Peritoneal Fluid Cytology of Disseminated Large Cell Neuroendocrine Carcinoma Combined with Endometrioid Adenocarcinoma of the Endometrium
Article information
Primary large cell neuroendocrine carcinoma (LCNEC) of the endometrium is extremely rare [1-3] and the cytomorphology has not been well described. Recently, we experienced a case of combined LCNEC with endometrioid carcinoma (ECa) showing peritoneal dissemination that was confirmed by histology and peritoneal fluid cytology. The purpose of this report is to delineate cytologic characteristics of LCNEC in an effusion specimen.
CASE REPORT
A 62-year-old woman was admitted for evaluation of continuous vaginal bleeding for 1 month. She was in a menopausal state since the age of 50 years with gravida 2-para 2, and had no specific remarkable past medical history. Her initial laboratory test was unremarkable. Ascites was noticed on physical examination. On ultrasonography, the uterine corpus was enlarged with the endometrium thickened to 15 mm. Endometrial curettage showed low-grade ECa. Intraoperative peritoneal fluid sampling demonstrated small-sized tumor cell clusters measuring approximately 100–150 μm in diameter and discohesive polyhedral single tumor cells admixed with karyorrhectic debris, which made a definitive diagnosis difficult. In contrast to mesothelial cells and lymphocytes in the background, tumor cells had large nuclei with an irregular nuclear membrane, vesicular nuclei, relatively prominent nucleoli, and notable cytoplasm (Fig. 1A–C). A panel of immunohistochemical stains was performed on the cell block material, and atypical cells were positive for CD56 (Fig. 1D) and synaptophysin, but not for chromogranin and CD45. These findings were consistent with neuroendocrine carcinoma (NEC). An en-bloc resection was performed. On microscopic examination, there were foci of transition between a low-grade ECa and a loosely cohesive carcinoma component in the endometrium (Fig. 2A) invading the superficial myometrium (Fig. 2B). The loosely cohesive tumor component showed pseudoglandular and cord-like growth patterns. The tumor cells had relatively abundant cytoplasm, vesicular nuclei, and prominent nucleoli (Fig. 2C). There were tumor emboli in lymphovascular spaces of the myometrium. These cells were positive for CD56 (Fig. 2D) and synaptophysin (Fig. 2E), but negative for CD45 and CD99. Some tumor cells were positive for epithelial membrane antigen (Fig. 2F) and cytokeratin. According to these findings, a diagnosis of combined LCNEC with a low-grade ECa was made. The patient died of the disease 32 days after operation.
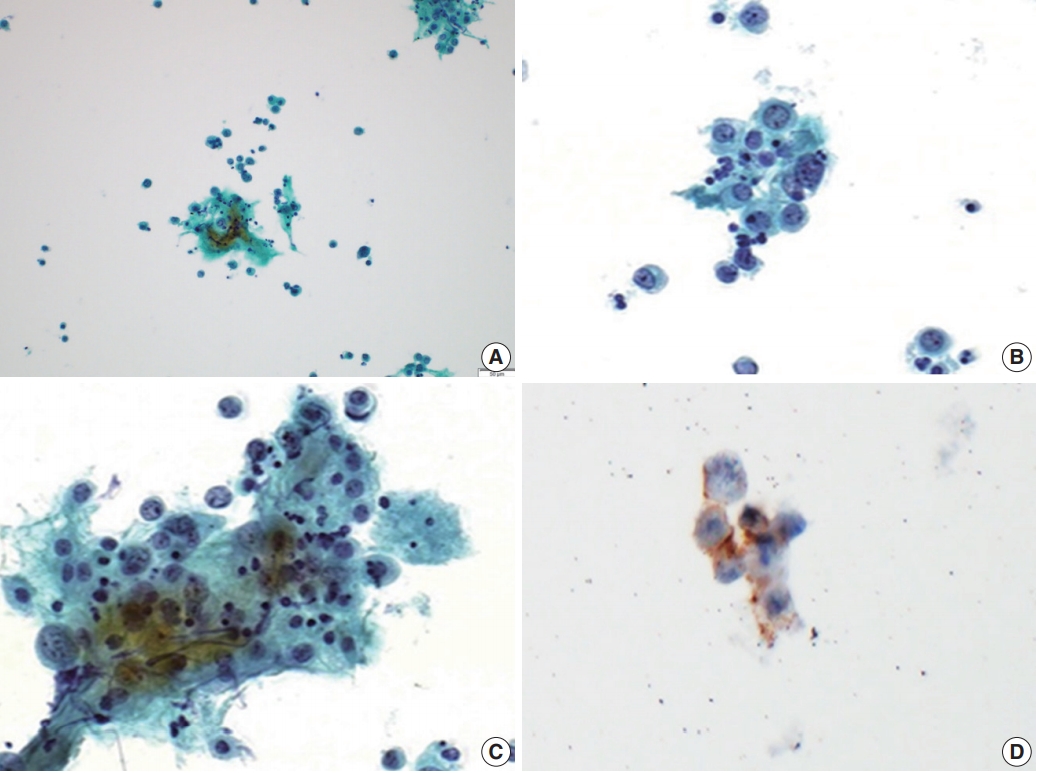
Cytologic features of large cell neuroendocrine carcinoma in a peritoneal fluid smear. (A) Loose clusters of tumor cells measuring 100 to 150 μm are present. (B, C) These tumor cells are polyhedral with abundant eosinophilic cytoplasm, nuclei are either vesicular or hyperchromatic, chromatin is heterogeneous, and nucleoli are variably prominent. (D) These cells show positive reactions for CD56.
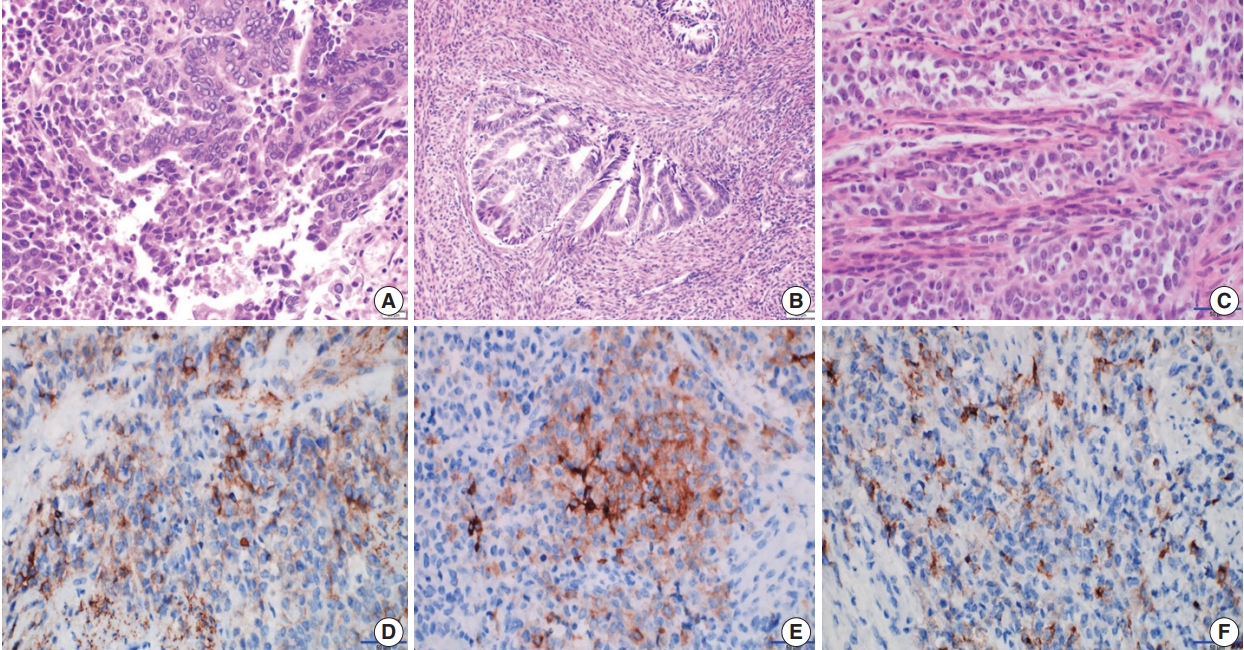
Microscopic and immunohistochemical findings. The endometrium shows foci of transition between a low-grade endometrioid adenocarcinoma and a loosely cohesive carcinoma component (A) with a cribriform pattern of growth invading the myometrium (B). (C) The loosely cohesive tumor component shows a cord-like growth pattern and the tumor cells have relatively abundant eosinophilic cytoplasm with a large polyhedral nucleus and prominent nucleoli. Immunohistochemically, these tumor cells show positive reactions for CD56 (D) and synaptophysin (E). (F) Some tumor cells are positive for epithelial membrane antigen.
Ethics statement
Approval for this case report was obtained from the Institutional Review Board (IRB) of Chungnam National University Hospital (CNUH-IRB 2017-9-49) with a waiver of informed consent.
DISCUSSION
LCNEC is characterized by the presence of polygonal cells with a neuroendocrine growth pattern in at least part of the tumor and expression of one or more of the neuroendocrine markers (chromogranin, CD56, and synaptophysin) in more than 10% of tumor cells [1]. Due to the rarity of LCNECs in the female genital tract [1-3], a specific diagnosis of LCNEC is usually not possible in effusion specimens. Additional difficulties that may be encountered in effusion cytology include overlapping morphology among similar neoplastic entities, scant cellularity, and predominance of apoptosis or cellular debris. Cytopathologic diagnosis of LCNEC is more challenging than small cell neuroendocrine carcinoma due to rare nuclear molding (13%), frequent apoptosis (67%), and prominent nucleoli (86%) in LCNEC.4 The cytologic findings of LCNEC of the uterine cervix are characterized by loosely cohesive clusters or single tumor cells with hyperchromatic nuclei and necrotic materials in the background. The ovoid nuclei are 3–5 times larger than nuclei of small lymphocytes and have coarsely clumped chromatin and two or more prominent nucleoli. The tumor cells have a moderate amount of cytoplasm [5-7].
A cytopathologic differential diagnosis of our case included serous carcinoma, small cell NEC (SCNEC), and undifferentiated carcinoma. Serous carcinoma is characterized by marked exfoliation of high-grade tumor cells either in the form of cellular clusters or single cells. Psammoma bodies can be seen [8]. SCNEC is composed of relatively uniform small hyperchromatic nuclei with characteristic nuclear molding and scant cyanophilic cytoplasm [9]. In this case, cellular overlapping, an acinar arrangement, and papillary configuration were not distinctive. Dedifferentiated carcinoma is composed of a mixture of undifferentiated carcinoma and either the International Federation of Gynecology and Obstetrics grade 1 or 2 ECa [1]. Undifferentiated carcinoma grows as sheets of noncohesive atypical tumor cells without any nested or trabecular architecture and displays chromogranin and/or synaptophysin staining in a minority of tumor cells [10]. In this case, the tumor components showed a cord-like growth pattern and were positive for CD56 and synaptophysin.
In high-grade NEC metastases, the three architectural patterns of the cytomorphologic spectrum that have been presented within body cavities are (1) a predominance of small clusters of tumor cells (seen more often in LCNEC cases), (2) a predominance of large clusters of tumor cells (mainly in SCNEC), and (3) a predominance of single tumor cells (seen in both SCNECs and LCNECs) [4]. The small clustering pattern is seen in 73% of LCNEC and 41% of SCNEC cases [4]. Our case presented predominantly with small clusters of tumor cells measuring less than 150 μm (approximately 100 to 150 μm) and discohesive single tumor cells.
Notes
Author contributions
Conceptualization: KHK, KSS.
Data curation: YML, MKY
Funding acquisition: KSS.
Writing—original draft: YML, KSS.
Writing—review & editing: MKY, SYC, KSS.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding
This study was supported by the research fund of Chungnam National University
