Duodenal Adenocarcinoma of Brunner Gland Origin: A Case Report
Article information
Abstract
We report a case of adenocarcinoma originating from the duodenal Brunner glands in a 47-year-old female patient. The lesion was 0.8 cm in extent and located at the posterior wall of the first part of the duodenum. Histologically, the tumor showed transition from non-neoplastic Brunner glands through dysplastic epithelium into adenocarcinoma. The carcinoma cells were strongly positive for MUC6 protein, which is an epithelial marker for the Brunner glands. Tumor protein p53 was overexpressed in the carcinoma cells, but not in the non-neoplastic or dysplastic epithelium. Dystrophic calcification was predominant. This is the first case report of duodenal adenocarcinoma of Brunner gland origin in Korea.
Proliferative Brunner gland lesions in the duodenum are commonly found as submucosal masses upon endoscopy [1], and most of the lesions are hyperplasia. Rarely found are adenomas or hamartomas. Adenocarcinoma arising from Brunner glands is very rare. Primary duodenal adenocarcinoma accounts for 0.3% of carcinomas of the gastrointestinal tract and among them, adenocarcinoma arising from Brunner glands constitutes only a minority [2]. Here, we present a case of adenocarcinoma arising from Brunner glands through dysplasia.
CASE REPORT
A 47-year-old female patient visited hospital for a national health screening program. Esophagogastroduodenoscopy revealed a 0.8-cm-sized depressed lesion at the posterior wall of the first part of the duodenum (Fig. 1A, B). Histologic analysis of the biopsy specimen revealed atypical glands forming cell clusters beneath the intact mucosal layer. The atypical clusters were arranged in tubular structures of columnar epithelium consisting of mucin-producing cells. Dystrophic calcification was scattered within the lesion, and foreign body reaction with giant cell formation was noted around the calcified materials (Fig. 1C, D). Immunohistochemistry (IHC) for CD56, chromogranin A and synaptophysin showed negative results, excluding the possibility of a neuroendocrine tumor, which is prevalent in this region. The histologic diagnosis was well-differentiated adenocarcinoma, and the patient underwent routine preoperative work-up. Physical examination, blood tests and abdominal computed tomography (CT) scan showed no abnormal findings. A minute duodenal lesion with submucosal invasion was suspected on endoscopic ultrasonography. Since the calcification was minute, it was not recognized by simple X-ray or by CT scan even upon retrospective review.
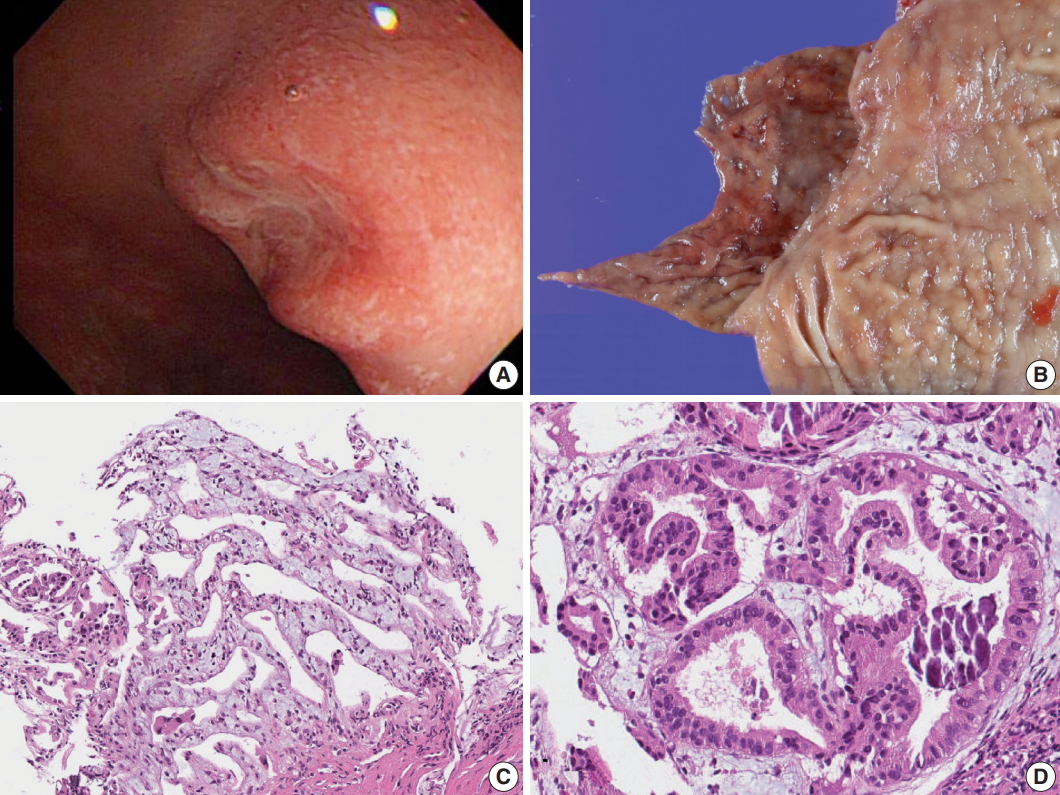
Gross and microscopic images of Brunner gland adenocarcinoma. (A) Endoscopy of the duodenal carcinoma showing a 0.8-cm lesion with central depression in the posterior wall of the first segment of duodenum. (B) Gross view. (C) Microscopic image of the endoscopic biopsy showing well-differentiated adenocarcinoma consisting of branching glands. (D) High-power view demonstrates psammoma-like structures of dystrophic calcification.
Endoscopic resection was not an option because it is usually difficult to remove a lesion by endoscopy at the posterior wall of the duodenum. The possibility of submucosal invasion was also considered; hence, the patient received distal gastrectomy. Since the lesion was located in the duodenal bulb, the duodenal stump was made near the pancreas and the distal resection margin was close to the lesion. D1+ lymph node dissection was performed.
On gross examination, the specimen consisted of 2.7 cm long duodenum and 6.5 cm long stomach. On duodenal mucosa, a 0.8 × 0.8-cm-sized superficial depressed lesion was found (Fig. 2A). The lesion was located at the posterior wall of the duodenum, 1.9 cm distal to the pyloric ring and 0.3 cm proximal to the distal resection margin. Pathologic examination revealed well-differentiated adenocarcinoma involving mucosal and submucosal layers. Around the carcinoma, dysplastic Brunner gland epithelium was noted. (Fig. 2B)
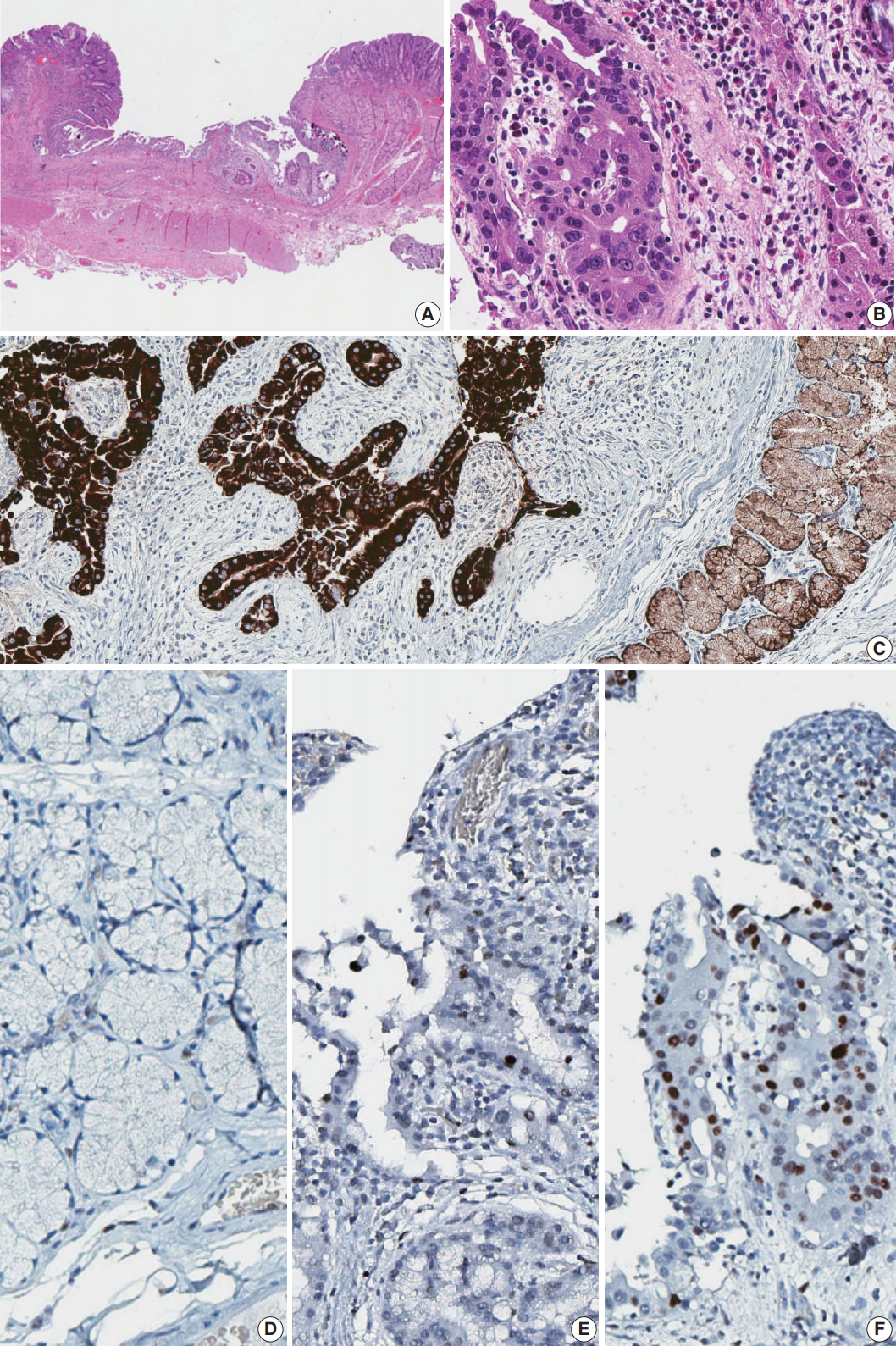
Histology and immunohistochemistry of the lesion. (A) Low power view of resected duodenal segment. (B) Higher power view of adenocarcinoma. (C) MUC6 stain showed diffuse positivity in adenocarcinoma as well as adjacent Brunner glands. (D) Negative p53 staining in normal Brunner glands. (E) Focal positive p53 staining in dysplasia. (F) Strong p53 staining in adenocarcinoma.
IHC was done for further evaluation. Gastric foveolar type mucin (MUC5AC) was focally positive and Brunner gland type mucin (MUC6) was diffusely positive (Fig. 2C). Carcinoembryonic antigen (CEA) was negative and p53 was positive in an increasing order of intensity from normal to adenoma-adenocarcinoma spectrum (Fig. 2D–F). A microsatellite instability (MSI) test was performed to investigate the involvement of mismatch repair failure during carcinogenesis. None of the five markers, which include BAT25, BAT26, D2S123, D5S346, and D17S250, showed instability.
This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-1706-098-860) and performed in accordance with the principles of the Declaration of Helsinki. Patient informed consent was waived.
DISCUSSION
Adenocarcinoma from Brunner gland origin is extremely rare, and this is the first case report in Korea. Among the 108 duodenal carcinomas collected from 22 institutes in South Korea, none was suspected to arise from Brunner glands [3]. Our case presents a unique Brunner gland lesion showing dysplasia and transformation into adenocarcinoma, suggesting the cell of origin to be Brunner glands. Strong positivity for the IHC of MUC6 in the dysplastic and carcinomatous epithelia also supports this speculation.
Additional ancillary tests were performed to evaluate the tumor characteristics. IHC for CEA was negative, but p53 protein was overexpressed in the tumor cells. Interestingly, p53 protein was completely negative in non-neoplastic Brunner glands and focally positive in dysplastic epithelium. The MSI test using five markers revealed the tumor to be microsatellite stable.
Since adenocarcinomas arising from Brunner glands are exceedingly rare, reported IHC results are diverse, though quite few, and there is no consensus as yet. Since 1994, 25 cases have been reported in Japan [2,4-7]. Some of them, including a recent case reported by Iwamuro et al. (2017) [7], were consistent with our case; positive for MUC6 in the carcinoma component of the tumor. However, Kamei et al. (2013) [2] reported negative staining of MUC6 and MUC5AC in the tumor cells. Another report also demonstrated loss of MUC5AC and MUC6 in the Brunner glands showing epithelial atypia [8]. The pathogenesis is unknown, but this discordance may be due to tumor cell heterogeneity, implying that some of the tumor cells may have lost their nature during progression.
There is no exclusive marker for the Brunner glands, and for this reason, the diagnosis of adenocarcinoma arising from Brunner glands depends on its distinct histology. The tumor can be treated by surgical resection, or a minimally invasive technique, such as endoscopic submucosal dissection, can be attempted if the tumor size is small and its depth shallow. Although located in the submucosal layer, its behavior is predicted to be the same as adenocarcinomas of mucosal origin, but more cases must be reported to build consensus on the prognosis and determine the standard treatment.
Here, we report a rare case of adenocarcinoma arising from Brunner glands of the duodenum. Despite the rarity of the tumor, the diagnosis was possible due to its histologic and immunohistochemical similarity with non-neoplastic Brunner glands and pre-neoplastic lesions.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.