High Cytoplasmic CXCR4 Expression Predicts Prolonged Survival in Triple-Negative Breast Cancer Patients Treated with Adjuvant Chemotherapy
Article information
Abstract
Background
Chemokine receptor CXC chemokine receptor type 4 (CXCR4) and its ligand CXC motif chemokine 12 (CXCL12; stromal cell-derived factor-1) are implicated in tumor growth, metastasis, and tumor cell-microenvironment interaction. A number of studies have reported that increased CXCR4 expression is associated with worse prognosis in triple-negative breast cancer (TNBC), but its prognostic significance has not been studied in TNBC patients treated with adjuvant chemotherapy.
Methods
Two hundred eighty-three TNBC patients who received adjuvant chemotherapy were retrospectively analyzed. Tissue microarray was constructed from formalin-fixed, paraffin-embedded tumor tissue and immunohistochemistry for CXCR4 and CXCL12 was performed. Expression of each marker was compared with clinicopathologic characteristics and outcome.
Results
High cytoplasmic CXCR4 expression was associated with younger age (p = .008), higher histologic grade (p = .007) and lower pathologic stage (p = .045), while high CXCL12 expression was related to larger tumor size (p = .045), positive lymph node metastasis (p = .005), and higher pathologic stage (p = .017). The patients with high cytoplasmic CXCR4 experienced lower distant recurrence (p = .006) and better recurrence-free survival (RFS) (log-rank p = .020) after adjuvant chemotherapy. Cytoplasmic CXCR4 expression remained an independent factor of distant recurrence (p = .019) and RFS (p = .038) after multivariate analysis.
Conclusions
High cytoplasmic CXCR4 expression was associated with lower distant recurrence and better RFS in TNBC patients treated with adjuvant chemotherapy. This is the first study to correlate high CXCR4 expression to better TNBC prognosis, and the underlying mechanism needs to be elucidated in further studies.
Triple-negative breast cancer (TNBC) refers to the breast cancer subtype which does not express estrogen receptor (ER), progesterone receptor (PR), and lacks overexpression of human epidermal growth factor receptor 2 (HER2). It comprises 10%–20% of all breast cancer cases and is associated with aggressive behavior and poor prognosis [1]. TNBC is generally considered an individual subtype of breast cancer, but it is also a highly heterogeneous disease which consists of various subgroups of tumors with different molecular, histologic, and clinical characteristics [2]. Advances in endocrine therapy and HER2-targeted therapy have greatly improved the survival of the patients with hormone receptor–positive and HER2-positive tumors, but TNBC patients still suffer from absence of specific treatment target. Systemic chemotherapy continues to be the mainstay of TNBC treatment, and there is an urgent need for novel biomarkers which can be used to predict prognosis, identify patients who will benefit from therapy, and provide potential treatment target [2].
CXC chemokine receptor type 4 (CXCR4) is a member of G protein-coupled receptors which is bound by its only ligand CXC motif chemokine 12 (CXCL12), also known as stromal cell-derived factor-1 [3]. It is physiologically involved in embryonic development, leukocyte trafficking and homing of hematopoietic cells to bone marrow [4-6]. In tumor biology, the CXCR4/CXCL12 axis is known to promote proliferation of tumor cells, direct metastasis by attracting CXCR4-positive tumor cells to CXCL12-rich organs, and mediate the interaction between the tumor cell and their microenvironment [7-9]. CXCR4 is expressed in different cancer types, and its overexpression and association with distant metastasis and unfavorable prognosis have been reported in breast cancer [3,9-11]. In addition, targeting of CXCR4 significantly reduced both primary and metastatic breast cancer in the mouse model, suggesting that CXCR4/CXCL12 axis may be a promising therapeutic target in breast cancer treatment [12,13].
A number of studies have reported the negative prognostic significance of CXCR4 expression in TNBC, but adjuvant treatment information is not clearly documented in most of these reports, leaving the possibility of confounding [14-16]. Moreover, although systemic chemotherapy is an essential element of TNBC treatment, the prognostic significance of CXCR4 has not been studied in TNBC patients treated with adjuvant chemotherapy. Therefore, we aimed to evaluate the expression of CXCR4 in TNBC tumor tissue, compare it with clinicopathologic parameters, and investigate its relationship with the outcome of the patients who received adjuvant chemotherapy. Since the expression of CXCL12 has not been well-addressed in TNBC, we planned to evaluate its expression as well.
MATERIALS AND METHODS
Patients and tissue samples
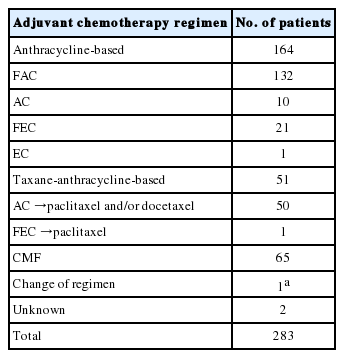
The study group consisted of primary unilateral TNBC patients who underwent surgical resection in Seoul National University Hospital between December 2000 and December 2006 and received adjuvant chemotherapy. The cases with available formalin-fixed, paraffin-embedded (FFPE) tissue for tissue microarray (TMA) were retrospectively collected. The patients who had distant metastasis at initial diagnosis, received neoadjuvant chemotherapy, underwent surgical resection for bilateral breast cancer, or had a history of ipsilateral or contralateral breast cancer were excluded from the study. Immunohistochemistry (IHC) for ER, PR, and HER2 was routinely performed on resection specimen at the time of diagnosis, and the IHC slides were reviewed. According to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines, ER and PR negativity was defined as nuclear staining in < 1% of tumor cells in IHC, and HER2 was considered negative if a tumor was scored 0 or 1+ in HER2 IHC or 2+ with a negative HER2 fluorescence in situ hybridization result [17,18]. Among the 313 primary TNBC patients identified, 283 patients (90.4%) received adjuvant chemotherapy. The patients were treated with standard chemotherapy and the regimens were classified into three categories: anthracycline-based regimen, taxane-anthracycline-based regimen and CMF (cyclophosphamide, methotrexate, and 5-fluorouracil). Use of taxane-anthracycline-based regimen was limited to node-positive tumors due to insurance constraints. More detailed information on chemotherapy regimen is shown in Table 1.
Clinicopathologic characteristics, treatment details, and follow-up data were retrieved from medical records and the original pathology report. Histologic grade was scored according to the Nottingham grading system and the pathologic stage was determined based on the American Joint Committee on Cancer staging system, seventh edition [19]. Follow-up and survival data were collected until the end of 2014. The date of recurrence, death, and last follow-up were obtained from medical records, and recurrence-free survival (RFS) and overall survival (OS) were assessed according to STEEP criteria [20]. Recurrence was diagnosed either pathologically or radiologically and was classified as locoregional or distant. This study was approved by the Institutional Review Board of Seoul National University Hospital with a waiver of informed consent (IRB No. 1512-076-728).
TMA construction and IHC
Hematoxylin and eosin–stained slides of each tumor were reviewed and the representative area was marked. Cylindrical tissue core with a diameter of 2 mm was extracted from the corresponding area of the FFPE tumor block and transferred into recipient paraffin block (SuperBioChips Laboratories, Seoul, Korea). Each TMA block included a maximum of 59 cores. TMA blocks were sectioned at the 3-μm thickness and IHC for CXCR4 and CXCL12 was performed with automated staining system (Bench-Mark XT, Ventana Medical Systems, Tucson, AZ, USA) following the manufacturer’s protocols. TMA sections were first deparaffinized, and antigen retrieval was done using cell conditioning solution (CC1, Ventana Medical Systems). Then sections were incubated with primary rabbit polyclonal anti-CXCR4 (1:50, ab2074, Abcam, Cambridge, UK) and mouse monoclonal anti-CXCL12 (1:10, MAB350, R&D Systems, Minneapolis, MN, USA) antibodies. The positive antigen-antibody reaction was visualized using diaminobenzidine detection kit (OptiView DAB, Ventana Medical Systems), and counterstaining was performed with hematoxylin and bluing reagent.
The IHC slides were examined blindly without knowledge of clinicopathologic information, and the expression of CXCR4 and CXCL12 was assessed by IHC using a semiquantitative scoring system. The staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong), and percentage of positively stained cells were scored as 0 (0%), 1 (1%–25%), 2 (26%–50%), 3 (51%–75%), and 4 (> 75%). The final score was calculated by multiplying the intensity and percentage scores, ranging from 0 to 12. The patients were divided into high or low expression groups using the median score of each marker as a cutoff point.
Statistical analysis
Differences in clinicopathologic variables and outcomes between high and low expression groups were compared using the chi-square test, or Fischer exact test when applicable. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazards model and logistic regression analysis were used to evaluate the prognostic significance of each variable in the univariate and multivariate analysis. Variables with a p-value of < .20 in univariate analysis were included in multivariate analysis, and forward conditional method was used to select the significant variables. All statistical analyses were performed using SPSS Statistics software ver. 22.0 (IBM Corp., Armonk, NY, USA), and a p-value of < .05 was considered statistically significant.
RESULTS
Expression of CXCR4 and CXCL12 in TNBC tissues
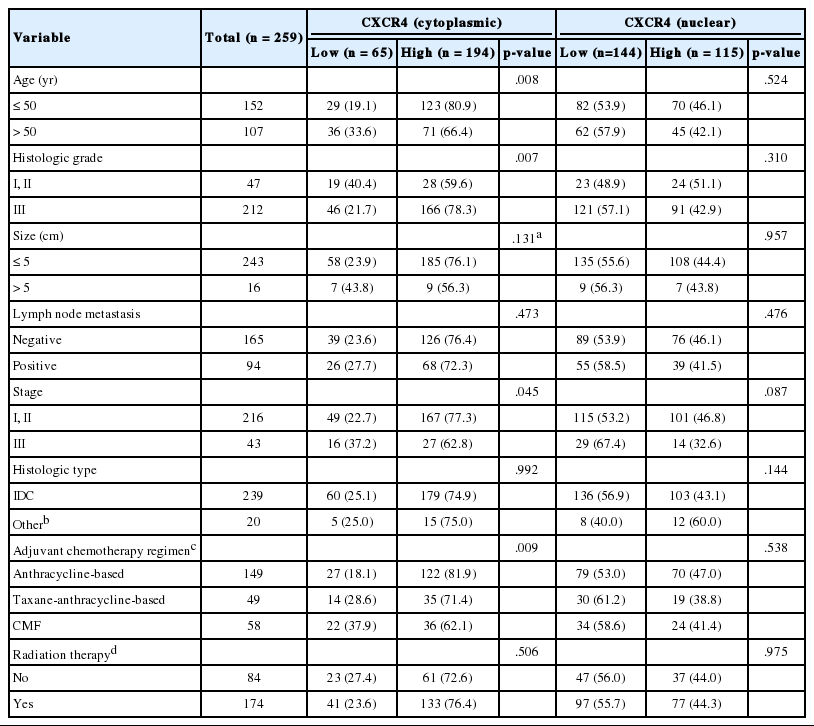
IHC for CXCR4 and CXCL12 was performed on TMA section, and due to core loss and noninformative cores with no invasive carcinoma, expression of CXCR4 and CXCL12 were evaluable in 259 (91.5%) and 238 (84.1%) cases, respectively. Immunostaining for CXCR4 was observed in tumor cells, stromal cells, and inflammatory cells, but staining was most prominent in the cytoplasm and nucleus of tumor cells (Fig. 1A–F). Staining for CXCL12 was mainly observed in tumor cell cytoplasm (Fig. 1G–I). Expression of each marker was scored in tumor cells, and cytoplasmic and nuclear expression were separately assessed for CXCR4. Based on the median IHC score, high cytoplasmic CXCR4, high nuclear CXCR4, and high CXCL12 expression were defined as IHC score of > 7, > 6 and > 2, and the tumors were classified into high cytoplasmic CXCR4, high nuclear CXCR4, and high CXCL12 groups in 194 (74.9%), 115 (44.4%), and 115 (48.3%) cases, respectively.
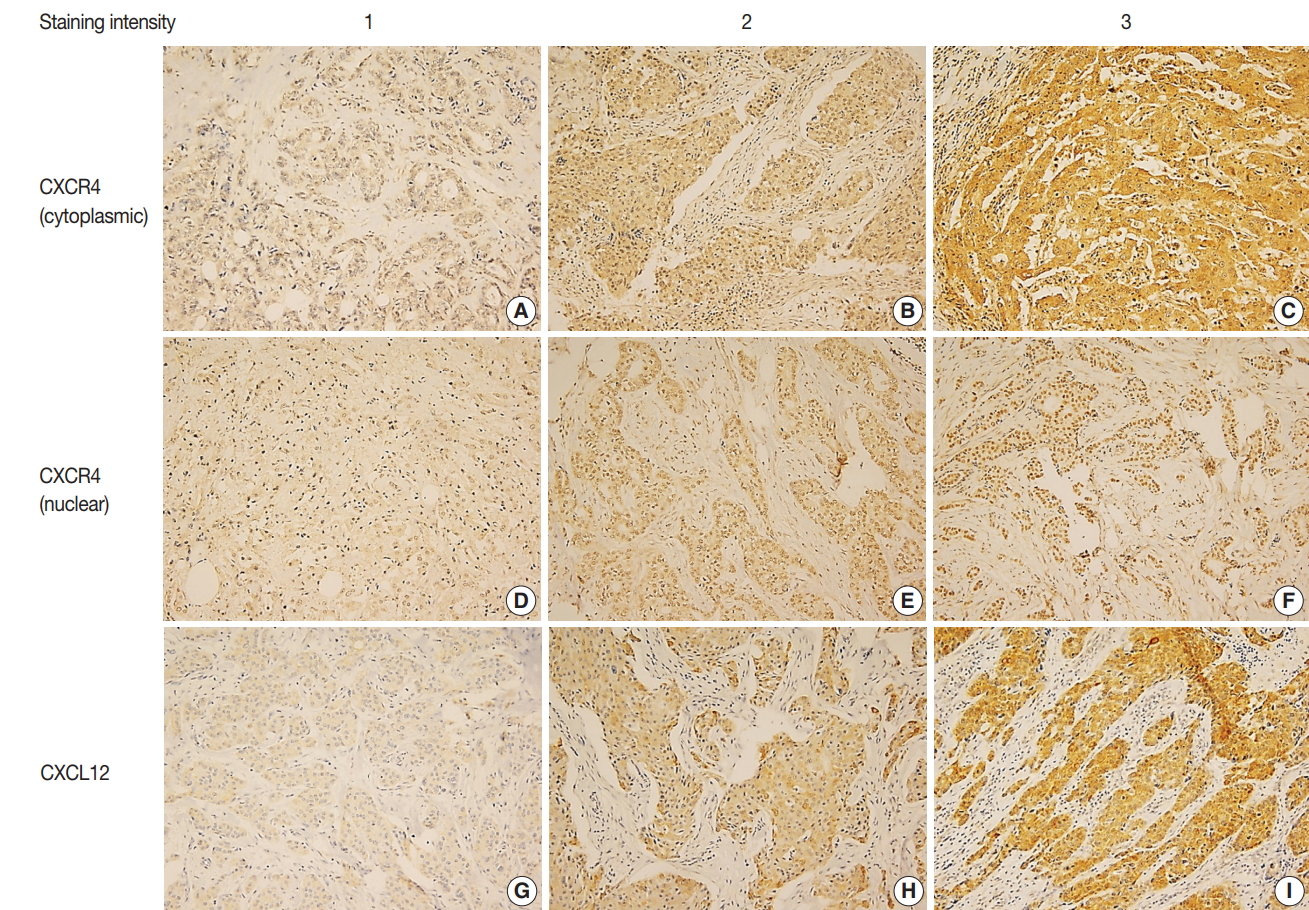
Immunohistochemistry for CXC chemokine receptor type 4 (CXCR4) and CXC motif chemokine 12 (CXCL12). Representative immunohistochemistry images of cytoplasmic CXCR4 (A–C), nuclear CXCR4 (D–F), and CXCL12 (G–I) in order of staining intensity. CXCR4 and CXCL12 expression are mainly observed in tumor cells. CXCR4 shows cytoplasmic and nuclear staining, and CXCL12 shows cytoplasmic staining.
Clinicopathologic characteristics and expression of CXCR4 and CXCL12
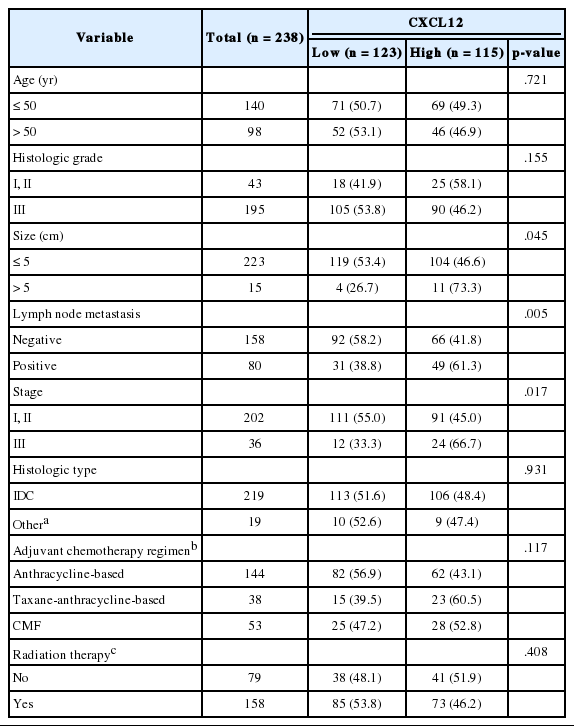
Correlations between clinicopathologic characteristics and expression of each marker are shown in Tables 2 and 3. All patients were female and the median age at surgery was 48 years (range, 21 to 71 years). High cytoplasmic CXCR4 expression was significantly associated with younger age (p = .008), higher histologic grade (p = .007), and lower pathologic stage (p = .045), but it was not related to tumor size or lymph node metastasis. On the other hand, high CXCL12 expression showed a significant correlation with larger tumor size (p = .045), positive lymph node metastasis (p = .005), and higher pathologic stage (p = .017). Nuclear CXCR4 expression was not associated with any of the clinicopathologic parameters studied. There was a significant difference in adjuvant chemotherapy regimen between high and low cytoplasmic CXCR4 groups (p = .009), but otherwise, no significant difference in adjuvant chemotherapy regimen or radiation therapy was observed between groups in other markers.
Clinical outcome and pattern of recurrence after adjuvant chemotherapy
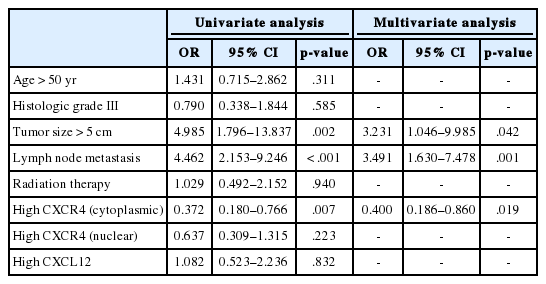
The median follow-up time was 100 months (range, 1 to 141 months). During the follow-up period, the tumor recurred in 53 patients (18.7%), and 17 patients (6.0%) died. Locoregional and distant recurrence occurred in 18 (6.4%) and 37 (13.1%) patients, respectively. Distant recurrence was less frequent in the high cytoplasmic CXCR4 group (p = .006), whereas nuclear CXCR4 and CXCL12 showed no significant association with any pattern of recurrence (Fig. 2A, B). Univariate logistic regression analysis revealed that high cytoplasmic CXCR4 expression was associated with lower distant recurrence (p = .007). After multivariate analysis, high cytoplasmic CXCR4 expression remained an independent variable for lower distant recurrence (p = .019) along with smaller tumor size (p = .042) and negative lymph node metastasis (p = .001) (Table 4).
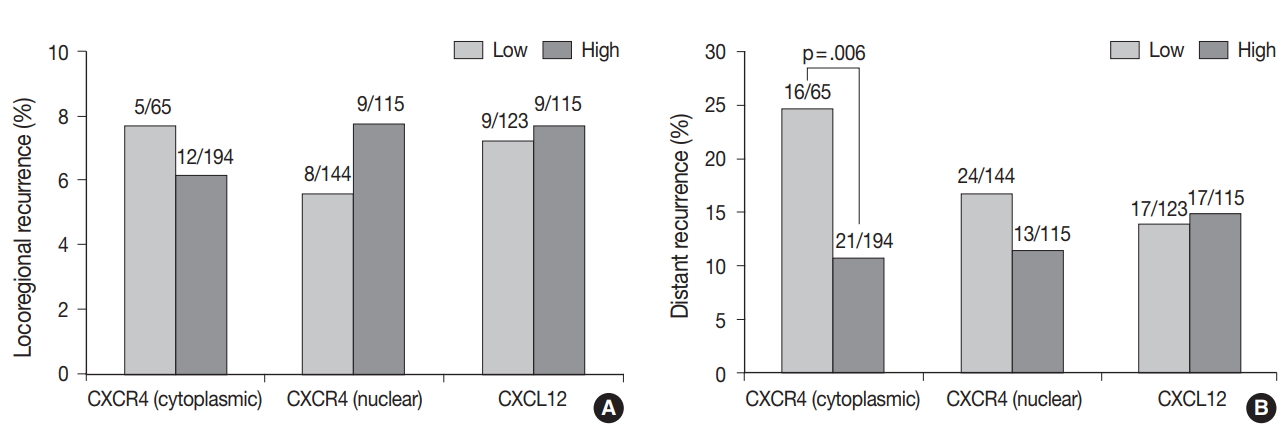
The pattern of recurrence after adjuvant chemotherapy according to CXC chemokine receptor type 4 (CXCR4) and CXC motif chemokine 12 (CXCL12) expression. CXCR4 and CXCL12 expression do not show a significant association with locoregional recurrence (A), while high cytoplasmic CXCR4 expression is significantly associated with lower distant recurrence (B). Figures above each bar refer to the number of recurrences/the number of patients in each group.
RFS and OS after adjuvant chemotherapy
Kaplan-Meier curves for RFS and OS was plotted according to the expression of each marker. A significant difference in RFS was observed between high and low cytoplasmic CXCR4 groups (log-rank p = .020), but the difference was not significant in OS (log-rank p =. 076) (Fig. 3A, B). The 5-year RFS in high and low cytoplasmic CXCR4 groups were 86.0% and 75.1%, respectively. The 5-year OS in high and low cytoplasmic CXCR4 groups were 96.0% and 90.7%, respectively. No significant difference in survival was observed between groups in nuclear CXCR4 (logrank p = .637 for RFS, p = .121 for OS) and CXCL12 (log-rank p = .521 for RFS, p = .538 for OS). In univariate Cox regression analysis, high cytoplasmic CXCR4 expression was associated with better RFS (p = .022). Multivariate analysis revealed that cytoplasmic CXCR4 expression (p = .038) and lymph node metastasis (p < .001) were independent factors of RFS (Table 5).
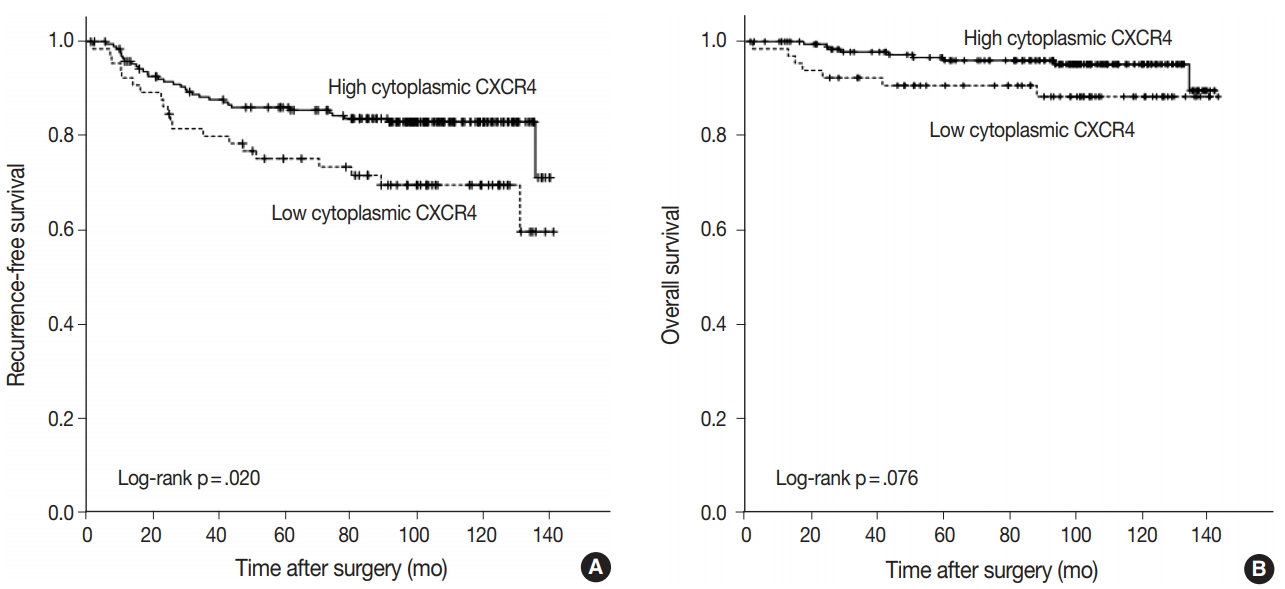
Recurrence-free and overall survival after adjuvant chemotherapy according to cytoplasmic CXC chemokine receptor type 4 (CXCR4) expression. Recurrence-free survival is significantly better in the high cytoplasmic CXCR4 group (A), but the difference in overall survival is not significant between high and low cytoplasmic CXCR4 groups (B).
DISCUSSION
Expression of CXCR4 is reported in various types of tumors, and its ligand CXCL12 is expressed widely in tumor and normal tissues by cancer cells, stromal cells, endothelial cells, and immune cells [3,21]. Binding of CXCL12 to CXCR4 activates multiple signaling pathways promoting tumor growth and metastasis, and CXCR4/CXCL12 axis has a role in tumor cell-microenvironment interaction [21]. In the present study, we evaluated the expression of CXCR4 and CXCL12 on TMA constructed from TNBC tissue using the immunohistochemical method. Immunoreactivity for CXCR4 was observed primarily in the cytoplasm and nucleus of tumor cells, and CXCL12 was stained mostly in tumor cell cytoplasm. While CXCR4 is a membrane-bound G protein–coupled receptor, it is rapidly internalized by binding of its ligand CXCL12, and cytoplasmic and nuclear expression of CXCR4 was previously demonstrated in breast cancer by IHC [22-24]. Our study also revealed that high cytoplasmic CXCR4 expression was associated with higher histologic grade, and this finding was consistent with previous studies regarding breast cancer and TNBC [15,16,23-25]. With respect to CXCL12 expression, Kobayashi et al. [26] previously demonstrated that cytoplasmic-dominant CXCL12 immunoreactivity is associated with higher CXCL12 mRNA level in resected breast cancer.
Currently, there are a number of studies which evaluated the expression of CXCL12 in breast cancer. High CXCL12 expression correlated with better survival in most of these studies, but discrepant report exists [26-29]. Ours is the first study to evaluate the expression of CXCL12 in TNBC subtype and showed that high CXCL12 expression was associated with known negative prognostic markers such as large tumor size, positive lymph node metastasis and higher stage in TNBC, although no significant difference in survival was observed between high and low CXCL12 groups. Loss of CXCL12 has been reported to have a role in distant metastasis of tumor cells, but overexpression of CXCL12 correlates with increased invasiveness, higher tumor grade and stage in several human cancers [30]. The discordant result between the present and previous studies seems to suggest the different role of CXCL12 in TNBC subtype, and the different proportion of breast cancer subtypes in study population might have resulted in the discrepant results between previous studies.
In the evaluation of outcome and survival, the present study showed better survival compared with previous reports which studied the expression of CXCR4 in TNBC [14-16]. TNBC is known to be more sensitive to chemotherapy than other breast cancer subtypes, and since the study group was restricted to the patients who received adjuvant chemotherapy, it is likely that the effect of chemotherapy has contributed to the better survival observed in our study [2]. The patient group with lower pathologic stage might have affected the survival as well. Our study also revealed that increased cytoplasmic expression of CXCR4 was associated with a better prognosis in TNBC patients treated with adjuvant chemotherapy in terms of lower distant recurrence and better RFS. However, previous studies have reported high CXCR4 expression as a poor prognostic marker of TNBC, and antitumor effect of CXCR4 inhibitors has been studied on breast cancer and shown efficacies in preclinical studies [12-16]. On the other hand, Lefort et al. [31] recently reported that CXCR4 inhibitors may not benefit TNBC patients and could even be detrimental in the study using patient-derived xenograft (PDX) model. In previous studies which evaluated the expression of CXCR4 in TNBC, Chu et al. [14] used western blot analysis on 151 frozen tissue, and Yu et al. [15] and Chen et al. [16] performed IHC on 148 and 75 FFPE samples, respectively. The difference in patient demographics, tissue preservation method, protein detection method, and scoring system may have caused the discordant results. However, this is the largest series of TNBC cases studied for the expression of CXCR4 and CXCL12 to date, with 259 and 238 cases studied for CXCR4 and CXCL12, respectively. Therefore, despite the limitation of retrospective study, we assumed that our data might have implications regarding the prognosis of TNBC.
The expression level of CXCR4 and CXCL12 has been correlated with different hormone receptor and HER2 status in breast cancer. For example, high CXCL12 expression was associated with ER positivity in resected breast cancer, and CXCL12 expression was induced by estradiol treatment in ER-positive breast cancer cell lines [26,27]. Salvucci et al. [23] demonstrated that cytoplasmic CXCR4 expression was correlated with ER negativity, PR negativity, and HER2 expression, and Hassan et al. [24] and Chen et al. [16] reported that CXCR4 expression level correlates with triplenegative status in breast cancer. Additionally, in the PDX model which recapitulated the stromal components of human breast cancer, CXCR4 inhibition did not reduce tumor growth and even increased the distant metastasis of TNBC [31]. Taken together, the difference in expression level and response to CXCR4 inhibition suggests that CXCR4/CXCL12 axis may exert a different effect on metastasis and prognosis of TNBC compared with other breast cancer subtypes. Other components of tumor microenvironment may have a role in this phenomenon, but the exact mechanism needs to be investigated in future studies.
The study population in our study was restricted to the TNBC patients treated with adjuvant chemotherapy, and the result revealed that high cytoplasmic CXCR4 expression is associated with lower distant recurrence and better RFS. Since adjuvant chemotherapy is indicated or recommended in most TNBC cases under current practice guideline, our result is clinically relevant and CXCR4 expression might be useful in predicting outcome in TNBC patients after adjuvant chemotherapy [32]. More importantly, we demonstrate for the first time that high CXCR4 expression may be associated with better prognosis in TNBC patients, and suggest the possibility of the different mechanism underlying the metastasis and prognosis of TNBC.
This study has limitations. As previously noted, this was a retrospective study with known disadvantages. In addition, the patient population was heterogeneous in terms of chemotherapy regimen, and there was a significant difference in chemotherapy regimen between high and low cytoplasmic CXCR4 expression groups. Use of taxane-anthracycline-based regimen was limited to node-positive cases, and since the patients received different chemotherapy based on nodal status, we could not compare the outcomes according to the chemotherapy regimen. Therefore, our results should be validated in prospective controlled cohort studies, and it would be beneficial to re-evaluate the prognostic significance of CXCR4 in patients treated with the same chemotherapy regimen.
In conclusion, the present study indicates that high cytoplasmic expression of CXCR4 may have a prognostic value in TNBC patients and predict lower distant recurrence and better RFS after adjuvant chemotherapy. To our knowledge, this is the first study to correlate high CXCR4 expression with better prognosis in TNBC, and the underlying mechanism needs to be explored in further studies.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.