Liquid-Based Cytology Features of Papillary Squamotransitional Cell Carcinoma of the Uterine Cervix
Article information
Papillary squamotransitional cell carcinoma (PSTCC) of the uterine cervix is an uncommon variant of cervical squamous cell carcinoma (SCC) [1]. Here we describe the SurePath liquid-based cytology (LBC) findings of a case of PSTCC which showed a combination of cytological features of low-grade papillary urothelial carcinoma (LGPUC) and SCC.
CASE REPORT
A 77-year-old previously healthy woman presented with bloody vaginal discharge at the outpatient clinic. A colposcopy revealed a 2.5 cm-sized papillary mass (Fig. 1), and a punch biopsy was performed. After the possibility of a PSTCC or papillary SCC was suggested on the biopsy, she underwent a total abdominal hysterectomy with bilateral salpingo-oophorectomy. Two years later, she returned to the clinic complaining of vaginal discharge. A 6- mm-sized enhancing nodular lesion was noted at the right vaginal stump on pelvic computed tomography, and a cervicovaginal smear and punch biopsy was taken to confirm recurrence. However, further treatment of the vaginal lesion was not performed, as imaging incidentally revealed a pancreatic tail neoplasm with peritoneal seeding. The patient was subsequently referred to hospice care.
At low-power magnification, the LBC preparation was highly cellular, showing a few small hyperchromatic crowded groups (HCGs) in a background of abundant scattered cells (Fig. 2A). At higher magnification, the HCGs were 3-dimensional irregularly-shaped papillary fragments containing fibrovascular cores. The atypical cells lining the papillary clusters were relatively uniformly distributed with mild nuclear overlapping (Fig. 2B). The nuclei were smaller than those of parabasal cells, and were round-to-oval with smooth nuclear membranes. Nucleoli were small and almost inconspicuous. Mitotic figures were only occasionally seen. Similar-looking atypical cells were individually scattered in the background (Fig. 2B, C). Some cells were spindled with long and tapered cytoplasmic processes (Fig. 2C). Altogether, most of the neoplastic cells demonstrated the cytological features of LGPUC. There were also a few interspersed cells with increased nuclear-to-cytoplasmic ratio, hyperchromasia and nuclear membrane irregularity, suggestive of high-grade squamous intraepithelial lesion (HSIL) (Fig. 2D). A small cluster of dyskeratotic cells was seen (Fig. 2E, F). Tumor diathesis was not identified.

Liquid-based cytology findings. (A) Low-power magnification shows some scattered hyperchromatic crowded groups and abundant individually scattered cells. (B) A papillary cluster (center) is lined by small neoplastic cells with round-to-oval nuclei and minimal nuclear atypia. (C) The scattered neoplastic cells demonstrate similar features, with eccentrically located, round-to-oval nuclei. Long cytoplasmic processes are seen in some neoplastic cells (“cercariform” cells) at the edge of papillary fragments (C, inset). A mitotic figure is seen (arrow). (D) A high-grade squamous intraepithelial lesion cell with coarse chromatin and irregular nuclear membrane (center) is seen among the scattered low-grade urothelial carcinoma-like cells. (E, F) Dyskeratotic cells and focal koilocytotic atypia are occasionally seen.
The biopsied tissue demonstrated papillary excrescences containing fibrovascular cores (Fig. 3A). The lining epithelium showed hybrid features of LGPUC and SCC (Fig. 3B, C). The LGPUClike component was seen towards the basal layers and showed mild cytological atypia. Towards the surface of the papillary stuctures, there was evidence of keratinization and high-grade cytologic atypia. Koilocytosis was focally seen (Fig. 3D). Although stromal invasion was not observed in the biopsied tissue, the subsequent hysterectomy specimen demonstrated residual tumor with stromal invasion and vascular invasion in the parametrial vessels. Immunohistochemistry revealed diffuse nuclear p63 expression, and the tumor cells were cytokeratin 7 (CK7)–positive and CK20-negative. A human papillomavirus (HPV) test (LG AdvanSure GenoBlot Assay, LG Life Sciences, Seoul, Korea) detected “other type” HPV. The histologic findings of the recurrent tumor were similar to that of the initial tumor.
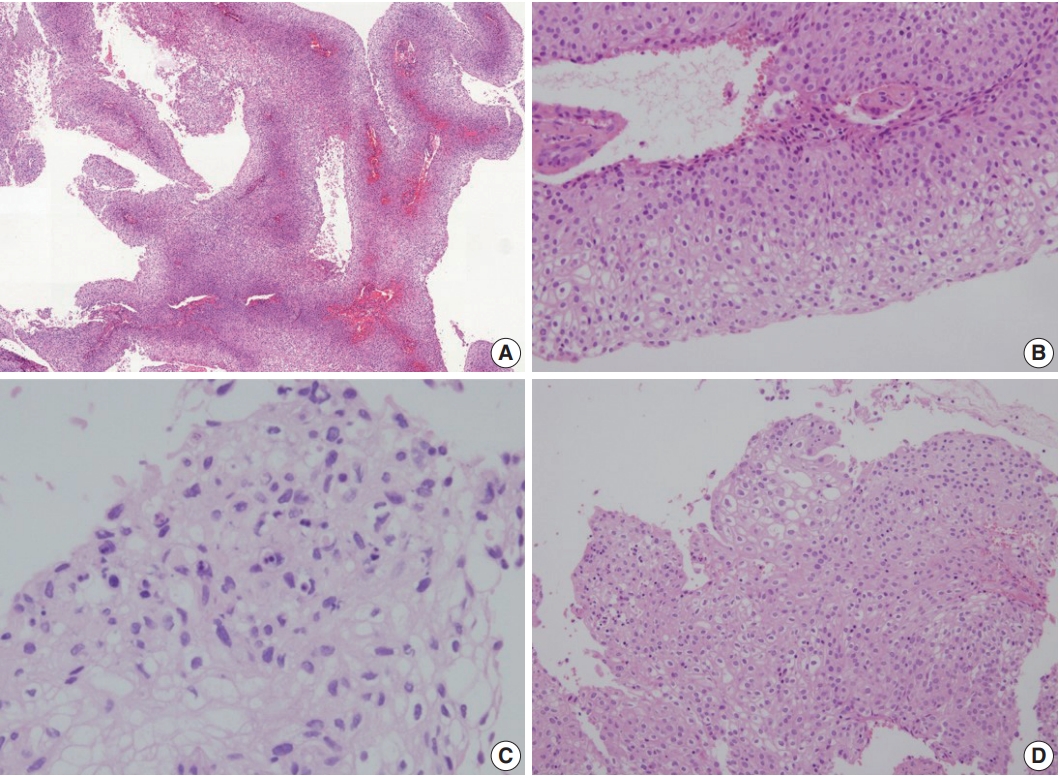
Histological findings. (A) Low-power view demonstrates papillary structures with fibrovascular cores. (B) The papillae are mostly lined by urothelial-like cells. (C, D) Focal areas of keratinization (C) and koilocytotic atypia (D) are seen in the surfaces of the papillae.
Ethics statement
This case study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB approval number B-1710-427-702) and informed consent was waived.
DISCUSSION
PSTCC was first reported in 1986 by Randall et al. [1] as a variant of SCC showing varying degrees of urothelial-like differentiation. Due to the predominantly exophytic nature of these tumors, invasion may not be detected on small biopsies or cervicovaginal cytology. However, despite the surface papillary growth pattern, PSTCCs have been frequently associated with deep invasion, local recurrence and distant metastasis [1-3].
While the clinicopathological features of PSTCC have been discussed in several case reports, the cytological findings of PSTCC have only been described in two reports: one study described the LBC features of six cases [4], and the other one is a single case report describing the conventional smear features [5]. The LBC features of PSTCC in this case could be summarized as follows: (1) a moderate-to-high cellularity, (2) HCGs characterized by 3-dimensional papillary fragments with branching architecture, (3) urothelial-like features, and (4) loosely dispersed hyperchromatic parabasal-like cells in the background [4,5].
The differential diagnosis of PSTCC includes a spectrum of lesions ranging from reactive to neoplastic, including papillary immature metaplasia and HSIL with papillary configuration [6,7]. Immature metaplastic and repair cells are immature parabasal-like cells with cytoplasmic projections, 2-dimensional sheets and fine chromatin, while PSTCCs frequently demonstrate 3-dimensional HCG with papillary architecture and hyperchromatic nuclei. Although papillary architecture may be seen in HSILs, the papillary fragments of PSTCC have been described to show more complex arborizing patterns [4]. In addition, the presence of horizontally aligned cells on the surface of papillary structures—similar to that seen in LGPUC in urine cytology—has been shown to be a characteristic cytological feature of PSTCC [4,8].
Despite the urothelial-like features, PSTCCs have been suggested to be metaplastic variants of SCC. The CK7+/CK20–profile of PSTCC (including this case) is identical to that of müllerian epithelium [3]. Moreover, similarly to SCC, HPV and allelic losses at 3p have been implicated in the pathogenesis of PSTCCs, while chromosome 9 alterations (common in bladder cancer) were not detected [3,9,10].
Notes
Author contributions
Conceptualization: HK (Haeryoung Kim).
Data curation: YL (Yangkyu Lee), YC.
Investigation: YL (Yangkyu Lee), KL, YL (Youngeun Lee).
Methodology: YL (Yangkyu Lee), HK (Haeryoung Kim).
Resources: HK (Hyojin Kim), JYC, HSL, YBK, HK (Haeryoung Kim).
Supervision: HK (Haeryoung Kim).
Visualization: YL (Yangkyu Lee), HK (Haeryoung Kim).
Writing—original draft: YL (Yangkyu Lee), YC, KL.
Writing—review & editing: YL (Yangkyu Lee), KL, HK (Haeryoung Kim).
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding
No funding to declare.

