Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
Article information
Abstract
Background
Pure mucinous carcinoma (PMC) is a rare type of breast cancer, estimated to represent 2% of invasive breast cancer. PMC is typically positive for estrogen receptors (ER) and progesterone receptors (PR) and negative for human epidermal growth factor receptor 2 (HER2). The clinicopathologic characteristics of HER2-positive PMC have not been investigated.
Methods
Pathology archives were searched for PMC diagnosed from January 1999 to April 2018. Clinicopathologic data and microscopic findings were reviewed and compared between HER2-positive PMC and HER2-negative PMC. We also analyzed the differences in disease-free survival (DFS) and overall survival according to clinicopathologic parameters including HER2 status in overall PMC cases.
Results
There were 21 HER2-positive cases (4.8%) in 438 PMCs. The average tumor size of HER2-positive PMC was 32.21 mm (± 26.55). Lymph node metastasis was present in seven cases. Compared to HER2-negative PMC, HER2-positive PMC presented with a more advanced T category (p < .001), more frequent lymph node metastasis (p = .009), and a higher nuclear and histologic grade (p < .001). Microscopically, signet ring cells were frequently observed in HER2-positive PMC (p < .001), whereas a micropapillary pattern was more frequent in HER2-negative PMC (p = .012). HER2-positive PMC was more frequently negative for ER (33.3% vs. 1.2%) and PR (28.6% vs. 7.2%) than HER2-negative PMC and showed a high Ki-67 labeling index. During follow-up, distant metastasis and recurrence developed in three HER2-positive PMC patients. Multivariate analysis revealed that only HER2-positivity and lymph node status were significantly associated with DFS.
Conclusions
Our results suggest that HER2-positive PMC is a more aggressive subgroup of PMC. HER2 positivity should be considered for adequate management of PMC.
Breast mucinous carcinoma (MC) is defined as floating tumor clusters within extracellular mucin pools [1]. MC is divided into two subtypes, pure and mixed, according to the proportion of mucinous component in the tumor [1]. Pure MC (PMC) is composed of more than 90% mucinous component, is a rare type of breast cancer accounting for approximately 2% of invasive cancers, and usually occurs in elderly patients (> 55 years) [1,2]. PMC generally has a low nuclear grade and good prognosis [1,3].
Typical PMC is positive for estrogen receptor (ER) and progesterone receptor (PR), whereas it is negative for human epidermal growth factor receptor 2 (HER2) [1,3,4]. While HER2 overexpression occurs in 15%–20% of invasive breast cancers [5], it has been reported in only 2.6–9.0% of PMC [4,6-8]. Clinicopathologic characteristics of HER2-positive PMC have not been studied well because of the rarity of this entity. Herein, we investigate the clinicopathologic characteristics of HER2-positive PMC.
MATERIALS AND METHODS
Patients and data
The pathology archives of Samsung Medical Center were searched, and 637 PMC cases were diagnosed from January 1999 to April 2018, which accounted for 2.9% of 22,318 invasive breast cancer cases. Clinicopathologic data were archived from electronic medical records and pathology reports and comprised of sex, age, type of procedure, tumor size, nuclear grade, histologic grade, lymph node status, presence of lymphovascular invasion (LVI), extensive intraductal component (EIC), chemotherapy, hormonal therapy, radiotherapy, recurrence status, distant metastasis, and death. TNM classification was designated according to the 8th edition of the American Joint Committee on Cancer.
Cases were excluded if (1) the tumor were composed of less than 90% MC (i.e., mixed MC), (2) multiple synchronous carcinomas occurred with one or more tumors of non-mucinous histology that were of comparable size or larger than the PMC, (3) tumor size was less than 0.1 cm (microinvasive carcinoma), (4) surgery was not performed, or (5) tumor slides were not available for review. Ultimately, 438 PMC cases were included.
Histologic review
Tumor slides were re-evaluated by two pathologists (Y.J. Jang and S.Y. Cho). Nuclear grade and histologic grade were classified on the basis of the Bloom-Richardson grading system [1]. EIC was defined as more than 25% ductal carcinoma in situ area within the invasive carcinoma [1]. Cases were subdivided into type A or type B depending on the cellularity of tumor cells, i.e., classic, hypocellular tumors with more extracellular mucin were classified as type A (Fig. 1A), and hypercellular PMC with large cell clusters were classified as type B (Fig. 1B) [1]. The presence of a micropapillary component or signet ring cells was also evaluated.
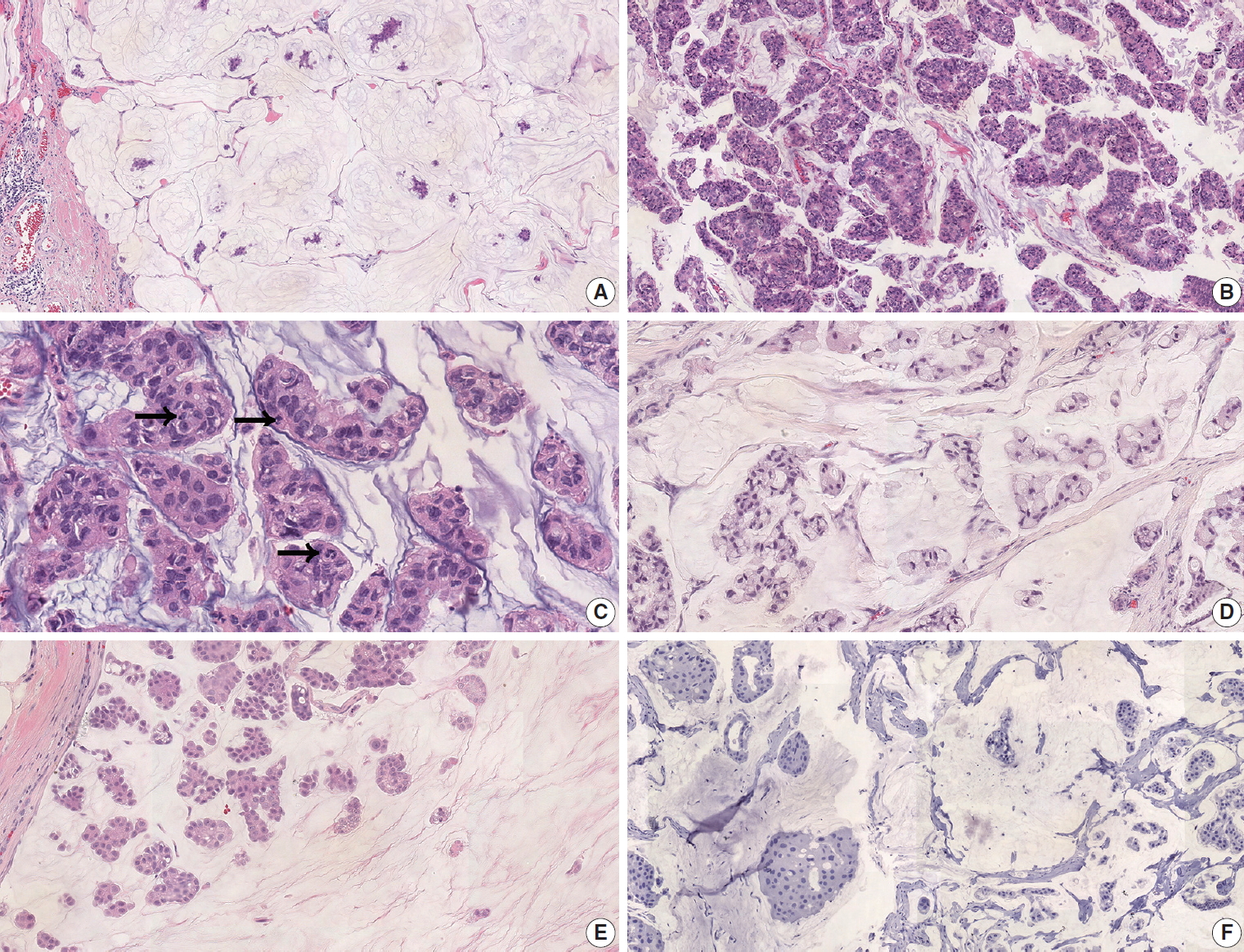
Pure mucinous carcinoma (PMC). (A) Hypocellular type A PMC with abundant extracellular mucin pool. (B) Hypercellular type B pattern. (C) Human epidermal growth factor receptor 2 (HER2)–positive PMC shows high nuclear and histologic grade. There are frequent mitoses (arrows). (D) Some HER2-positive PMC shows extensive signet ring cell differentiation. (E) Some PMC presents a micropapillary pattern. (F) HER2-positive PMC is frequently negative for estrogen receptor.
Immunohistochemistry and silver in situ hybridization
Immunohistochemistry (IHC) for ER (1:200, clone 6F11, Bond-Max System, Novocastra, Newcastle upon Tyne, UK), PR (1:800, clone 16, Bond-Max System, Novocastra), HER2 (4B5, BenchMark XT, Ventana, Tucson, AZ, USA), and Ki-67 (1:200, clone MIB-1, Bond-III, Dako, Glostrup, Denmark) was performed on formalin-fixed paraffin-embedded (FFPE) tissue. Silver in situ hybridization (SISH) analysis was performed using INFORM DDISH HER-2 SISH probe kits (BenchMark XT, Ventana) on FFPE tissue. ER and PR were considered positive when at least 1% of tumor cells showed nuclear staining according to the American Society of Clinical Oncology/College of American Pathologists guidelines [9]. HER2 was considered positive if ≥ 10% of tumor cells showed 3+ staining by IHC or 2+ staining by IHC with amplification using SISH [10]. The Ki-67 labeling index was determined via automated image analysis. After the Ki-67–stained slide was scanned under × 20 magnification (Ventana iScan), the percentage of positively stained cells was calculated using image analysis software (Ventana Virtuoso, ver. 5.6).
Statistical analysis
Categorical variables were analyzed using t tests and chi-square tests. Disease-free survival (DFS) and overall survival (OS) were defined as the duration from pathologic diagnosis to recurrence/progression and death, respectively. DFS and OS were plotted using the Kaplan-Meier method. A log-rank test was used to evaluate differences in survival. Multivariate analyses were performed to assess the prognostic factors for survival using a Cox proportional hazards model. A p-value less than .05 was considered statistically significant. All statistical analyses were performed using SPSS software ver. 25.0 (IBM Corp., Armonk, NY, USA).
Ethics statement
This study was approved by the Institutional Review Board of Samsung Medical Center, and the need for informed consent was waived (IRB No. 2019-08-051).
RESULTS
Basic characteristics of HER2-positive pure mucinous carcinoma
Basic clinicopathologic characteristics are summarized in Table 1. Twenty-one HER2-positive cases were included, accounting for 4.8% of 438 PMC. Seventeen cases were HER2 IHC 3+, and the remaining four cases showed equivocal (2+) staining, of which HER2 amplification were confirmed using SISH. All 21 patients were female, and their mean age was 45 years. Ten patients underwent total mastectomy, and 11 patients underwent conserving surgery. Axillary lymph node dissection was performed in 10 cases, and sentinel node biopsy alone was performed in the remaining 11 cases. Six patients received neoadjuvant chemotherapy, and their T category before treatment was cT1 in one case, cT2 in one case, and cT3 in four cases. Among them, three patients (cases Nos. 11, 12, and 13) received neoadjuvant chemotherapy including trastuzumab. One patient showed pathologic complete response (case No. 11). The T category of the remaining 15 patients was pT1 in 10 cases, pT2 in four cases, and pT3 in one case. Nodal metastasis was present in seven cases (35.0%). Eleven patients received trastuzumab treatment after surgery. Distant metastasis occurred in two patients (9.5%) (lung and brain, one; skin and lung, one each), and local recurrence occurred in one patient (4.8%). Distant metastasis and local recurrence occurred in two of nine patients without trastuzumab treatment (22.2%) and one of 11 patients with trastuzumab treatment (9.1%) (p = .421).
Microscopic findings of HER2-positive pure mucinous carcinoma
Nuclear grade was two in 10 cases (50.0%) and three in 10 cases (50.0%). Histologic grade was II in 10 cases (50.0%) and III in 10 cases (50.0%) (Fig. 1C). EIC was present in 10 cases (50.0%). LVI was present in five cases (25%). Thirteen cases (68.4%) were classified as type A, and six cases (31.6%) were classified as type B. Signet ring cells were present in 12 cases (60.0%) (Fig. 1D). In two cases, the tumor was comprised of > 90% of signet ring cells. The micropapillary component was present in five cases (25.0%) (Fig. 1E). ER was positive in 14 cases (66.7%) and negative in seven cases (33.3%) (Fig. 1F). PR was positive in 15 cases (71.4%) and negative in six cases (28.6%).
Comparison of clinicopathologic characteristics between HER2-positive and HER2-negative pure mucinous carcinoma
Clinicopathologic characteristics of HER2-positive and HER2-negative PMC are summarized in Table 2. Average follow-up duration was 69 months (range, 1 to 227 months). Mean age was younger in the HER2-positive group without statistical significance (p = .073). Tumor size was larger (p < .001) and axillary lymph node metastasis was more common in the HER2-positive group (p = .009). Nuclear and histologic grades were higher in the HER2-positive group than in the HER2-negative group (p < .001 and p < .001). Distribution of tumor type (mucinous type A or B) was similar in the two groups (p = .940). The micropapillary pattern was more frequent in HER2-negative PMC (p = .012), whereas signet ring cells were frequently observed in the HER2-positive PMC (p < .001). In HER2-positive PMC, EIC was more common (50.0% vs. 21.9%, p = .011), and LVI tended to occur more frequently without statistical significance (25.0% vs. 12.2, p = .158). ER was negative in seven HER2-positive PMC (33.3%), while there were only five HER2-negative PMC (1.2%) (p < .001). PR was negative in six cases of HER2-positive PMC (28.6%); in contrast, PR was negative in 30 cases of HER2-negative PMC (7.2%) (p = .005). HER2-positive PMC cases showed a more frequent high Ki-67 labeling index (≥ 20%) (p = .006).
Prognosis of pure mucinous carcinoma
Recurrence or distant metastasis occurred in 16 cases (3.7%), and 17 deaths were observed. In three cases, the cause of death was breast cancer (HER2-negative PMC, two cases; HER2-positive PMC, one case). Nuclear and histologic grades, T category, lymph node status, LVI, and HER2 status were significantly associated with DFS in univariate analysis using a log-rank test (Table 3). The 10-year DFS was significantly lower in HER2-positive PMC (DFS, 78.1% vs 93.9%, p = .001) (Fig. 2). In multivariate analysis, only lymph node status and HER2 positivity were significantly associated with DFS (relative risk [RR], 4.818; p = .005 and RR, 7.822; p = .002) (Table 4). In univariate analysis of OS, lymph node status and HER2 positivity had significance. In multivariate analysis, only lymph node status retained significance (RR, 11.72; p = .045) (Table 4).

Univariate analysis of DFS and OS using Kaplan-Meier plot and log-rank test in pure mucinous carcinoma
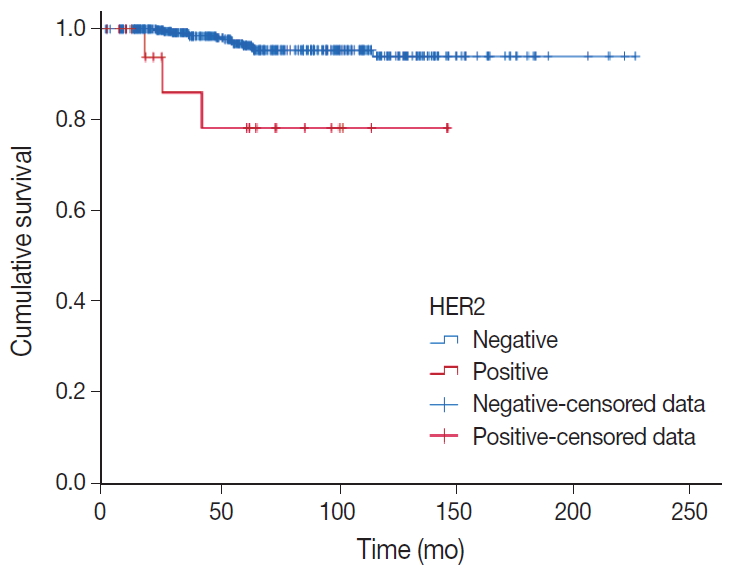
Kaplan-Meier plots for disease-free survival of pure mucinous carcinoma according to human epidermal growth factor receptor 2 (HER2) status.
DISCUSSION
PMC is a rare subtype of breast cancer characterized by extracellular mucin pools with floating tumor cells [1]. Typical PMC belongs to the luminal A molecular subtype; most PMC is low grade, positive for ER and/or PR, and negative for HER2 [1-4]. PMC generally shows favorable prognosis. However, some studies reported that ER/PR negativity and high grade are associated with a poor prognosis in PMC [11,12].
While HER2 amplification occurs in 15%–20% of invasive breast cancer [5], it is very rare in PMC and is reported to occur in 2.6%–9% of cases [4,6-8]. In our cohort, 4.8% of PMC showed HER2 positivity, consistent with previous studies. HER2-positive breast cancer is typically poorly differentiated and high grade, has high rates of cell proliferation and lymph node involvement, and is relatively resistant to certain types of chemotherapy [13]. Clinicopathologic characteristics of HER2-positive PMC have not been well investigated because of its very low incidence. This study was the first to describe clinicopathologic features of HER2-positive PMC in a large series. HER2-positive PMC showed higher nuclear and histologic grades, more frequent axillary lymph node metastasis, and a more advanced stage at the time of diagnosis than HER2-negative PMC. HER2 status was significantly associated with DFS in multivariate and univariate analyses. However, this would have to be further validated because of the few events in our series. Recently, Gwark et al. [14] also reported worse prognosis of HER2-positive PMC among PMC cases that were hormone receptor positive and node negative with a tumor size ≥ 3 cm.
Clinically, HER2-positive PMC has important implications for patient management. Although there was no difference in recurrence or survival according to trastuzumab treatment in this study, the effects of anti-HER2 agents including trastuzumab on survival of HER2-positive breast cancers are well known [5]. Our result might be due to the limited number of cases. Besides, we experienced a case of HER2-positive PMC with pathologic complete response after neoadjuvant chemotherapy with trastuzumab and pertuzumab (case No. 11) [15]. Gwark et al. [14] also reported better prognosis of patients treated with trastuzumab than without trastuzumab among HER2-positive hormone receptor–positive PMC. In our study population, only 11 patients received trastuzumab as either neoadjuvant or adjuvant treatment. None of the patients who were initially diagnosed before 2009 received trastuzumab, whereas all but three patients who were diagnosed after 2009 received trastuzumab treatment. Tumors of two patients were too small (0.4 cm and 0.45 cm), and in one patient, trastuzumab use was uncheckable because of her referral to an outside hospital. Clinicians should be aware of HER2-positive PMC and manage according to immunophenotype of PMC, as in usual breast cancers.
Previous case reports of our group and others reported signet ring cell components in HER2-positive PMC [15-17]. Micropapillary features have been suggested to have an association with HER2 positivity [18]. Thus, we evaluated micropapilla and signet ring cells in PMC. Interestingly, the presence of signet ring cells was significantly associated with HER2 positivity in PMC, with 12/20 cases showing signet ring cells (60.0%). In contrast, 48 HER2-negative PMC patients (11.5%) showed signet ring cell differentiation. Extracellular mucin pools are a characteristic feature of PMC, and intracellular accumulation of mucin is a feature of signet ring cells. Coexistence of extracellular and intracellular mucin with HER2 positivity remains unexplained. Although carcinomas with signet ring cell differentiation are not a distinct disease entity in the World Health Organization classification [1], our result suggests signet ring cell differentiation may represent a specific histologic feature associated with HER2 positivity in PMC [15-17]. Micropapillary features were quite common in overall PMC (53.8%) and were more frequently observed in HER2-negative PMCs than in HER2-positive PMCs. The prognostic significance of micropapillary features in PMC remains controversial. Some studies suggested more aggressive behavior of PMC with micropapillary features [19], whereas others showed no association [20]. Recently, Xu et al. [21] reported that micropapillary features are common in PMC (80%), and micropapillary features with low nuclear grade are associated with indolent biologic behavior. This might explain the discordant results of previous studies and frequent presence of micropapillary features in HER2-negative PMC in our study.
Typical PMC is a luminal A type breast cancer with low nuclear grade, high ER expression, and HER2 negativity. However, as shown in this study, there is a minor proportion of HER2-positive PMCs, which showed aggressive histologic and clinical features, including high nuclear grade, high histologic grade, large tumor size, a frequent extensive intraductal component, and lymph node metastasis. Signet ring cells were also significantly associated with HER2-positive PMC. Finally, HER2 positivity in PMC was significantly associated with poor DFS in univariate and multivariate analyses. Consideration of HER2 positivity in PMC is important for treatment decisions regarding the use of HER2 target therapy.
Notes
Author contributions
Conceptualization: SYC, EYC, JEL.
Data Curation: YJ.
Formal analysis: YJ, SYC.
Investigation: YJ, HJ, HNK, YS, EA, SJN, SWK, JEL, YHP.
Project administration: YJ, YS.
Supervision: SYC, EYC.
Writing—original draft: YJ, SYC.
Writing—review & editing: YJ, SYC, EYC.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding
No funding to declare.



