Imaging features of breast cancer molecular subtypes: state of the art
Article information
Abstract
Characterization of breast cancer molecular subtypes has been the standard of care for breast cancer management. We aimed to provide a review of imaging features of breast cancer molecular subtypes for the field of precision medicine. We also provide an update on the recent progress in precision medicine for breast cancer, implications for imaging, and recent observations in longitudinal functional imaging with radiomics.
Gene expression profiling has revealed that there are four major breast cancer subtypes: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, and basal-like tumors [1–3]. Each subtype has varied prognoses, risk of progression, response to treatment, and survival outcomes. In general, basal-like tumors have the worst prognosis, while luminal A tumors have the best prognosis. However, as full genomic analysis is costly and time consuming in clinical practice, the St. Gallen International Expert Consensus panel has suggested surrogate subtypes based on semiquantitative immunohistochemistry (IHC) scoring of estrogen receptor (ER), progesterone receptor (PR), and in situ hybridization tests for HER2 overexpression as follows: luminal A (ER and/or PR positive, HER2 negative), luminal B (ER and/or PR positive and HER2 positive or Ki67 ≥ 14%), HER2-enriched (HER2 amplified, ER and PR negative), and triple-negative breast cancer (TNBC; ER, PR, and HER2 negative) [4–6] (Table 1). Type determination by percutaneous image-guided biopsy is the first step in managing systemic therapy strategy for breast cancer, because traditional prognostic factors including tumor size, histologic grade, and lymph node status do not fully reflect the heterogeneity of breast cancer, and treatment guidelines are no longer based solely on anatomic stage. The biological diversity of tumors requires the continual refinement of treatment algorithms, which are more and more personalized in the recent St. Gallen Consensus Guidelines [6]. The escalating strategy includes longer duration of anti-estrogen therapy, ovarian function suppression, dual blockade with anti-HER2 therapy, and residual tumor treatment following neoadjuvant chemotherapy [6]. The de-escalating strategy includes omission of adjuvant chemotherapy, shortening of radiation therapy, and avoidance of axillary dissection [6]. However, percutaneous biopsy sampling does not represent the topographic heterogeneity of a whole tumor. Moreover, as breast cancer continuously evolves following systemic therapy, spatio-longitudinal monitoring of a whole tumor using imaging modalities during systemic therapy is crucial.

Treatment-oriented classification of subgroups of breast cancer from St. Gallen consensus guidelines
The earliest imaging studies have reported that the triple-negative subtype has non-calcified and circumscribed margins, the luminal subtype mass is irregular with spiculated margins, and the HER2-positive subtype mass has pleomorphic calcifications [7]. While repeated measurements of voxel-based signal intensity of whole tumor is feasible, with breast magnetic resonance imaging (MRI) there are several studies that link imaging phenotypes using radiomics analysis with breast cancer molecular subtypes. In this article, we aim to help readers to stay up-to-date and play a role as key members of a multidisciplinary team for breast cancer treatment.
RADIOMICS IN BREAST CANCER
Radiomics is a technique to extract and select the quantitative features of radiologic images, to create a high-dimensional data set, and to draw hypotheses, which will lead to better clinical decisions [8]. In breast radiomics analysis, determination of benign or malignant lesion, correlation with prognostic factors, prediction of the response to systemic chemotherapy or lymph node metastasis have been studied. Radiomics features are mathematically defined and classified into morphology, histogram, texture, or transformed features [8]. Morphology features are compactness, roundness, or convexity. Histogram features are median, entropy, uniformity, skewness, or kurtosis, in which spatial information is not included. Conversely, texture features include the spatial information. Gray-level co-occurrence matrix (GLCM)-based features are the most commonly used method for textural analysis [8]. The relationship between voxels and their neighborhoods are characterized in GLCM analysis. Entropy, contrast, and homogeneity, which reflect the uniformity or heterogeneity of the voxel signal intensities, are the main parameters of GLCM models. Transform-based features such as Laplacian of Gaussian and wavelet are commonly used; these transform the original image, creating a new image from which the features can be quantified.
Application of radiomics in distinguishing molecular subtypes is one of the most intensely studied areas (Table 2) [9–20]. Leithner et al. [9] reported accuracies of 81%–89% in distinguishing luminal A from luminal B, luminal B from triple-negative, luminal B from all others, and HER2-enriched from all others. In their study, the region of interest of a tumor was drawn, and gray-level normalization was performed to minimize the effect of contrast variations. Then, first-order histogram, GLCM, and transform-based features were calculated. Feature selection to reduce the dimensionality of texture features, to select the minimal numbers of features explaining the phenomenon, were performed. K-nearest neighbor classification with leave-one-out cross validation was performed for classification [9]. More recent studies using radiomic analysis reported an area under the receiver operating characteristic curve (AUC) of 0.844 in the prediction of disease-free survival of TNBC [10] and an AUC of 0.890 in the differentiation of HER2-positive tumors vs. −negative tumors [11]. The radiomics approach is based on the premise that microstructural variations between molecular subtypes would cause various gray-level textures on contrast enhanced MRI. The radiomics approach has the potential to provide prognostic information of spatio-longitudinal biology changes of the peritumoral parenchyma as well as of the tumor itself. Moreover, deep learning methods have increasingly been applied in recent radiomics studies [8,11]. The deep learning approach, which is data driven and capable of learning relevant features from the data themselves, has shown superior performance in various tasks in radiology [8,11]. In the near future, radiomics parameters based on a deep learning algorithm would be useful surrogate markers for precision medicine in breast cancer treatment.
LUMINAL SUBTYPE
About 70% of breast cancers are ER positive and show a more favorable prognosis than ER-negative cancers. Within ER-positive/HER2-negative breast cancer, 90%–95% are luminal A or B subtypes [3]. The luminal B subtype shows higher proliferation gene expression [2] and worse recurrence-free survival outcomes compared with the luminal A subtype, although the luminal B subtype shows higher pathological complete response (pCR) rate following neoadjuvant chemotherapy [3]. Thus, differentiation between luminal A and B tumors is important for deciding the duration of endocrine therapy or to predict resistance to endocrine therapy [3]. There is a 30 to 44% discordance rate between the gene expression profiling and surrogate IHC classifications [3,21]. Within ER-positive/HER2-negative breast cancers, 5%–10% of tumors are non-luminal subtypes (HER2 enriched and basal-like tumors) by gene expression profiling [3]. Non-luminal (ER positive/HER2 negative) breast cancers show worse outcomes compared with the luminal A subtype when they were treated with 5 years of adjuvant tamoxifen-only [22]. One study reported that 80% of tumors showing low expression ER positive (1%–9%) were non-luminal subtypes [23].
For patients with ER-positive tumors, prognostic signatures including 70-gene MammaPrint microarray assay (Agendia, Amsterdam, The Netherlands), the 50-gene PAM50 assay (Prosigna, Nanostring Technologies, Seattle, WA, USA), and the 21-gene Oncotype DX assay (Genomic Health, Redwood City, CA, USA) are commercially available [24]. These signatures allow us to distinguish prognosis of patients according to the proliferation-associated genes expression levels [24]. However, the signatures do not apply to patients with ER-negative tumors, because more than 95% of them already have high expression of proliferation-associated genes [24,25]. The oncotype DX assay analyzes a panel of 21 genes to decide a recurrence score (RS) representing the possibility of recurrent cancer within 10 years. Adjuvant chemotherapy provides greater benefits for patients with high-RS tumors and does very little for patients with low-RS tumors (Fig. 1) [26].
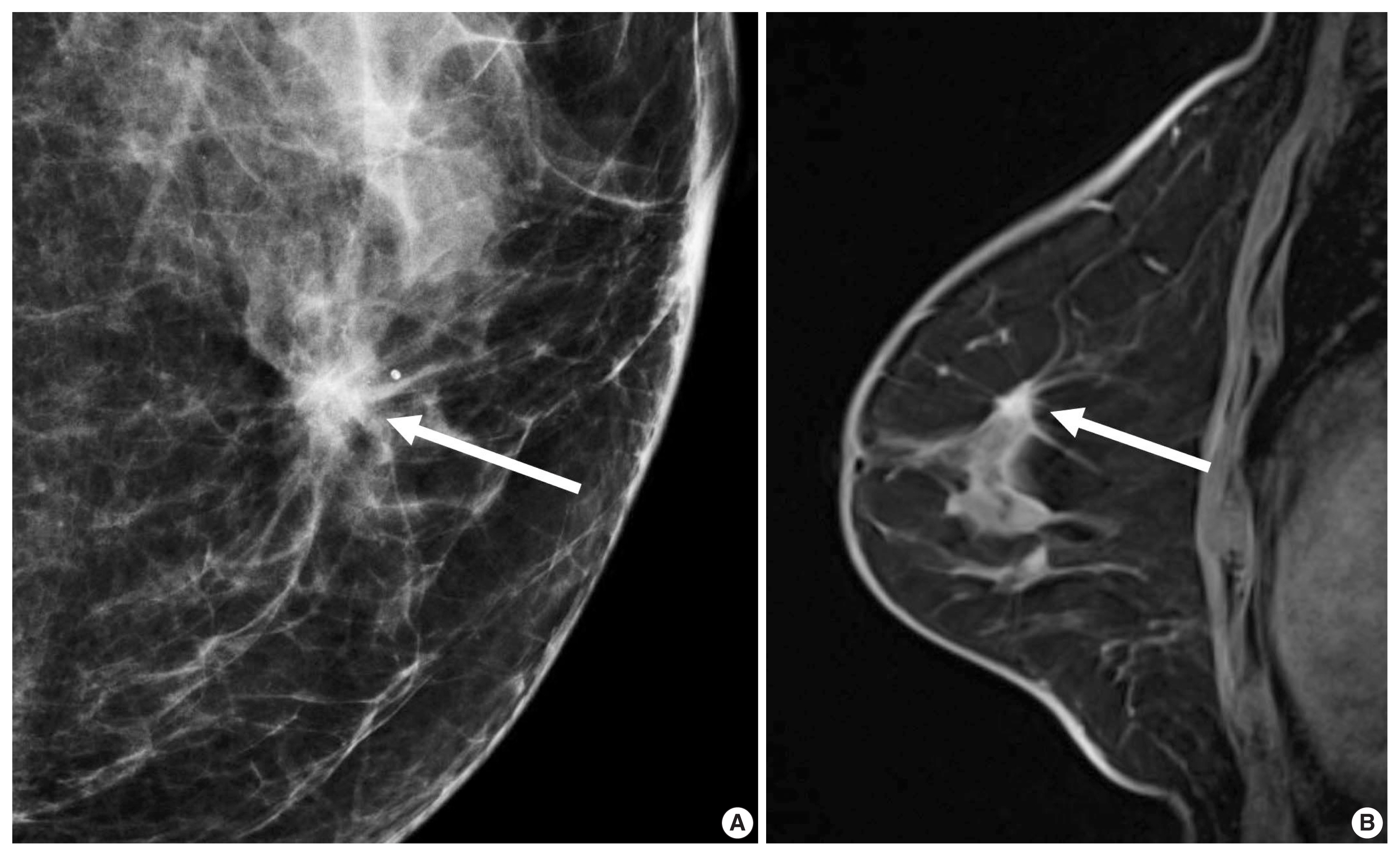
A 56-year-old woman with a luminal A-like breast cancer. (A) Mammography shows a spiculated mass with calcifications (arrow). (B) Enhanced T1-weighted magnetic resonance imaging shows an irregular, spiculated mass (arrow). Histopathology revealed a 1.5-cm invasive ductal carcinoma with low histologic grade. American Joint Committee on Cancer (AJCC) anatomic stage was T1N0M0. Immunohistochemistry analysis revealed that estrogen receptor 90% positive, progesterone receptor 1% positive, human epidermal growth factor receptor 2–negative, and Ki-67, 1% positive. Multigene assay recurrence score was 10 and low risk. The 9-year distant recurrence risk was estimated as 3%. She did not receive adjuvant chemotherapy, but received aromatase inhibitor.
In morphologic analyses for mammography, ER-positive tumor tends to show a not-circumscribed margin (Fig. 1), which is in contrast to ER-negative tumors [12,13,27]. Tumor roundness score, quantifying the relative similarity to a perfect circle, has been shown to have an inverse correlation with the ER expression (%) and a positive correlation with the Ki-67 index [14]. By ultrasonography, parallel orientation (odds ratio [OR], 5.53; p = .02) and tumor roundness (OR, 1.70 per 10 increase in the roundness value; p = .01) were independent features associated with high RS on Oncotype DX [28]. The high-risk group was also associated with the presence of calcifications, similar to a previous study in which a mass with pleomorphic microcalcifications might be associated with an intermediate to high RS in ER-positive, HER2-negative early breast cancer at mammography [29].
A previous report on MRI results demonstrated that patients with luminal B subtype tended to have multifocal or multicentric cancer 2.8 times more often than patients with luminal A subtype [30]. Sutton et al. [15] reported that an increased kurtosis was associated with high RS on Oncotype DX for ER-positive/HER2-negative tumors (Fig. 2). Kurtosis is a second order parameter quantifying the amount of histogram deviating from a Gaussian shape. High kurtosis might reflect the amount of heterogeneity in a complex way, and these tumors are believed to be more biologically aggressive [19]. This result is in line with a study reporting that both ER-positive and ER-negative tumors showed statistically different entropy levels [16]. The entropy reflecting spatial distribution pattern of grayness of voxel is also believed to be an important biomarker at textural analysis of medical image.
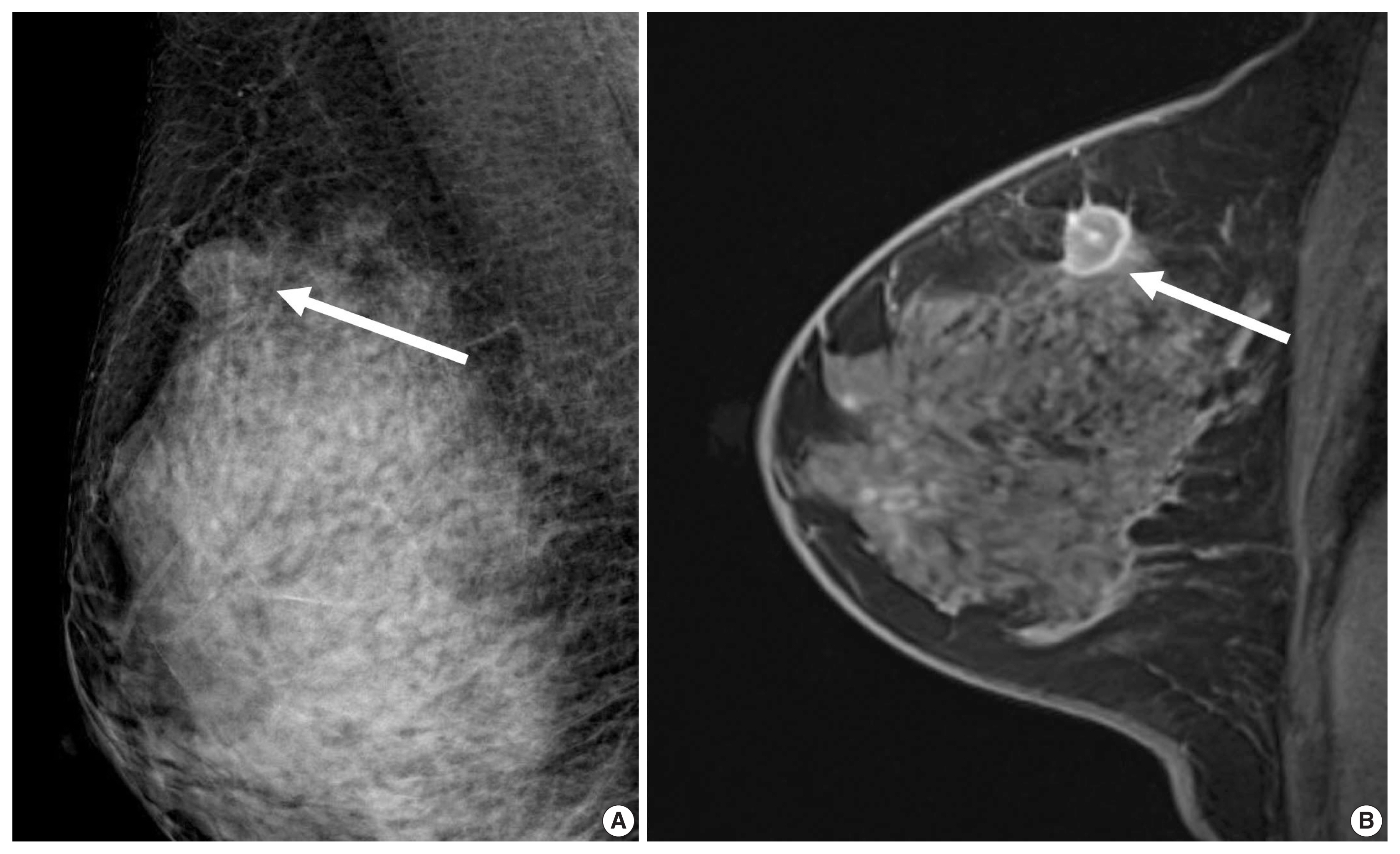
A 44-year-old woman with a luminal A-like breast cancer. (A) Mammography shows an oval non-calcified mass (arrow). (B) Enhanced T1-weighted magnetic resonance imaging shows an irregular mass with internal rim-enhancement (arrow). Histopathology revealed a 1.7-cm invasive ductal carcinoma with intermediate histologic grade. American Joint Committee on Cancer (AJCC) anatomic stage was T1N0M0. Immunohistochemistry analysis revealed that estrogen receptor 90% positive, progesterone receptor 5% positive, human epidermal growth factor receptor 2 negative, and Ki-67, 4% positive. Multigene assay recurrence score was 23. The 10-year distant recurrence risk was estimated as 12% and high risk. She received adjuvant chemotherapy and tamoxifen.
HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2–ENRICHED SUBTYPE
HER2 overexpression is found in approximately 20% of invasive breast cancers. It is associated with worse prognosis but good response to HER2-targeted therapies [31] and is reported to increase cell proliferation, survival, mobility, and invasiveness, as well as neo-angiogenesis at the cellular level [32]. The Cancer Genome Atlas (TCGA) and clinical trials have suggested that HER2-positive tumors are a heterogeneous group of cancers [3]. Compared with ER-positive/HER2-positive tumors, patients with ER-negative/HER2-positive tumors show a higher risk of death within 5 years of diagnosis; the first recurrence in brain was higher and in bone was lower, and the response rate to neoadjuvant chemotherapy was higher [33,34]. HER2-targeted agents combined with chemotherapy are recommended for ER-negative/HER2-positive tumors, and HER2-targeted agents with endocrine therapy are recommended for ER-positive/HER2-positive tumors [4]. A higher pCR rate was observed in patients with ER-negative/HER2-positive tumors than in patients with ER-positive/HER2-positive tumors [35]. The pCR rate was over 70% using the dual HER2 blockade, either with trastuzumab with lapatinib or trastuzumab with pertuzumab in combination with chemotherapy [6].
HER2 overexpression was also associated with the presence of calcifications, branching or fine linear shape calcifications, high level of suspicion on mammography, and a washout or fast early enhancement kinetic curve pattern on MRI (Fig. 3) [36]. MRI showed that patients with the HER2 subtype tended to have 4.1 times more multifocal or multicentric cancers than patients with the luminal A subtype [30]. Fine pleomorphic/fine linear or linear branching calcification morphology on mammography (OR, 7.23), PR negativity (OR, 6.76), and a high TILs (tumor infiltrating lymphocytes) level (OR, 5.92) were independent factors associated with pCR in patients receiving neoadjuvant chemotherapy with dual HER2 blockade (Fig. 3) [37]. Low tumor peak enhancement at MRI indicating less aggressiveness was significantly associated with high TILs (OR, 1.01; p = .020) [38]. These results are consistent with previous studies showing that TILs observed in breast cancers were associated with higher rate of pCR or improved overall survival outcomes [5,6]. Moreover, increasing TILs during systemic therapy was reported to be correlated with pCR [39], which suggests that MRI could provide valuable information regarding response during treatment.
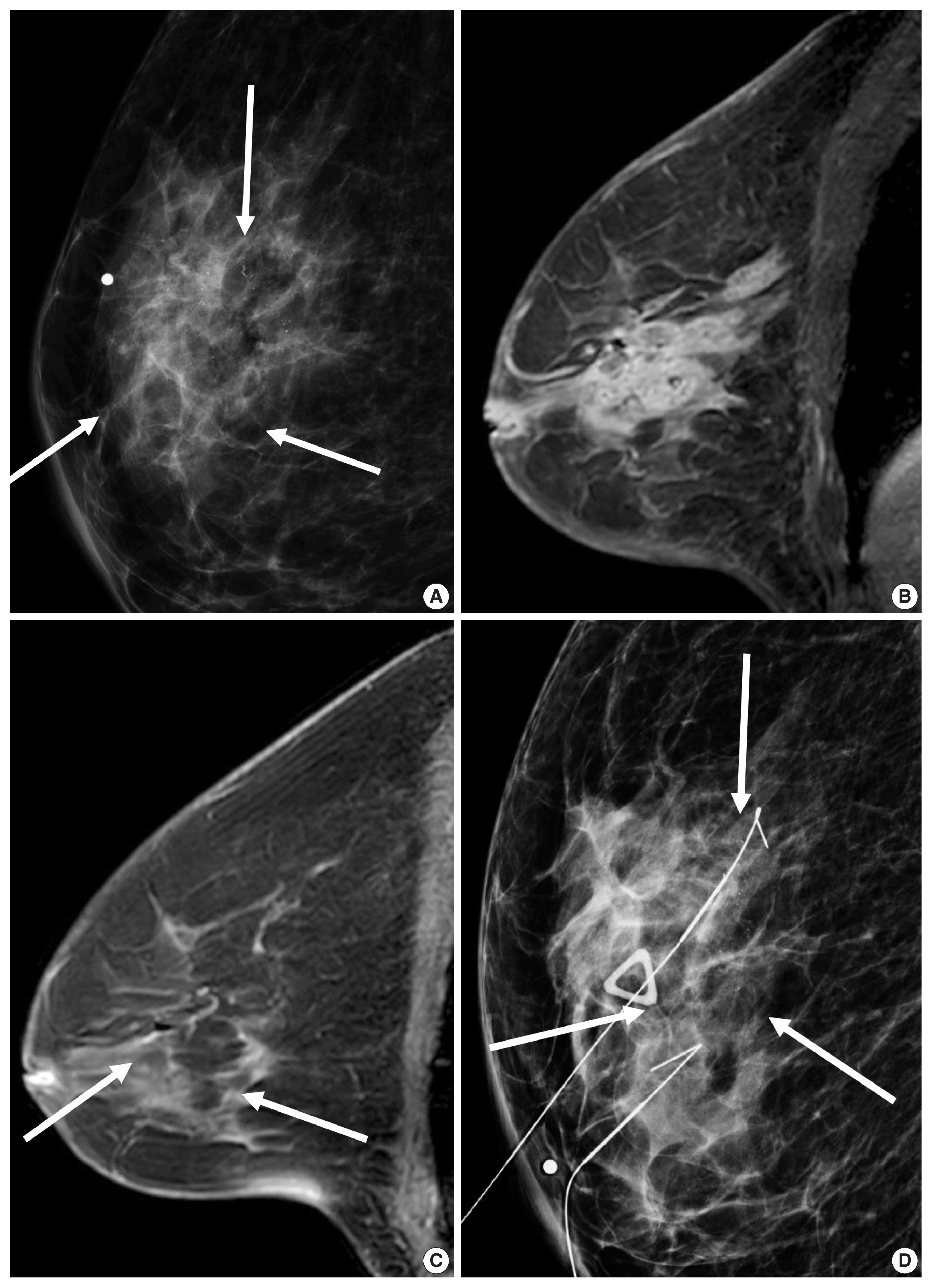
A 67-year-old woman with a human epidermal growth factor receptor 2 (HER2)–positive breast cancer. (A) Mammography shows ill-defined asymmetry with pleomorphic microcalcifications (arrows). (B) Enhanced T1-weighted magnetic resonance imaging (MRI) shows an 8.2-cm ill-defined, diffuse irregular mass with internal heterogeneous enhancement. Needle biopsy revealed an invasive ductal carcinoma with high histologic grade. Immunohistochemistry analysis revealed that estrogen receptor and progesterone receptor negative, and HER2 positive. (C) Following combined docetaxel, carboplatin and dual HER2 blockade, there is no residual mass and but subtle enhancements in the breast on MRI (arrows). (D) Mammography shows two hookwires around the residual calcifications (arrows). Surgical histopathology revealed pathological complete response in the breast and axilla.
BASAL-LIKE SUBTYPES
TNBC comprises 10%–20% of all breast cancers. The term of TNBC and basal-like tumors are interchangeably used because 86% of TNBC are the basal-like subtype [3]. However, each of the intrinsic subtypes exist within a TNBC [40], and TNBC is a very heterogeneous group of tumors based on genetic profiling [41]. Rates of pCR after receiving anthracycline/taxane regimen are 25%–35% and patients with pCR show a better outcome in patients with TNBC [25]. Recent St. Gallen Consensus Conference Guidelines recommended that TILs should be routinely characterized for TNBC in view of their prognostic value [6]. Tumor programmed death-ligand 1 and immune-cell programmed death-1 expression are considered as markers to predict benefit from immunotherapy for advanced TNBC [6]. Also, in TNBC with residual disease following neoadjuvant chemotherapy, post-neoadjuvant treatment with capecitabine showed survival benefits [42].
Unifocal, circumscribed margin, round shape, and no associated calcifications are signatures of TNBC [43]. Circumscribed margin and round shape are more commonly found in high-grade tumors and spiculation is more frequently found in low-grade tumors [44]. TNBC has been shown to have a higher tumor roundness score compared with the other subtypes, reflecting a more biologically aggressive tumor type. Absence of calcifications is also considered representative of rapid malignant transformation of TNBC with bypassing of the in-situ stage [43]. TNBC shows a round mass with rim enhancement on MRI and frequently has internal high signal intensity on T2-weighted MRI [45–47] (Fig. 4). Recent studies using radiomics analysis have reported that TNBC masses tend to be larger, have a more heterogeneous enhancement texture, are more irregularly shaped, and have a rapid enhancement rate compared with other subtypes [17,18]. Notably, the heterogeneous enhancement texture, quantified at the first post-contrast enhanced MRI, has emerged as a discriminatory indicator for tumor subtype, regardless of tumor size [17,18]. Waugh et al. [16] also reported that TNBC and HER2 subtypes showed increased entropy values compared with luminal A and luminal B subtypes.
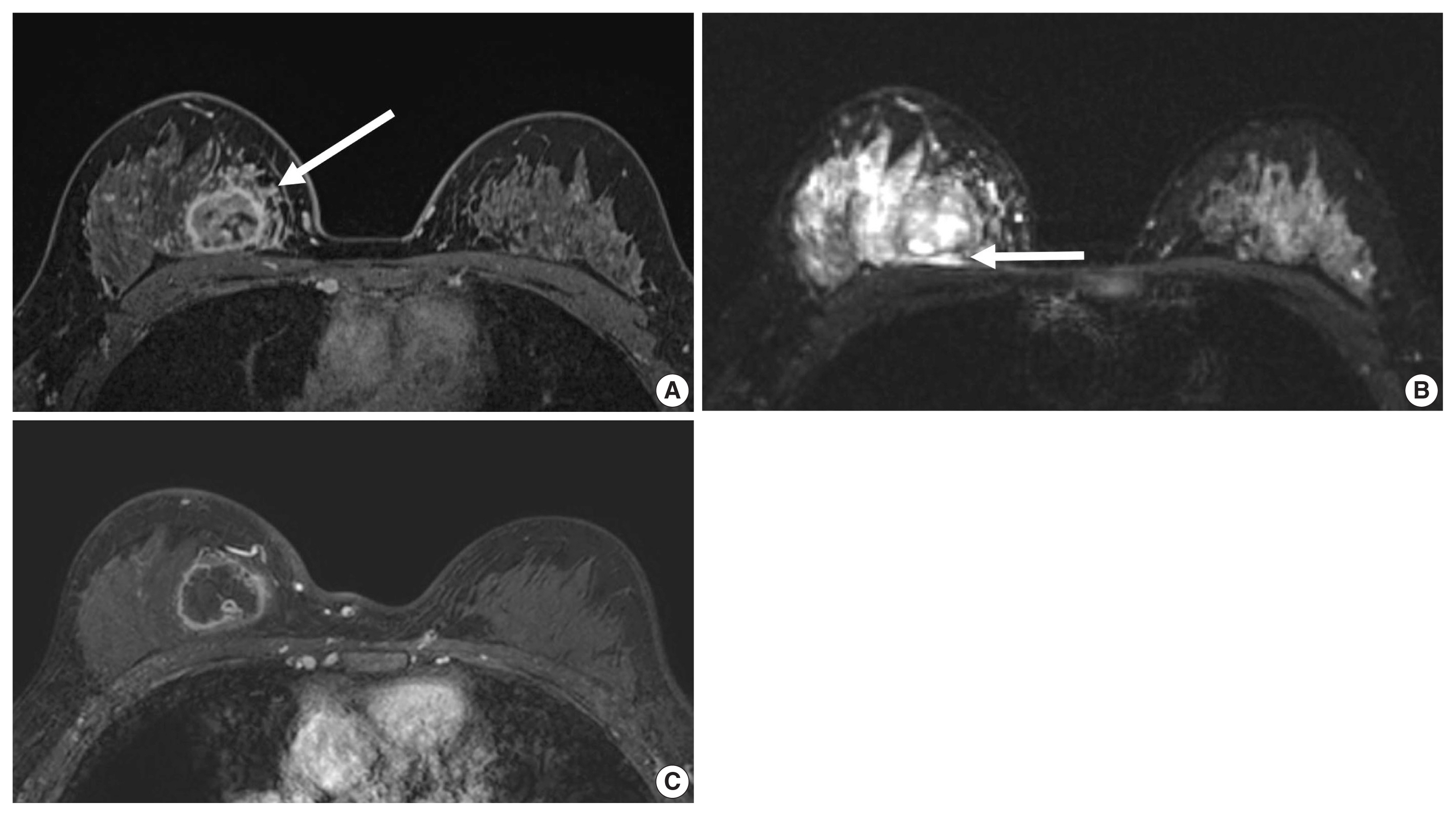
A 35-year-old woman with a triple-negative breast cancer. (A) Enhanced T1-weighted magnetic resonance imaging (MRI) shows a 3.3 cm round mass with an internal rim enhancement and peritumoral heterogeneous enhancement (arrow) (B) T2-weighted MRI shows a central cystic necrosis and peritumoral edema (arrow). Needle biopsy revealed an invasive ductal carcinoma with high histologic grade. Immunohistochemistry analysis revealed that estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 negative. (C) Following chemotherapy, enhanced T1-weighted MRI shows a 3.4 cm round mass without response to chemotherapy.
In the neoadjuvant chemotherapy setting, intratumoral necrosis was associated with non-response to chemotherapy [48] and peritumoral edema was associated with worse recurrence-free-survival outcome of TNBC (Fig. 4) [49]. With textural analysis, increased kurtosis of non-TNBC on T2 weighted image was independently associated with pCR; however, the association between increased kurtosis and pCR was not found in TNBC [19]. In another study predicting pCR by using radiomics, Braman et al. reported that combined intratumoral and peritumoral radiomic features yielded a maximum AUC of 0.83 for the ER-positive/HER2-negative group and 0.93 for TNBC or HER2-positive group [20]. In Braman et al’s study [20], elevated peritumoral heterogeneity was associated with non-pCR in ER-positive/HER2-negative tumors, and peritumoral speckled enhancement pattern was associated with non-pCR in TNBC or HER2-positive tumors (Fig. 4A) [20]. These results are in line with peritumoral lymphatics or vascular invasion, and peritumoral immune response as predictors of survival. In addition, TILs, known as a favorable prognostic factor in TNBC, could be quantified by textural analysis [20]. Thus, peritumoral radiomics features on MRI could be valuable predictors of pCR in TNBC and HER2-positive tumors.
CONCLUSION
Breast cancer consists of heterogeneous subtypes and evolves continuously after systemic therapy. Earlier studies linking imaging features and molecular subtypes have reported presence of calcifications, margin or shape features, and enhancement features on dynamic contrast enhanced MRI according to each subtype. Recent studies using radiomics parameters, which are indiscernible by the human eye, have shown high accuracy in distinguishing molecular subtypes, prediction of responses to chemotherapy, and prediction of survival outcomes. Imaging biomarkers could be helpful in realizing better precision medicine due to the feasibility of repeated measurements for whole tumors and the applicability of deep-learning based algorithms.
Notes
Ethics Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.

