtain an adequate specimen for diagnosis, the experience of the operator may be more important for CNB than for FNA [48]. Whether CNB is appropriate as an initial test for thyroid nodules is not fully understood and could not be determined in the current systematic review. Despite a previous report that found follow-up CNB to have lower non-diagnostic and inconclusive rates than rFNA (7.2% vs 72.0%) [18], many previous studies have reported that CNB showed lower sensitivity than FNA. However, the diagnostic accuracy of follow-up CNB has not been fully elucidated. In the current DTA review, the pooled sensitivity and specificity of follow-up CNB were significantly high. For this reason, follow-up CNB after initial FNA might be useful for predicting malignancy and reducing the inconclusive rate in follow-up study.
There were some limitations in the current meta-analysis. First, most included studies were retrospective rather than prospective evaluations. Many thyroid nodules with initial inconclusive results only involved follow-up with ultrasonography. The conclusive and inconclusive rates of rFNA and follow-up CNB might have been affected by such cases. Therefore, cumulative prospective studies are needed to determine the effectiveness of follow-up procedures. Second, the current study included both non-diagnostic and AUS/FLUS subgroups, and subgroup analysis was performed. However, the DTA review could be not conducted for the rFNA subgroup due to insufficient information from eligible studies. Thus, a comparison of diagnostic accuracy between follow-up CNB and rFNA could not be performed. Third, because the current guidelines recommend initial FNA test for thyroid nodules [4-6], the current meta-analysis was performed only for studies with initial FNA. An investigation of the effectiveness of CNB as an initial test was therefore not conducted in the current study. Fourth, the current study was analyzed for initial non-diagnostic and AUS/FLUS categories. However, additional evaluations, including subgroup analysis for AUS and FLUS results, could not be performed due to lack of information on follow-up CNB from eligible studies [23]. Fifth, eligible studies used various criteria for nondiagnostic or indeterminate lesion. The rate of inconclusive results of CNB may have differed from the real value. Further studies are needed to establish guidelines of pathology reporting for thyroid CNB.
In conclusion, the current study showed that follow-up CNB had a higher conclusive rate than rFNA after initial FNA with inconclusive results. In addition, follow-up CNB had greater diagnostic accuracy for prediction of malignancy than rFNA. Additional prospective studies are required to determine standardized application criteria of follow-up CNB in daily practice.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.