Chronic Placental Inflammation as a Risk Factor of Severe Retinopathy of Prematurity
Article information
Abstract
Background
Chronic placental inflammation (CPI) has been implicated in the pathogenesis of diseases in premature infants, whereas retinopathy of prematurity (ROP) is a major complication primarily affecting preterm and very low-birth-weight (VLBW) infants. This study aims to investigate the association between CPI and ROP in VLBW infants.
Methods
We performed a retrospective review of clinical records of VLBW infants born between 2013 and 2016. Placental pathology findings including CPI cases were analyzed using logistic regression to study infants’ morbidities and other clinical characteristics.
Results
A total of 402 infants with a mean (standard deviation) gestational age of 28.5 (2.8) weeks and birth weight of 1,027.2 (304.4) g were included. The incidence of ROP was 24.1%. CPI was found in 90 infants (22.4%), among which 28.9% (26 of 90) developed ROP, and 21.1% (19 of 90) underwent laser photocoagulation. Lower gestational age, lower birth weight, longer duration of oxygen supply, and presence of CPI were associated with the development of ROP. After adjustment for gestational age, birth weight, sex, duration of oxygen supply, and other overlapping placental pathology, CPI was associated with the odds for type 1 ROP that required laser photocoagulation (adjusted odds ratio, 2.739; 95% confidence interval, 1.112 to 6.749; p = .029).
Conclusions
CPI was associated with severe ROP requiring treatment with laser photocoagulation in VLBW infants.
Preterm birth is associated with an increased risk of perinatal mortality and long-term morbidity [1]. The pathological findings of the placenta often provide valuable information regarding the clinical outcomes of preterm infants because the changes in the placenta mirror intrauterine environments affecting the fetus [2,3]. Acute and chronic placental inflammations are common pathology of preterm birth and are significantly associated with preterm birth and neonatal morbidity [1-4]. While acute placental inflammation (API) (acute chorioamnionitis and acute funisitis) is caused by the infiltration of neutrophils into the placental compartments, chronic placental inflammation (CPI) (chronic chorioamnionitis, chronic villitis, and chronic deciduitis) is characterized by the infiltration of lymphocytes, plasma cells, and/or macrophages [5,6]. API accompanies elevation of proinflammatory cytokines such as interleukin (IL)-6 and IL-8 in the amniotic fluid and fetal plasma. On the other hand, CPI is associated with elevation of antiangiogenic T-cell chemokines CXCL9, CXCL10, and CXCL11 in the amniotic fluid and fetal plasma [6]. Therefore, it is very plausible that API and CPI affect the systemic biology of preterm newborns.
Illnesses associated with prematurity include respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), bronchopulmonary dysplasia (BPD), and retinopathy of prematurity (ROP). The prevalence of ROP has increased because of progressive improvement in the survival of preterm infants. The overall incidence of ROP has been reported to be 66% for any severity of ROP, 18% for moderately severe ROP, and 6% for severe ROP in preterm infants born weighing ≤ 1,250 g [7]. Lower gestational age (GA), lower birth weight, and longer oxygen supply in the postnatal period are known major risk factors for the development of ROP [8]. Recent studies have suggested an association between chorioamnionitis and ROP [8,9]. They reported that the incidence of ROP has increased more in infants born to mothers with histologic chorioamnionitis than in infants born to mothers without chorioamnionitis [1,8,9]. An exposure of the immature retina to changing levels of oxygen leading to dysregulated expression of retinal growth factors such as vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF) seems to play a major role in the development of ROP [9]. Maternal systemic inflammation, as in acute histologic chorioamnionitis, affects the development of ROP by decreasing levels of IGF-1 [9,10], which causes abnormal vascularization of the retina. In view of the relationship between acute chorioamnionitis and ROP, we postulated that CPI representing maternal antifetal rejection with derangement in antiangiogenic chemokines can be associated with the development of ROP. This study was conducted to determine if there is a relationship between CPI and ROP in very low-birth-weight (VLBW) infants who were born less than 1,500 g.
MATERIALS AND METHODS
Study population
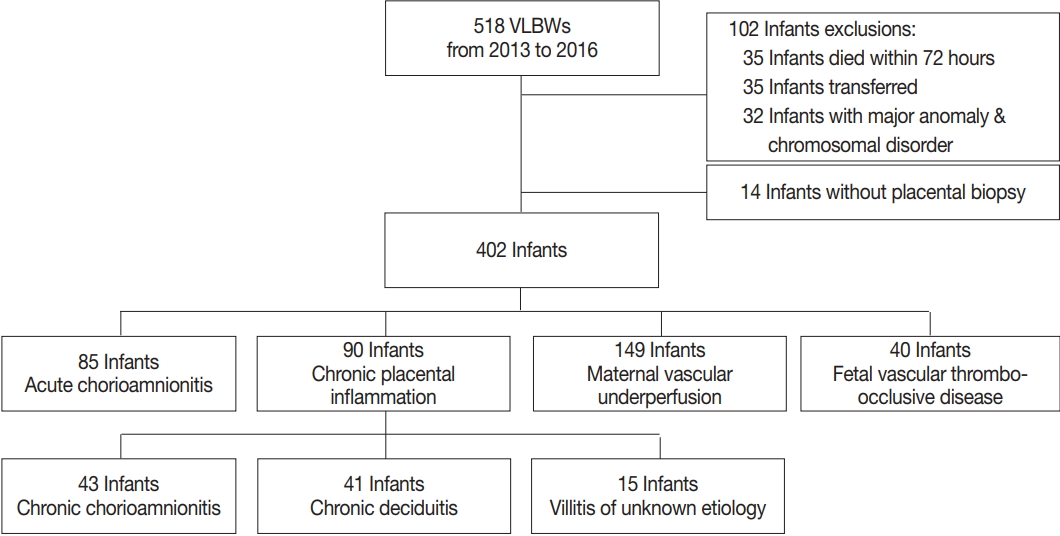
This was a retrospective cohort study of VLBW infants born at Asan Medical Center Children’s Hospital, a tertiary academic center, and admitted to neonatal intensive care unit between January 2013 and December 2016. Infants with major congenital malformations or chromosomal abnormalities who died within 72 hours after birth, transferred to other hospitals, or did not have placental biopsies were excluded from analysis (Fig. 1).
Study design
Perinatal and neonatal clinicopathologic data on each infant including placental biopsies and presence of ROP were retrieved from the electronic medical records. The Institutional Review Board of Asan Medical Center (2017-1058) approved the collection and use of the clinical information for research purposes before the investigation was started and waived the requirement for informed consent.
Placental pathology
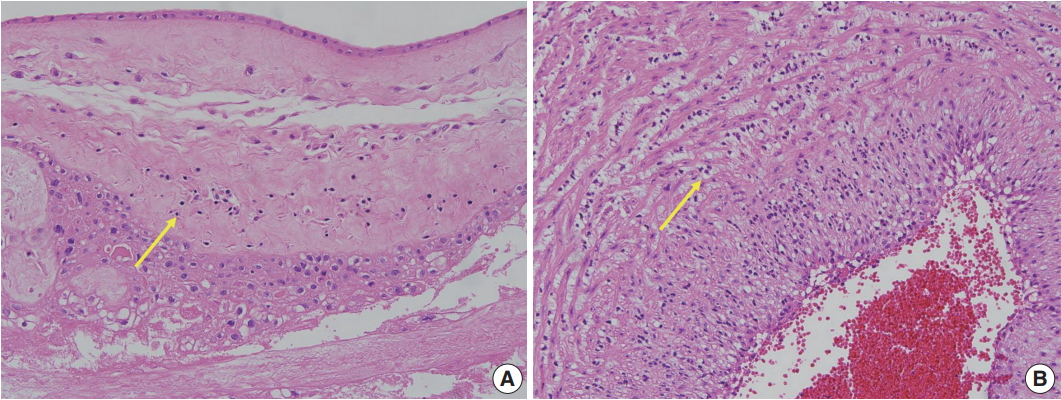
Placental examinations were routinely performed after delivery of VLBW infants. Hematoxylin and eosin–stained sections of the amnion, choriodecidua, umbilical cord, and chorionic plate were examined by pathologists at the same hospital. Histopathological screening of the placenta was performed according to the diagnostic criteria recommend by the Perinatal Section of the Society for Pediatric Pathology [11]. The placental pathological findings were grouped into four categories: amniotic fluid infection/inflammation (ACA), maternal vascular underperfusion (MVU), fetal vascular thrombo-occlusive disease (FVTOD), and CPI. The subtypes of CPI included chronic chorioamnionitis (CCA), chronic deciduitis, and villitis of unknown etiology (VUE) [12,13].
Examination and treatment of retinopathy of prematurity
We had been performing routine eye examinations of neonates whose GA was < 32 weeks or whose birth weight was < 1,800 g. Eye examinations were performed at postnatal day 28 or at 32 weeks in postmenstrual age by an ophthalmologist. Performance of follow-up eye examinations depended on the disease severity. Diagnoses of ROP were based on the international classification [14]. Laser treatment was performed for eyes reaching type 1 ROP. Type 1 ROP was divided into zone I, any stage ROP with plus disease; zone I, stage 3 ROP without plus disease; and zone II, stage 2 or 3 ROP with plus disease [15]. We also included severe ROP as type 1 ROP that required laser photocoagulation.
Clinical data on neonates
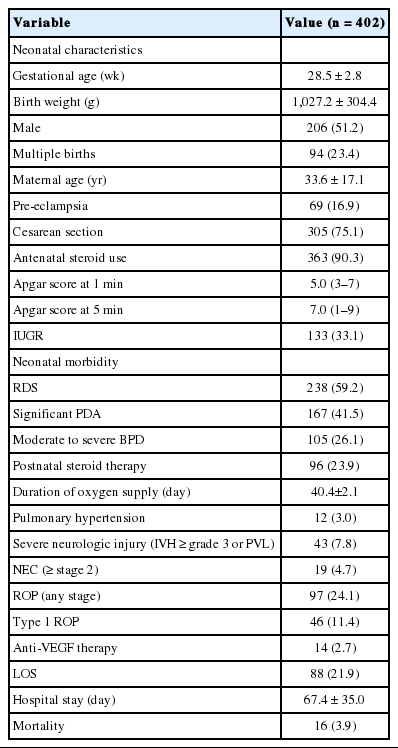
Demographic characteristics such as GA, birth weight, sex, history of multiple births, incidence of maternal preeclampsia, type of delivery, antenatal steroid use, Apgar scores at 1 and 5 minutes, presence of major morbidities such as RDS, patent ductus arteriosus (PDA), BPD, pulmonary arterial hypertension (PAH), significant neurological injury (SNI), necrotizing enterocolitis (NEC), and late-onset sepsis (LOS), duration of hospital stay, and mortality were obtained for each subject. Significant PDA was defined as a condition requiring pharmacological and/or surgical treatment for the mitigation of hemodynamic disturbance. PAH was diagnosed based on the use of medication according to echocardiographic results. SNI included IVH severer than grade III or periventricular leukomalacia. LOS was defined as bacterial infection confirmed by a positive blood or cerebrospinal fluid culture after postnatal day 7.
Statistical analysis
Statistical analysis was performed using SPSS ver. 23.0 (IBM Corp, Armonk, NY, USA). Correlations between CPI and clinical outcomes were determined by performing the chi-square test or Fisher exact test for categorical data and the t test for continuous data. Logistic regression analysis was performed to estimate the independent effects of the main explanatory variables of interest. Statistical significance was defined as p < .05.
RESULTS
Clinical characteristics of the study population
A total of 518 VLBW infants were eligible for the study. Of these 518 infants, 35 infants who died within 72 hours, 35 infants who were transferred to other hospitals, 32 infants with major anomalies and chromosomal disorders, and 14 infants without placental biopsies were excluded from analysis (Fig. 1). A total of 402 VLBW infants were included in the analysis. The mean (standard deviation) GA, birth weight, and percentage of males were 28.5 (2.8) weeks, 1,027.2 (304.4) g, and 51.2%, respectively. The mean maternal age was 33.6 (17.1) years. The incidence of preeclampsia and intrauterine growth retardation (IUGR) were 16.9% and 33.1%, respectively. Regarding neonatal morbidity, the incidence of RDS, significant PDA, moderate to severe BPD, PAH, SNI, NEC ≥ stage 2, and LOS was 59.2%, 41.5%, 26.1%, 3.0%, 7.8%, 4.7%, and 21.9%, respectively. The incidence of any stage ROP and type 1 ROP requiring laser photocoagulation was 24.1% and 11.4%, respectively (Table 1). The mean duration of hospital stay of the infants was 67.4 (35.0) days, and the mortality rate was 3.9%.
Placental pathology
Of the cases included in this study, 122 did not show any placental pathological finding. Among the remaining 280 cases, 85 cases showed findings consistent with ACA, 90 cases showed CPI, 149 cases showed MVU, and 40 cases showed FVTOD (Fig. 1). Two or more placental pathological findings were superimposed in 82 cases, and one placental pathological finding was superimposed in 198 cases (Fig. 2). Of the 90 cases with CPI, 43 were with CCA, 41 were with chronic deciduitis, 15 were with VUE, and 16 showed overlapping patterns (Fig. 1).
Association of CPI and retinopathy of prematurity
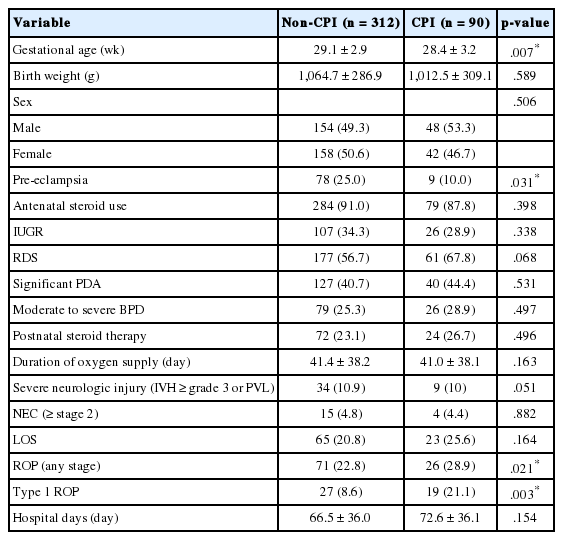
Of the 90 cases with CPI, 28.9% (26 of 90) developed any stage ROP, and 21.1% (19 of 90) underwent laser photocoagulation (Table 2). The infants with CPI showed a significantly lower GA (p = .007) and had a higher incidence of any stage ROP and history of laser photocoagulation treatment than the infants without CPI did (p = .021 and p = .003). Other characteristics were not significantly different between the two groups (Table 2).
Lower GA, lower birth weight, severe BPD, and longer duration of oxygenation treatment were associated with greater increases in the incidence of type 1 ROP. According to the pathological findings of the placenta, CPI and ACA statistically correlated with any stage ROP (odds ratio [OR], 2.050; 95% confidence interval [CI], 1.170 to 3.592; p = .038 and OR, 2.079; 95% CI, 1.177 to 3.671; p = .012) and type 1 ROP for laser photocoagulation (OR, 2.825; 95% CI, 1.487 to 5.367; p = .002 and OR, 1.977; 95% CI, 1.012 to 3.862; p = .046) in univariate analysis (Table 3).
Table 4 compares the placental pathological findings in terms of their effect on any stage ROP and type 1 ROP. Multiple logistic regression analysis adjusted for GA, birth weight, sex, incidence of preeclampsia and IUGR, duration of oxygen supply, and overlapping placental pathology was performed. CPI was found to be independently associated with type 1 ROP (adjusted OR, 2.739; 95% CI, 1.112 to 6.749; p = .029). ACA was significantly associated with type 1 ROP in the univariate analysis, but not with type 1 ROP after adjustment analysis. Any stage ROP was not significantly associated with any placental finding after adjustment analysis.
DISCUSSION
Many investigators have studied the relationship between placental lesions in VLBW infants and clinical complications because examinations frequently provide information valuable for the management of neonates [2,4-6]. For example, the presence of acute chorioamnionitis and funisitis indicates in utero fetal systemic inflammation as a consequence of ACA [16]. In this study, we showed a significant correlation between CPI and severe ROP that required laser photocoagulation (type 1 ROP) in VLBW infants for the first time.
A prominent cause of visual impairment and blindness in children, ROP is a developmental perturbation of the retina [9,17,18]. The structural characteristics of ROP include the initial cessation of retinal vascularization and subsequent proliferation of retinal vessels [17,19]. ROP is caused by impaired autoregulation of the retinal blood vessels and sudden postnatal oxygen tension. Retinal neovascularization in ROP cases involves angiogenic factors such as VEGF in preterm infants and VLBW infants [20-22]. Recent studies have reported that lower GA, lower birth weight, and excessive oxygen exposure are major risk factors for ROP [20-23]. Other studies demonstrated that both antenatal and neonatal exposures to inflammation are consistently associated with an increased risk of ROP as well as cerebral white matter damage and BPD in preterm infants [9,24,25]. The formation of new blood vessels from existing vessels, angiogenesis, is affected not only by normal physiological growth, but also by pathological events such as inflammation [24,26]. Inflammation is a host defense mechanism serving to protect tissues from infection and injury, and studies have shown that proinflammatory cytokines such as tumor necrosis factor α and IL-1 function as proangiogenic factors [24,27-29].
Although oxygen supply is far better managed by using noninvasive ventilators today, ROP remains a major issue because survival rates of infants with extremely low GA and birth weight and immature retinas at high risk of ROP have substantially increased. The lack of in utero angiogenic factors such as VEGF, IGF-1, and erythropoietin significantly increases the risk of ROP [20,30]. Omega long-chain polyunsaturated fatty acids, which are provided by maternal and fetal interaction, are also important for retinal development [30-32]. Therefore, the risk of ROP may increase in cases of intrauterine insufficiency of angiogenic factors in infants with lower GA and birth weight.
It has been recently proposed that idiopathic CPI is a feature of maternal antifetal rejection mimicking allograft rejection [12]. The placenta and fetus are semiallografts to the mother, and fetal (paternal) human leukocyte antigen-specific antibodies are detected in the sera of subsets of pregnant women [6,12]. CPI is characterized by the infiltration of maternal CD8+ T cells into fetal placental tissues and associated with the overexpression of C-X-C–motif chemokines such as CXCL9, CXCL10, and CXCL11 in placental tissues [6,12]. Also a marker of CPI coupled with maternal-fetal rejection [33,34], elevation of CXCL10 level has been shown to occur in the event of rejection of allografts such as the liver and kidneys [35].
The findings in this study suggest that CPI may be at least partially responsible for the increased incidence of severe ROP that required laser photocoagulation after adjustment for GA, birth weight, sex, duration of oxygen supply, and other overlapping placental pathologies. We did not adjust for BPD because the condition is a confounding variable overlapping with the duration of oxygen supply. The association between CPI and severe ROP suggests that maternal-fetal rejection associated with increased fetal plasma antiangiogenic CXCL10 level contributes to abnormal angiogenesis of the retina in VLBW infants. A relationship between the subtypes of ROP and the intrauterine environment that triggers preterm birth also has been proposed [36]. Likewise, acute chorioamnionitis may increase the risk of ROP by directly sensitizing the developing retina to oxygen-induced changes in VEGF availability and subsequent vascular development or by causing systemic hypotension resulting in retinal hypoperfusion or ischemia [8,9,25].
Decreased production of placental growth factors (PGF), 45- to 50-kDa dimeric glycoproteins with 53% sequence homology with VEGF, plays a key role in placental angiogenesis [37-40]. PGF may have therapeutic potential in cases of disorders associated with problems of angiogenesis such as cancer, retinopathy, and certain inflammatory disorders [40]. This is another piece of evidence indicating the importance of placenta-derived growth factors for developmental angiogenesis. Therefore, it is plausible that CPI-mediating antiangiogenic effects lead to deregulated angiogenesis of ROP in VLBW infants.
There are some limitations of our study. First, it was a retrospective study of a relatively small number of inborn infants at a single hospital; thus, the findings might not extrapolate to a larger population. Second, multiple pathological diagnoses were given in some cases, which can interfere with the strength of the association. Third, in the association between CPI and type 1 ROP, we had a wide confidence interval (1.112–6.749) because many factors may play a contributory role in ROP development. Despite these limitations, this study demonstrates an association between CPI and severe ROP in VLBW infants. Future studies that examine the direct causality of CPI in the development of retinal damage and ROP are needed.
In conclusion, the pathological findings of the placenta are important in predicting the outcomes of preterm infants and VLBW infants. This study shows for the first time that CPI may affect the development of type 1 ROP requiring treatment of laser photocoagulation in VLBW infants.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.