Abrupt Dyskeratotic and Squamoid Cells in Poorly Differentiated Carcinoma: Case Study of Two Thoracic NUT Midline Carcinomas with Cytohistologic Correlation
Article information
Abstract
Cytologic diagnosis of nuclear protein in testis (NUT) midline carcinoma (NMC) is important due to its aggressive behavior and miserable prognosis. Early diagnosis of NMC can facilitate proper management, and here we report two rare cases of thoracic NMC with cytohistologic correlation. In aspiration cytology, the tumor presented with mixed cohesive clusters and dispersed single cells, diffuse background necrosis and many neutrophils. Most of the tumor cells had scanty cytoplasm and medium-sized irregular nuclei, which had fine to granular nuclear chromatin. Interestingly, a few dyskeratotic cells or squamoid cell clusters were present in each case. Biopsy specimen histology revealed more frequent squamous differentiation, and additional immunohistochemistry tests showed nuclear expression of NUT. Because this tumor has a notorious progression and has been previously underestimated in terms of its prevalence, awareness of characteristic findings and proper ancillary tests should be considered in all suspicious cases.
Nuclear protein in testis (NUT) midline carcinoma (NMC) is one of the arduous differential diagnoses with histologic features of poorly differentiated or undifferentiated carcinoma [1,2]. Fluorescence in situ hybridization (FISH) and a specific antibody to identify gene rearrangement allow rapid diagnosis of NMC [3]. However, delays in diagnosis without any suspicion of NMC can affect proper management and survival due to the extremely aggressive behavior and dismal prognosis for this cancer [1]. Previous studies of cytologic findings in NMC are limited because of its rarity; herein, we present two interesting cases of NMC arising in the lung with cytohistologic findings. The Institutional Review Board (IRB) of Samsung Medical Center approved this case report (IRB file No. 2017-09-050) and informed consent was waived.
CASE REPORT
Case 1
A 45-year-old male presented with a 3-month history of cough. Chest X-ray and computed tomography (CT) showed a 3.8-cm-sized mass in the right lower lobe and multiple enlarged mediastinal lymph nodes (Fig. 1A–C) in which 18F-fluorodeoxyglucose uptake was detected (Fig. 1D). For diagnosis and nodal staging, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and biopsy were performed. Aspirates from both lymph nodes and lung mass were highly cellular. Some tumor cells were cohesive enough to form 3-dimensional clusters, but others were dispersed in single cells (Fig. 1E). Frequent crush artifacts and naked nuclei were easily identified. Tumor cells were poorly to undifferentiated with scanty cytoplasm and irregular nuclear contour. Nuclear chromatin was fine to granular and nucleoli were small but occasionally identified (Fig. 1F). Unexpectedly, a few scattered dyskeratotic cells were noticed (Fig. 1E, F). Many neutrophils and necrosis were present in the background (Fig. 1F). Biopsy of mediastinal lymph nodes showed sheets of metastatic undifferentiated tumor cells with large area of necrosis and peritumoral neutrophilic infiltration (Fig. 1G). Tumor cells had scant and delicate amphophilic cytoplasm. Nuclear chromatin and contour were similar to the findings of aspirate smears. Foci of abrupt squamous differentiation were identified more often than in cytology (Fig. 1H). Under additional immunohistochemistry (IHC) staining, these tumor cells showed a very high proliferation index of up to 70% in Ki-67 (1:200, Dako, Glostrup, Denmark) and were positive for pan-cytokeratin (1:500, Dako), p53 (1:4,000, Thermo Fisher, Rockford, IL, USA), p63 (1:200, Biocare, Concord, CA, USA), and NUT (1:100, C52B1, Cell Signaling Technology, Danvers, MA, USA), and up to 70% of tumor cells expressed programmed death-ligand 1 (PD-L1; RTU, 22C3, Dako) (Fig. 1S, T). Tumor cells were negative for Oct3/4 (1:50, Santa Cruz Biotechnology, Dallas, TX, USA), chromogranin (1:400, Dako), CD99 (1:50, Dako), CD45 (1:1000, Dako), CD56 (1:200, Leica Biosystems, Novocastra, Newcastle upon Tyne, UK), and thyroid transcription factor 1 (TTF-1; 1:50, Dako), which can help rule out the possibilities of other malignancies.
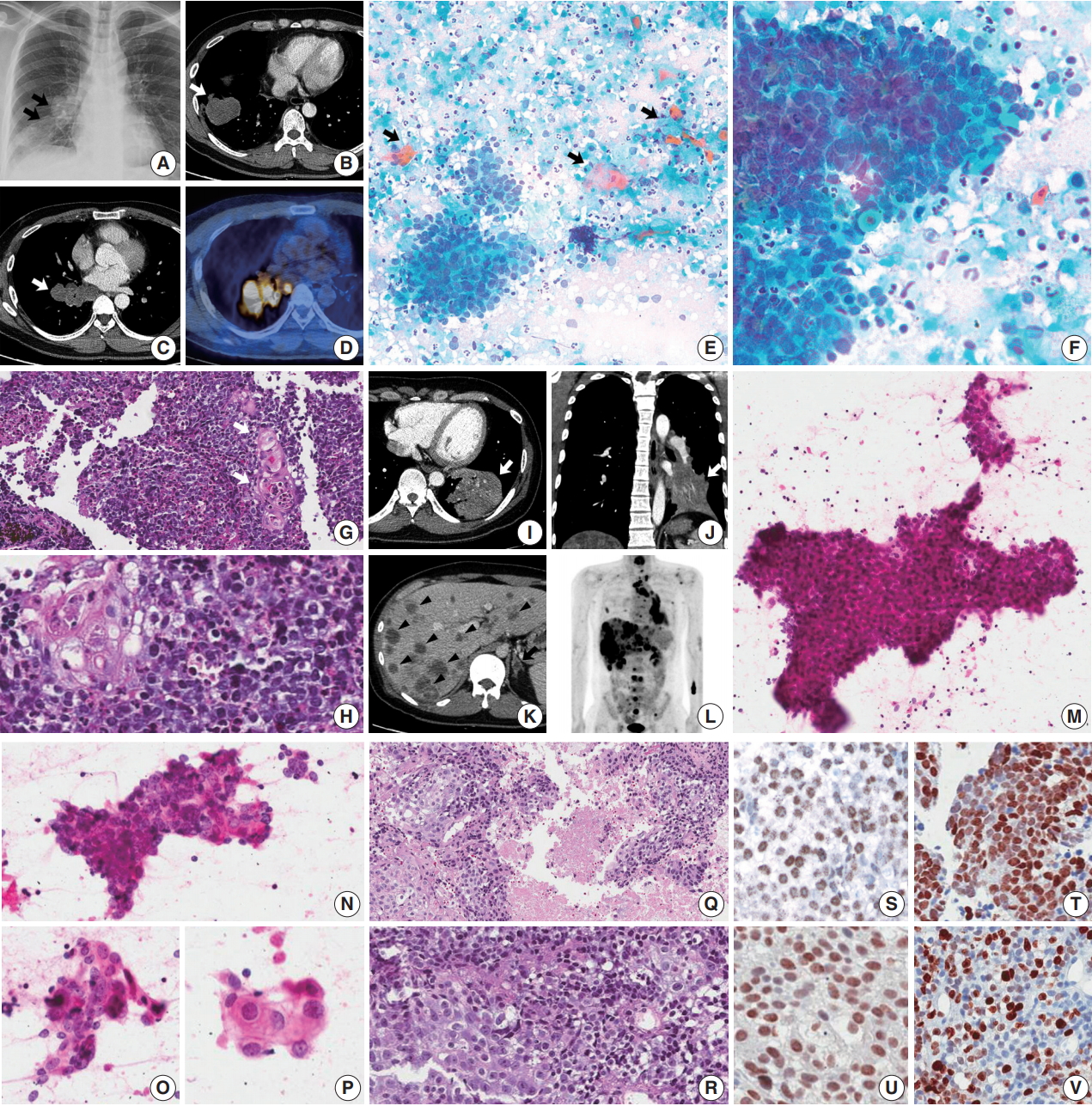
Thoracic nuclear protein in testis (NUT) midline carcinoma and cytohistologic findings. (A–H) Case 1. (A–D) Chest radiograph and computed tomography (CT) shows 3.8-cm-sized mass in the right lower lobe with multiple enlarged lymph nodes (arrows). Positron emission tomography (PET) reveals 18F-fluorodeoxyglucose uptake of the mass and lymph nodes. (E) Mediastinal lymph node aspiration smears are highly cellular with cohesive clusters and dispersed single cells. Scattered dyskeratotic cells and squamous differentiations are noticed (arrows). (F) Tumor cells have scanty cytoplasm, nuclear molding, irregular nuclear contours, and fine to granular nuclear chromatin. Nucleoli are small but occasionally identified. A few dyskeratotic cells are also identified. Background of the smear shows many neutrophils and necrosis. (G, H) Biopsy of a mediastinal lymph node shows monotonous tumor cells and hyperchromatic nuclei. Nuclei have fine to granular chromatin and the occasional small nucleolus, and the cytoplasm is scant and delicately amphophilic. Foci of squamous differentiation are more often identified than in cytology. (I–R) Case 2. (I–L) Chest and abdomen CT show a 5.5-cm-sized mass (white arrow) in the left lower lobe, and multiple metastases in both a lobe of the liver (arrowheads) and the adrenal glands (black arrow). PET highlights multiple hypermetabolic lesions in the whole spine, ribs, pelvic bone and scapulae, in addition to the lung and liver masses. (M–P) In aspiration cytology of a mediastinal lymph node, some tumor cells are medium-sized and poorly differentiated with scanty cytoplasm and hyperchromatic nuclei. However, others have more abundant amphophilic to eosinophilic cytoplasm and a lower nuclear to cytoplasmic ratio, which can be considered squamous differentiation. (Q, R) Histology of a mediastinal lymph node shows two different tumor cell components with extensive necrosis, poorly differentiated cells and squamous cells, which are similar to the findings of aspiration cytology. (S–V) Immunohistochemistry shows nuclear expression of NUT in case 1 (S) and case 2 (U). Tumor cells are positive for p63 (T, case 1) and there is a variable proliferation index of Ki-67 (V, case 2).
Case 2
A previously healthy 32-year-old male presented with a persistent cough for 3 months, blood-tinged sputum and chest pain. On chest CT, a 5.5-cm-sized consolidative lung mass accompanied by enlarged mediastinal lymph nodes was identified at the left lower lobe (Fig. 1I, J). Abdomen-pelvis CT showed multiple necrotic masses scattered throughout both lobes of the liver and bilateral adrenal glands (Fig. 1K), and positron emission tomography highlighted multiple hypermetabolic bony lesions in the whole spine, bilateral ribs, pelvic bones, sternum and both scapulae (Fig. 1L). Cytology of lymph node aspirates from EBUS-TBNA showed dispersed and clustered tumor cells in the background of necrosis (Fig. 1M). Some tumor cells had large vesicular nuclei with open chromatin, prominent nucleoli, scanty cytoplasm and a high nuclear to cytoplasmic (N/C) ratio. Naked nuclei were frequently identified in scattered individual cells. However, others exhibited more abundant amphophilic to eosinophilic cytoplasm and granular nuclear chromatin with a lower N/C ratio, which could be considered squamous differentiation (Fig. 1N–P). Biopsy of the mediastinal lymph nodes and liver showed metastatic carcinoma in a necrotic background composed of two morphologically different tumor cell components: poorly differentiated cells with hyperchromatic nuclei and scanty cytoplasm, and squamoid cells with less hyperchromatic nuclei, prominent nucleoli and ample cytoplasm (Fig. 1Q, R). By IHC, tumor cells were positive for p63, p53, and NUT, negative for TTF-1 and CD56, and had a relatively low Ki-67 proliferation index of approximately 25% (Fig. 1U, V). PD-L1 IHC test revealed low expression of about 1% of the tumor.
DISCUSSION
Diagnosis of NMC in cytology is challenging. Cytologic findings are characterized by high cellularity with medium-sized poorly to undifferentiated cells. Cohesiveness of these tumor cells is variable [2] and while clusters of tumor cells are usually patternless, some can be arranged in a pseudoglandular pattern in focal areas [4]. Round to oval nuclei have irregular contours, but their size is relatively uniform and monotonous rather than pleomorphic. Nuclear chromatin is not coarse or condensed but fine to granular and vesicular. Nucleoli are usually single, small and prominent [5]. Scant cytoplasm is amphophilic to eosinophilic and nuclear molding is frequent. The absence of cytoplasm makes nuclei naked and squeezing artifacts are common, especially in smears rather than liquid-based preparations. Mitosis is frequent, but atypical mitosis is rare. Necrotic debris, apoptotic bodies and nuclear dust with many neutrophils fill the background of the tumor cells. Previous reports have described difficulty in finding squamous differentiation in cytology specimens [2,5], but tumor cell clusters with a squamoid appearance or a few dyskeratotic cells were easily noticed in our cases. This finding can be variable, from subtle to very distinct with obvious keratinization.
The cytogenetic rearrangement of this tumor is unique and characteristic. Two-thirds of such tumors have chromosomal translocation t(15;19), which forms the BRD4-NUT fusion oncogene, and subsets have BRD3-NUT and other NUT-variant rearrangement [1]. In recent studies, knockdown of BRD-NUT induces rapid squamous differentiation with a halt in proliferation in patient-derived NMC cells [6,7]. This result strongly suggests the role of BRD-NUT as a fusion protein that blocks differentiation, and is compatible with its characteristic cytologic and histologic findings. The tumor histology is usually made of sheets of undifferentiated cells. Nuclear size and shape, chromatin pattern, nucleoli and cytoplasm are similar to the cytology findings. Frequent areas of necrosis are common, and diffuse infiltrations of neutrophils among tumor cells are easily identified. Despite the simple and uniform chromosomal abnormality, some tumor cells might be out of influence from the cytogenetic alteration. Accordingly, there may be focal areas of abrupt squamous differentiation that are more commonly identified than in cytology specimens.
Differential diagnosis of NMC in cytology includes small cell carcinoma (SCC), basaloid squamous cell carcinoma (BSqCC), and small cell variant of squamous cell carcinoma (SCVSqCC). The cytology of SCC shows predominantly single cells, but has some cell clustering with nuclear molding exhibiting fine, open chromatin, and was inconspicuous to small nucleoli [8,9]. Scant cytoplasm, artifactually crushed and naked nuclei, and background necrosis are common, and all of these findings mimic NMC. However, keratinized squamous cells are rare, even if some aspirate smears have bronchial epithelial cells with squamous metaplasia.
BSqCC and SCVSqCC are more problematic diagnoses because both have small cells with some dyskeratosis and squamous differentiation. The cytology of BSqCC has small to medium-sized nuclei with fine chromatin, small to inconspicuous nucleoli, scant cytoplasm and nuclear molding with necrosis [9]. SCVSqCC shows similar small cells but slightly more abundant, bluish cytoplasm, with vesicular nuclei and prominent nucleoli [10]. Both basaloid and SCVSqCC have areas of necrosis, squamous differentiation and dyskeratosis, which is often noticed in NMC but usually composed of predominantly cohesive cell clusters rather than dispersed single cells [9]. It is important to note that all these tumors have similar cytologic findings and aggressive behaviors but require different management.
In IHC, NMC tumor cells are positive for pan-cytokeratin, p53, p63 and NUT, and usually have a high but variable proliferation index by Ki-67. It is hard to differentiate NMC from possible mimickers without diagnostic markers, which are not routinely screened. If the cytomorphology is suspicious for NMC, a FISH assay and IHC test should be performed in all cases due to the underestimation of its prevalence [1]. A previous study examined NUT IHC expression using a monoclonal antibody in 114 cases of poorly differentiated carcinoma or unclassified malignancy in the mediastinum, and 3.5% of cases showed nuclear expression with a median age of 50 [11]. Further studies are warranted in other anatomic sites, regardless of age.
Currently, conventional cytotoxic chemotherapy and radiation therapy are not effective in treating NMC. Unlike other usual carcinomas, this tumor has a typical chromosomal alteration and genetic stability. Novel targeted therapy using histone deacetylase inhibitors and BET inhibitors has been developed lately based on the idea that BRD-NUT fusion proteins specifically bind to acetylated histones [1]. These drugs are now in several clinical trials (ClinicalTrials.gov identifiers: NCT01587703, NCT01987362, NCT02711137, and NCT02307240). Not only doctors, but also patients, continue to struggle with NMC at this moment. To treat this disease properly, all pathologists should pay attention to this underestimated entity.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
Special thanks to Dr. Yoon Kyung Jeon, Department of Pathology of Seoul National University Hospital, for providing immunohistochemistry testing with anti-NUT antibody.
