A Case of Malignant PEComa of the Uterus Associated with Intramural Leiomyoma and Endometrial Carcinoma
Article information
Abstract
Perivascular epithelioid cell tumors (PEComas) refers to a family of mesenchymal neoplasms composed of angiomyolipomas, clear cell “sugar” tumors of the lung, and lymphangioleiomyomatoses. These tumors have a distinctive and common component of perivascular epithelioid cells that show an association with blood vessel walls and immunohistochemically display myomelanocytic differentiation. The unique neoplasms have been shown to have an expanded range through a variety of case reports, including visceral, intra-abdominal, soft tissue, and bone tumors. The retroperitoneum, abdominopelvic region, and uterus have been reported to be the most common sites. Most PEComas follow a benign course. However, reports of malignant PEComas are increasing. Many papers have described uterine PEComas, but to our knowledge, there have not yet been any reports of a malignant PEComa arising concomitant with another epithelial tumor and mesenchymal tumor. We report herein the case of a 67-year-old woman who experienced a malignant uterine PEComa infiltrating a preexisting intramural leiomyoma with synchronous well differentiated endometrial carcinoma and multiple liver and lung metastases.
Perivascular epithelioid cell tumors (PEComas) have a distinctive and common component of perivascular epithelioid cells that show an association with blood vessel walls and immunohistochemically display myomelanocytic differentiation [1-4]. In this report, we present a unique case of a malignant uterine PEComa infiltrating a preexisting intramural leiomyoma with synchronous well differentiated endometrial carcinoma and multiple liver and lung metastases.
CASE REPORT
A 67-year-old post-menopausal woman visited the clinic due to vaginal bleeding. Ultrasonography revealed one myometrial mass and one endometrial lesion. Levels of serum tumor markers, including carbohydrate antigen 125 and carbohydrate antigen 19-9, were normal. Curettage of the endometrial lesion showed endometrioid adenocarcinoma, the International Federation of Gynecology and Obstetrics (FIGO) G1. Imaging studies revealed multiple suspicious metastatic lesions in the liver and lung. A total abdominal hysterectomy and bilateral salpingo-oophorectomy were performed.
There was an ill-demarcated grayish mass, measuring 4×3.7×3.5 cm, on the anterior wall of the endometrium considered to be endometrial origin. There was another well-demarcated heterogeneous white to yellowish partially hemorrhagic and fibrotic mass, measuring 6×5×4.4 cm, on the posterior wall of the myometrium. The cut surface of the mass showed an ill-demarcated yellowish necrotic lesion, enclosed by a whitish fibrotic area. It measured 3.7×2.5×2.5 cm (Fig. 1).
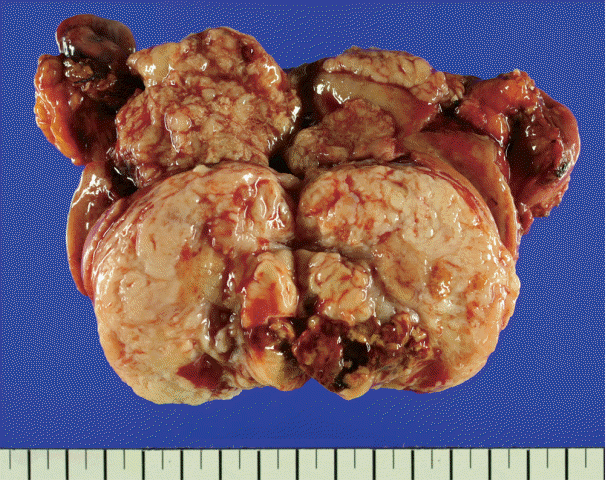
Total hysterectomy showing endometriod carcinoma on anterior wall to fundus of the endometrium, and whitish leiomyoma encircling perivascular epithelioid cell tumors in the myometrium.
The endometrial lesion had the histologic appearance of endometrioid carcinoma, the same as the previous curettage (Fig. 2A). It invaded the myometrium 16 mm of the total 20 mm thickness.
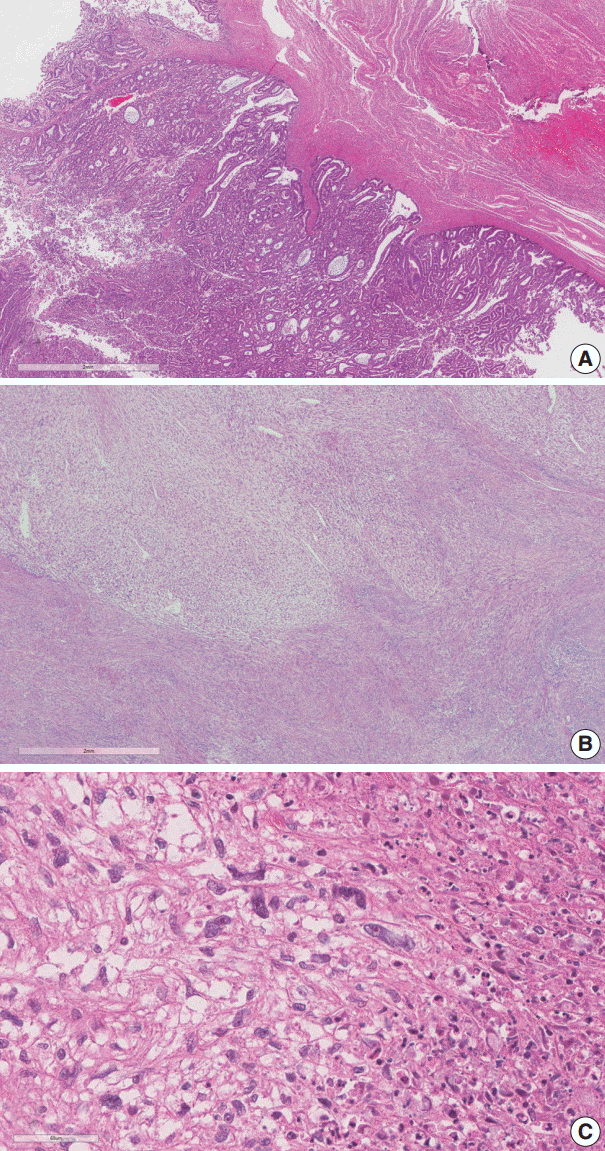
(A) Endometrioid carcinoma, International Federation of Gynecology and Obstetrics (FIGO) G1. (B) Infiltrating spindle perivascular epithelioid cell tumor (PEComa) cells into surrounding leiomyoma. (C) The PEComa cells have elongated nuclei, prominent nucleoli, clear cytoplasm, cytological atypia, and necrosis.
The histologic findings of the myometrial mass were characterized by infiltration of spindle to ovoid cells into the surrounding leiomyoma. The infiltrating cells showed clear cytoplasm and elongated nuclei with cytological atypia. Frequent mitotic activity was encountered in up to 14/50 high-power fields (HPF). Coagulative tumor cell necrosis was present (Fig. 2B, C).
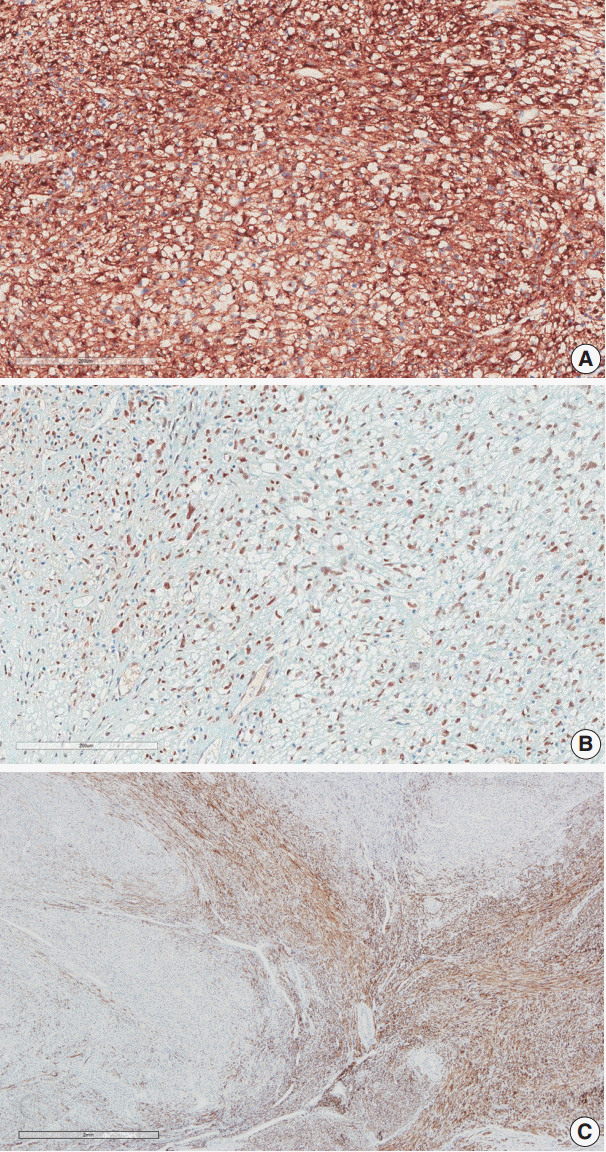
Immunohistochemical staining was performed on the myometrial lesion for CD10, caldesmon, desmin, α-smooth muscle actin (SMA), human melanoma black 45 (HMB-45), Melan-A, transcription factor E3 (TFE3), and Ki-67. The infiltrating tumor cells were positive for SMA, HMB-45, and TFE3 with a low Ki-67 index of about 5%, but they were negative for CD10, caldesmon, and desmin (Fig. 3A, B). In contrast, the surrounding leiomyoma showed positivity for caldesmon and desmin (Fig. 3C).
DISCUSSION
PEComas, which have no known normal tissue counterpart, are unique in that they react immunohistochemically for both melanocytic and myoid markers [2]. Folpe et al. [5] reviewed 26 cases of PEComas of soft tissue and gynecologic origin. According to that report, all cases demonstrated expression of at least one of the melanocytic markers (HMB-45, Melan-A, and microphthalmia transcription factor), and HMB-45 showed the highest rate of positivity at 92%. They also showed positivity for myoid markers with SMA (80%) and desmin (36%).
Our case demonstrated positivity for both HMB-45 and Melan-A, and melanin pigments were observed in some cells. Among the myoid markers, our case was positive for SMA and negative for desmin and caldesmon. The existing leiomyoma was positive for desmin and caldesmon, so the PEComa was clearly defined from the large preexisting leiomyoma at the peripheral portion of the bulky mass.
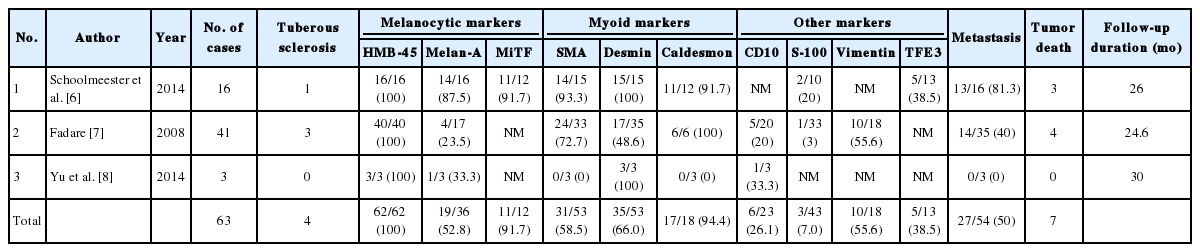
We reviewed all case series published in English available in PubMed that included more than three cases of PEComa in the female genital tract, for a total of 63 cases. The results (Table 1) [6-8] show that all (100%) of the tested cases showed positivity for HMB-45, and 52.8% were positive for Melan-A. Myoid markers including SMA, caldesmon, and desmin were positive in 74.5%, 66.0%, and 94.4% of tested cases, respectively. CD10 immunostaining was performed in 23 cases and showed positivity in six cases (26.1%). Five out of 13 cases (38.5%) were positive for TFE3. These results confirm the diagnostic value of immunohistochemistry profiling in uterine PEComa, especially the use of melanocytic and myoid markers, due to their high rates of positivity.
The differential diagnosis of uterine PEComa includes uterine smooth muscle tumor, endometrial stromal tumor, gastrointestinal stromal tumor (GIST) with secondary involvement of the uterus, and other sarcomas such as alveolar soft part sarcoma (ASPS). Paraganglioma, metastatic melanoma/clear cell sarcoma of soft part, and rarely, carcinoma should also be considered in the differential diagnosis due to their epithelioid cytomorphology. Immunohistochemical staining may be helpful in the differential diagnosis. HMB-45 positivity enables a differential diagnosis of PEComa from uterine smooth muscle tumor, endometrial stromal sarcoma, and ASPS. There was a report of late pulmonary and renal metastatic PEComas with initial misdiagnosis as uterine leiomyosarcomas [9]. CD10, which usually shows diffuse and strong immunoreactivity in endometrial stromal tumors, may be helpful in the differential diagnosis because 25% of uterine PEComas are reported to be positive for CD10 [7]. The exclusion of GIST from PEComa may be possible on the basis of CD34 staining, as well as c-Kit positivity and melanocytic marker negativity in GISTs. Metastatic melanoma and/or clear cell sarcoma can be distinguished from PEComa by the strong S-100 protein immunoreactivity of the former and their muscle marker negativity.
PEComas harboring TFE3 gene fusion are described in several reports [6,10,11]. TFE3 is a member of the MiT family of transcription factors, and TFE3 gene fusions have been found in some neoplasms such as ASPS and a subset group of renal cell carcinoma. Our case also showed strong TFE3 immunoreactivity. In PEComa, the group harboring TFE3 gene fusion is reported to be more common in young patients, to be unrelated to tuberous sclerosis, to show alveolar architecture, and to have more epithelioid than spindle cell cytology and minimal immunoreactivity for myocytic markers [11]. Because of the small number of cases, however, it is difficult to define these cases as a single distinct group showing specific clinical characteristics compared with conventional PEComas. More research and case studies are required.
There was a report of a concomitant PEComa and an endometrioid carcinoma with synchronous/metastatic bilateral ovary carcinomas and uterine leiomyoma [12]. The case had a finding common to ours in that there was a well differentiated endometrial carcinoma involving a depth of more than half of the myometrium with a synchronous separate PEComa. In that case, however, the pathologic examination revealed benign features of PEComa and it presented as a subserosal mass, unlike our case, which presented as a protruding endometrial mass and showed malignant features.
Criteria for malignancy of PEComa of the female genital tract are currently not clearly defined due to insufficient case studies. According to criteria that are currently accepted, PEComas are classified into three categories: benign, uncertain malignant potential, and malignant [5,13]. Benign is defined to exhibit the following gross or histologic features: gross size <5 cm, non-infiltrative growth, non-high-grade nuclear features, no necrosis or vascular invasion, and a mitotic rate <1/50 HPF. Tumors of uncertain malignant potential are defined as corresponding to one or more of the following features: nuclear pleomorphism or multinucleated giant cells, or gross size >5 cm regardless of cellular features. Satisfying the malignant category refers to cases which show two or more of the following features: gross size >5 cm, infiltrative growth, high-grade nuclear features, necrosis, vascular invasion, or a mitotic index ≥1/50 HPF. According to the largest single series of PEComas of gynecologic origin, the current classification system was very specific and sensitive for the diagnosis of malignant PEComa in the gynecologic tract [6]. The most common solid organ metastatic site according to the study was the lung. Multiple metastases were observed in the lung and liver in our case.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.