Clinicopathologic Significance of Survivin Expression in Relation to CD133 Expression in Surgically Resected Stage II or III Colorectal Cancer
Article information
Abstract
Background
Cancer stem cells have been investigated as new targets for colorectal cancer (CRC) treatment. We recently reported that CD133+ colon cancer cells showed chemoresistance to 5-fluorouracil through increased survivin expression and proposed the survivin inhibitor YM155 as an effective therapy for colon cancer in an in vitro study. Here, we investigate the relationship between survivin and CD133 expression in surgically resected CRC to identify whether the results obtained in our in vitro study are applicable to clinical samples.
Methods
We performed immunohistochemical staining for survivin and CD133 in surgically resected tissue from 187 stage II or III CRC patients. We also comparatively analyzed apoptosis according to survivin and CD133 expression using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling.
Results
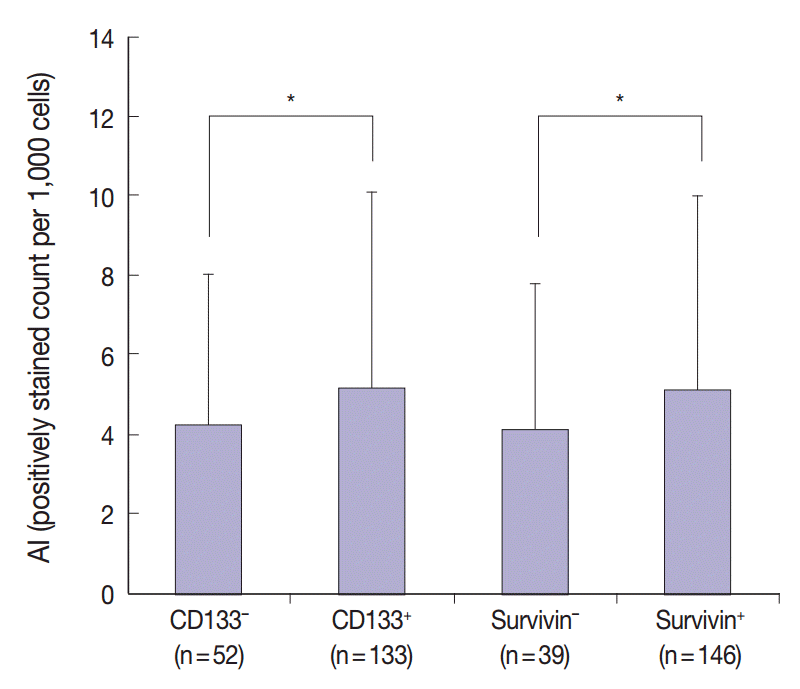
The results of the Mantel-Haenszel test established a linear association between nuclear survivin and CD133 expression (p = .018), although neither had prognostic significance, according to immunohistochemical expression level. No correlation was found between survivin expression and the following pathological parameters: invasion depth, lymph node metastasis, or histologic differentiation (p > .05). The mean apoptotic index in survivin+ and CD133+ tumors was higher than that in negative tumors: 5.116 ± 4.894 in survivin+ versus 4.103 ± 3.691 in survivin– (p = .044); 5.165 ± 4.961 in CD133+ versus 4.231 ± 3.812 in CD133– (p = .034).
Conclusions
As observed in our in vitro study, survivin expression is significantly related to CD133 expression. Survivin may be considered as a new therapeutic target for chemoresistant CRC.
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of cancer-related death worldwide [1]. Despite the availability of preventive measures, early screening tests, and improved surgical treatments and chemotherapy, the mortality rate remains high. The complexity of mechanisms involved in tumor recurrence, metastasis, and chemoresistance [2] has spurred cancer stem cell studies to seek novel and more effective targets for CRC treatment. In our in vitro study, we found that CD133+ cells showed high levels of survivin expression, which was related to chemoresistance to 5-fluorouracil (5-FU), and a survivin inhibitor was more effective than 5-FU in decreasing the viability of colon cancer cells [3,4].
CD133, a transmembrane glycoprotein, is a cancer stem cell marker in several human tumors [5], including CRC [6]. CD133 expression in CRC has been reported to be associated with Wnt [7,8], Notch [9], STAT3 [10], and transforming growth factor-beta signaling pathways [11], which are essential for self-renewal [2], tumorigenesis [2], progression [12], and chemoresistance in CRC [3]. However, the relationship of CD133 with survivin expression in clinical samples has not been evaluated.
Since tumor development and progression are based on the balance of cell apoptosis and proliferation, Alcaide et al. [13] described that a higher rate of apoptosis is associated with more aggressive behavior of tumors and poorer prognosis in CRC patients. Survivin, a member of the inhibitor of apoptosis family, plays a dual role in cell apoptosis and proliferation. As a subunit of the chromosomal passenger complex, nuclear survivin regulates the chromosomal central spindle in various cell division processes, whereas cytoplasmic survivin inhibits apoptosis. This suggests that survivin can be a good target for cancer therapy because it promotes cell survival [14]. In meta-analyses, survivin expression has shown a positive correlation with poorer prognosis in CRC patients [15,16]. However, a previous study has reported that survivin expression was associated with good prognosis [17]. Furthermore, most immunohistochemical studies of survivin did not clearly define the sublocation of survivin expression. Therefore, the clinical significance of survivin expression in CRC remains to be clarified.
The aim of the present study was to determine the clinicopathologic significance of survivin expression in relation to CD133 expression in CRC. In addition, we comparatively analyzed apoptosis according to survivin and CD133 expression in CRC.
MATERIALS AND METHODS
Patients and tissue samples
We used formalin-fixed and paraffin-embedded tissues from surgically resected stage II or III CRCs that were registered to the Department of Pathology at Wonju Severance Christian Hospital between January 2000 and December 2006. We analyzed the data from 187 patients with follow-up information. Patients who received preoperative chemotherapy or radiotherapy were excluded. Clinicopathological data of patient age, sex, tumor location, invasion depth, histologic differentiation, and lymph node metastasis were collected from pathology reports.
Ethics approval
This study was approved by the Institutional Ethics Committee of Yonsei University, Wonju College of Medicine (YWMR-14-4-102) and was carried out in compliance with the guidelines of the Declaration of Helsinki.
Immunohistochemical staining
Paraffin-embedded tissue sections from representative blocks were warmed to 75°C for 4 minutes. Slides were deparaffinized with EZPrep (Ventana Medical Systems, Tucson, AZ, USA), and an antigen-retrieval step was performed for 60 minutes with cell conditioning solution #1 for survivin staining and #2 for CD133 staining (Ventana Medical Systems). Then, endogenous peroxidase activity was blocked by applying an ultraviolet inhibitor for 4 minutes. After washing with the reaction buffer several times at room temperature (RT), an Ultra View Universal DAB Detection Kit (Ventana Medical Systems) was used for immunohistochemical (IHC) staining. The slides were incubated with monoclonal antibodies against survivin (Abcam, Cambridge, MA, USA) for 1 hour and against CD133/1 (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) for 2 hours at 37°C in an autostainer (Benchmark XT, Ventana Medical Systems). After incubating with the primary antibodies, the slides were rinsed with the reaction buffer. Drops of HRP UNIV MULT, DAB, and DAB H2O2 (Ventana Medical Systems) were applied sequentially on each slide (8 minutes per reagent), with intermittent rinsing with reaction buffer. The slides were then treated with a drop of COPPER for 4 minutes. Finally, a bluing agent was added and rinsed with reaction buffer. In addition, the sections were counterstained with hematoxylin for nuclei staining.
CD133 and survivin expression was manually counted in selected hot-spot fields.
IHC expression for CD133 was scored as 0 when there was no expression, 1+ when the expression for CD133 was detected in 1%–10% of the whole tumor area, and 2+ and 3+ when the expression was in 11%–50% and 51%–100% of the tumor area, respectively. We set a cut-off point at 10% between the positive and negative groups. Survivin expression was scored as 1+ (0%–25%), 2+ (26%–50%), 3+ (51%–75%), and 4+ (76%–100%) according to the level of nuclear reactivity among total tumor cells [18]. We set a cut-off point at 25% between the positive and negative groups. The IHC staining results were independently evaluated by two pathologists who were unaware of patient clinical and pathological information. Discrepancies between the pathologists were resolved by consensus.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling assay
All patient samples were stained using an In situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany) in formalin-fixed and paraffin-embedded sections. The slides were deparaffinized and rehydrated. After washing, the slides were incubated for 30 minutes at RT with 0.1 M Tris-HCl (pH 7.5), 3% bovine serum albumin, and 20% normal bovine serum for blocking. Then, the slides were washed twice with phosphate-buffered saline (PBS). The terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) reaction mixture (50 μL) was applied to each section for 1 hour at 37°C in a humidified chamber. After three washes with PBS, the slides were treated with 0.3% H2O2 in methanol for 10 minutes (RT). The blocking step was then repeated.
After washing with PBS, 50 mL Converter-POD was applied to each slide, and the slides were incubated for 30 minutes at 37°C in a humidified chamber. After three washes with PBS, 100 μL DAB substrate solution was added, and the slides were incubated for 1–3 minutes at RT. The slides were then washed thoroughly with tap water and counterstained with hematoxylin before mounting. Under light microscopy, we calculated the apoptotic index (AI) by counting the number of apoptotic nuclei per 1,000 cells in a high-power field. Cells were considered positive if the entire nuclear area of the cell was stained brown. Cells in areas with necrosis and the margins of sections were excluded from the analysis.
Statistical analysis
Data are displayed according to the properties of the variable. Continuous variables are presented as mean and standard deviation. Categorical variables are noted as frequency and percentage. The Mantel-Haenszel test was performed to evaluate the linear association between two variables on an ordinal scale. To compare the survivin IHC groups, we performed one-way analysis of variance or the chi-square test (Fisher exact test), as appropriate. The survival curve was estimated by the Kaplan-Meier method and was compared by the log-rank test. To estimate hazard ratio and 95% confidence interval, we used a Cox proportional hazard regression model. A p-value less than 0.05 was considered statistically significant, and all statistical analyses were performed with SAS ver. 9.2 (SAS Inc., Cary, NC, USA).
RESULTS
Relationships between survivin expression, clinicopathological parameters, and CD133 expression
The demography of cases examined in this study is presented in Table 1. Representative cases with high and low survivin expression are shown in Fig. 1A–D with matched CD133 expression. CD133 showed a luminal membranous expression pattern (Fig. 1A), whereas survivin showed distinct nuclear expression with weak and vague cytoplasmic expression in CRC cells (Fig. 1B). We analyzed only the nuclear survivin expression in this study because the cytoplasmic expression was too diffuse and weak to be scored.
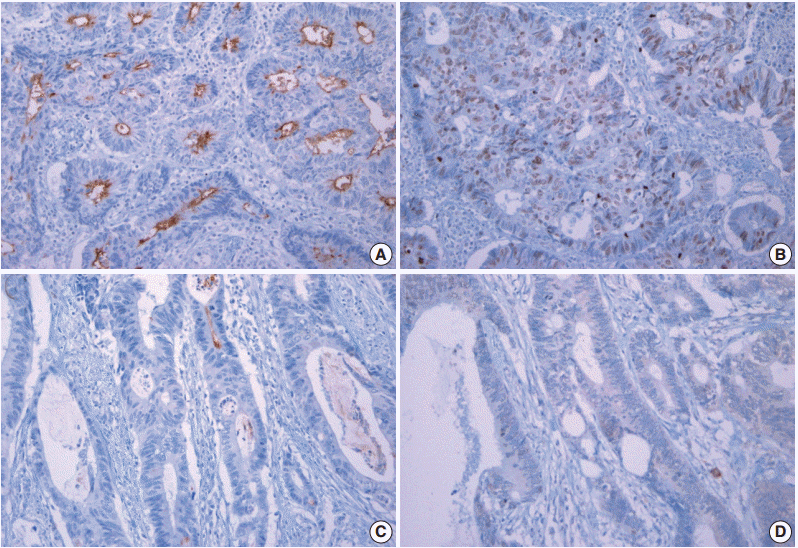
Photomicrographs showing representative cases of high CD133 (A) and survivin (B) expression and low CD133 (C) and survivin (D) expression in colorectal cancer tumor cells. CD133 expression localized to the luminal surface of the cytoplasm, and survivin expression localized to the nucleus.
Survivin expression showed a significant correlation with CD133 expression (p=.018) when analyzed by the Mantel-Haenszel test; however, survivin showed no significant relationship with other pathological parameters (Table 2).
Survival analysis according to survivin expression
The mean overall survival (OS) according to survivin expression was 113.1±9.1 months for score 1+, 47.0±1.7 months for score 2+, 41.8 ±1.4 months for score 3+, and 7.4 ±0.1 months for score 4+. However, survivin expression had no prognostic significance in the survival analysis (p=.480). Disease-free survival was also not related to survivin expression (p=.488).
Difference in apoptosis according to survivin and CD133 expression
Apoptosis detected by TUNEL analysis showed rare positive cells in normal mucosa (Fig. 2A), in contrast to occasionally scattered positive cells in CRC cells (Fig. 2B). AI was significantly higher in CD133+ tumors (5.165 ±4.961) than in CD133- tumors (4.231±3.812, p=.034). Further, compared with survivin- tumors, survivin+ tumors had a higher AI (5.116±4.894 vs 4.103±3.691, p=.044) (Fig. 3).
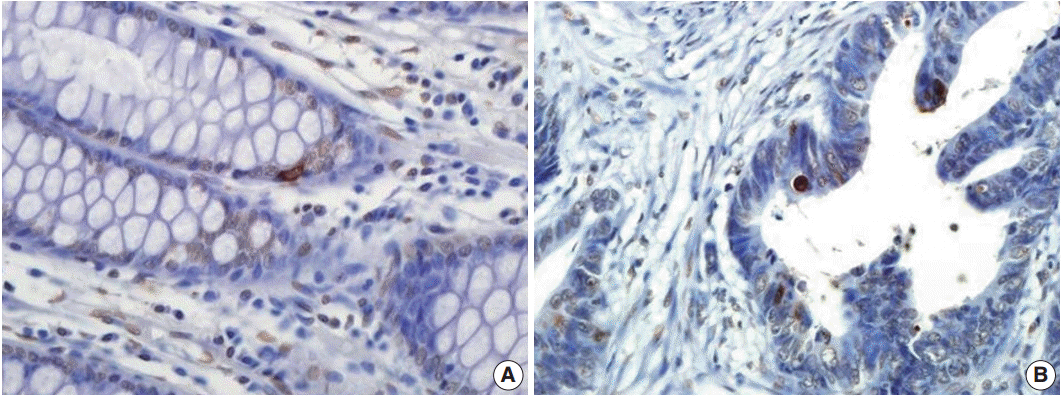
There was rare apoptosis in normal mucosa (A), but scattered positively-stained apoptotic cells in colorectal cancer (B) were observed by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling assay.
DISCUSSION
In our previous in vitro study, we described that CD133+ colon cancer cells showed chemoresistance to 5-FU through high survivin expression. In addition, we demonstrated that the survivin inhibitor YM155 was more effective than 5-FU in inducing cell death of colon cancer cells [4]. YM155 is a novel small-molecule survivin inhibitor that acts by inhibiting survivin promoter activity [19]. Inhibitors of apoptosis have been used as new targeted therapies in CRC [20]. Recently, Gyuraszova et al. [21] also reported that the combined use of YM155 with hypericin is effective in the treatment of CRC and lung adenocarcinoma through induction of apoptosis. To determine whether the results of our in vitro study are applicable to clinical samples, we examined the significance of survivin expression in relation to clinicopathological parameters and CD133 expression of CRC in the present study. We found a correlation between CD133 expression and nuclear survivin expression in CRC in a sample size of 187. It agrees with our previous in vitro study, which indicates that both the mRNA and protein expression levels of survivin in CD133+ cells are remarkably higher than those in small interfering RNA (siRNA)-induced CD133- cells [3], These findings are also concordant with those of the transcriptome analysis reported by Kim et al. [22].
In two recently published meta-analyses, the authors concluded that survivin expression is closely associated with high mortality [16,23]. However, these studies did not specify whether the positive correlation was observed with nuclear or cytoplasmic survivin expression. In most data used in these meta-analyses, the subcellular localization of survivin expression was unstated or cytoplasmic. As previously mentioned, nuclear survivin expression can improve cell proliferation by regulating chromosome alignment and segregation in mitosis, whereas cytoplasmic survivin expression inhibits the apoptosis pathway. Another recent report suggested that nuclear survivin expression is related to better patient prognosis [17]. However, this finding is contrasted with results reported by Kim et al. [22], who showed that nuclear survivin expression can be an independent poor prognostic factor for disease-free survival and OS. It remains controversial whether nuclear survivin expression is related to patient prognosis. Nuclear survivin expression has been reported to be significantly higher than cytoplasmic expression in CRC [24]. Therefore, further large-scale studies are required to confirm the prognostic significance of subcellular survivin expression in CRC.
In a previous study, we found that CD133 expression was related to survivin expression in CRC cell lines [3,4]. However, we found that survivin expression increased in Caco-2 cells after transfection with Ctrl-siRNA and CD133-siRNA. This may indicate that transfection affects survivin expression as a cell-damaging force. To avoid the bias induced by transfection, we analyzed survivin and CD133 expression together with apoptosis in clinical samples of CRC.
The balance between proliferation and apoptosis maintains the homeostasis of the colonic epithelium, but uncontrolled cell proliferation and apoptosis contribute to tumor development [18]. Alcaide et al. [13] reported that AI in CRC is higher than that in adenomas and normal tissue. We also found that AI in tumor tissue was higher than that in the surrounding normal mucosa. In addition, CD133+ and survivin+ tumors showed a higher mean AI than CD133- and survivin- tumors. However, AI was not significantly related with either survivin or CD133 expression in this study. Bedi et al. [25] reported that apoptosis is inhibited during CRC development, owing to abnormal expression of the BCL2 gene. Zhao et al. [26] reported that a low dose of bcl-2 inhibitor could up-regulate survivin expression in hepatocellular carcinoma. These results indicate that apoptosis is regulated by not only survivin, but also bcl-2. In addition, the effects of apoptotic proteins on CD133+ colon cancer cells have been rarely reported. In a study by Kemper et al. [27], activated caspase-9 was found to induce a high level of apoptosis in CD133+ CRC stem cells. Sam et al. [28] demonstrated that inhibiting survivin and caspase 3 triggers apoptosis in colon cancer stem-like cells.
In this study, we found that nuclear survivin expression was correlated with CD133 expression in CRC, although it was not significantly related to pathological parameters or patient prognosis. Further in vivo investigations of the effect of survivin inhibitors and the mechanism of survivin expression in relation to cancer stem cell markers are required for verifying whether survivin is a new target for more effective treatment of CRC.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a research grant from the Korea Science and Engineering Foundation funded by the Korean government (Ministry of Education, Science, and Technology, grant code: 2015-51-0329).