A sinonasal yolk sac tumor in an adult
Article information
Abstract
Yolk sac tumors (YSTs), which are also called endodermal sinus tumors, are malignant tumors of germ cell origin. These tumors usually occur in the gonads, but 20% of cases have been reported at extragonadal sites. The head and neck is a rarely affected region that accounts for just 1% of all malignant tumors of germ cell origin. In addition, YSTs arise mostly in childhood. We present a rare pathologically pure case of primary adult YST in the sinonasal area. A 45-year-old male patient presented with a rapidly growing mass in the nasal cavity, which caused nasal obstruction and bloody post-nasal drip. The histopathologic features indicated pure YST, and immunohistochemical analysis revealed positive reactivity for Sal-like protein 4 and alpha-fetoprotein. Herein, we discuss the clinical, radiologic, and histologic features of this YST and review other cases of sinonasal YST in adults.
Yolk sac tumors (YSTs), which are also called endodermal sinus tumors, are malignant tumors of germ cell origin. These tumors usually occur in the gonads, but 20% of cases have been reported at extragonadal sites. The head and neck is a rarely affected region that accounts for just 1% of all malignant tumors of germ cell origin [1]. In addition, YSTs arise mostly in childhood. To our knowledge, there are only eight reported cases of sinonasal YSTs in adult patients. We report a very rare case of a pathologically pure form of primary adult YST in the sinonasal area.
CASE REPORT
A 45-year-old man was referred to the Department of Otorhinolaryngology at Asan Medical Center in Seoul for persistent right nasal obstruction, bloody rhinorrhea, and post-nasal drip. He reported a history of bloody post-nasal drip for the past four months, facial pain for the last three months, visual disturbance in the right lower field, and diplopia. An endoscopic examination showed a 3-cm-sized mass obstructing the right nasal cavity with partially necrotic tissue.
Initial computed tomography and magnetic resonance imaging revealed a mass that obstructed the right nasal cavity and caused bone destruction of the bilateral frontal and ethmoidal sinuses with intracranial extension. Destruction of the bilateral superomedial orbital wall and invasion of the right medial orbital wall and right nasolacrimal duct and sac were also identified (Fig. 1).

Coronal (A) and transverse (B) magnetic resonance imaging shows a mass in the right nasal cavity and bilateral frontal sinus.
Endoscopic excisional biopsy was carried out. The specimen was removed in three pieces measuring up to 4 × 3 × 1.5 cm, with a pinkish brown color and a fleshy gelatinous texture. The submitted specimen was processed for histopathological examination and revealed typical YST features. The tumor showed mostly reticular and microcystic growth patterns with loose myxoid stroma, but there were also areas of glandular, solid sheet-like, and polyvesicular vitelline patterns. Some Schiller-Duval bodies were noted. Intra- and extra-cytoplasmic hyaline globules were identified (Fig. 2). No other tumor components were seen.
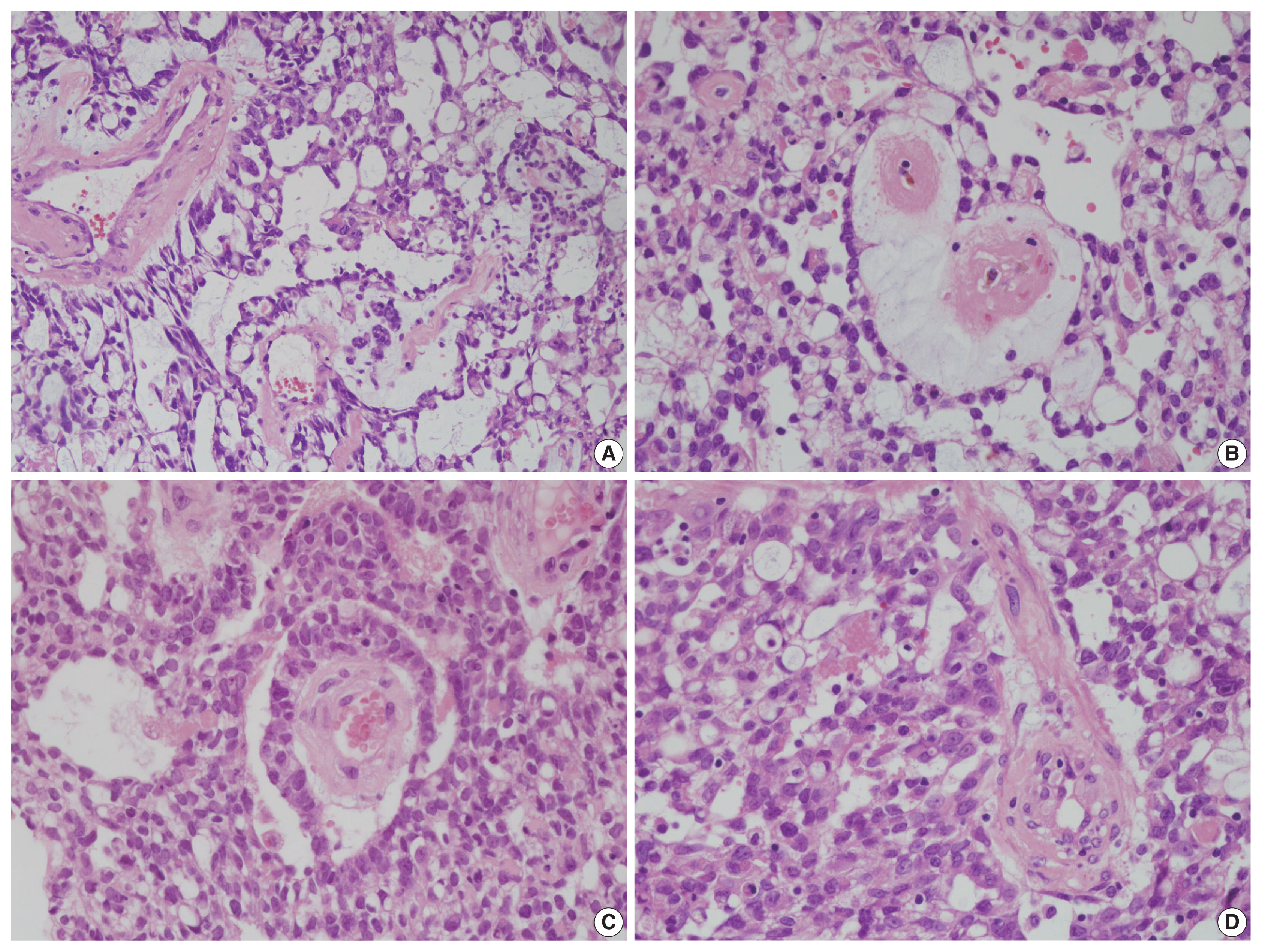
Histopathologic findings of a sinonasal yolk sac tumor. (A) Reticular and microcystic growth patterns are observed with loose myxoid stroma. (B) A polyvesicular vitelline pattern is occasionally observed. (C) Schiller-Duval bodies are noted focally. (D) Intra- and extra-cytoplasmic hyaline globules are observed.
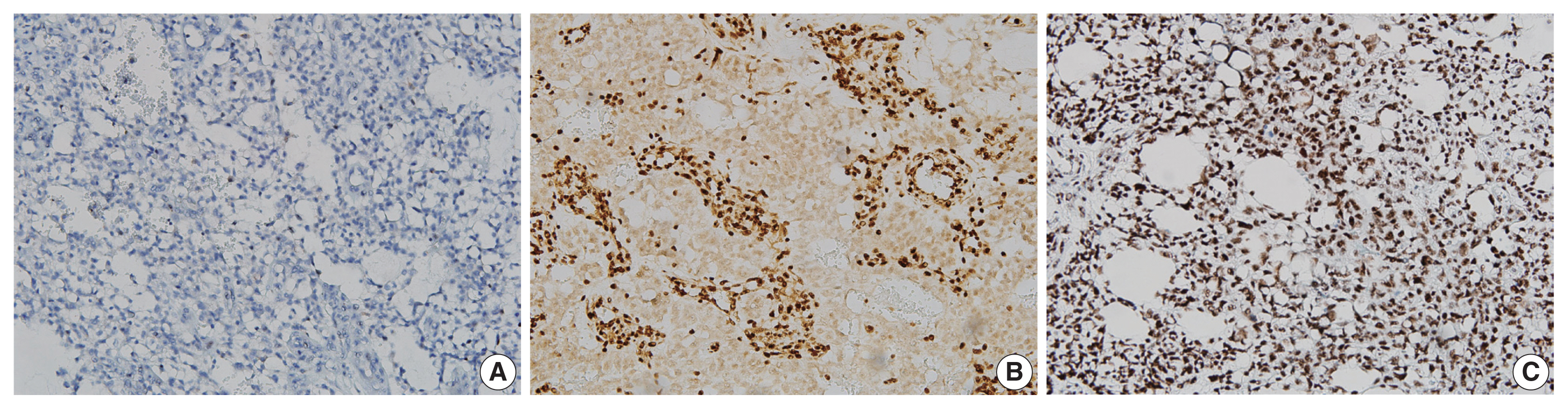
Immunohistochemical staining showed diffuse strong nuclear positivity for Sal-like protein 4 and diffuse strong cytoplasmic positivity for α-fetoprotein (Fig. 3). P40 was negative, but integrase interactor 1 (INI1) and Brahma-related gene-1 (BRG1) were both positive (Fig. 4). Therefore, the final diagnosis was pure YST. The patient was started on chemotherapy with a bleomycin, etoposide, and cisplatin regimen and palliative radiation therapy but showed a poor response. He died from the YST at 13 months after diagnosis.
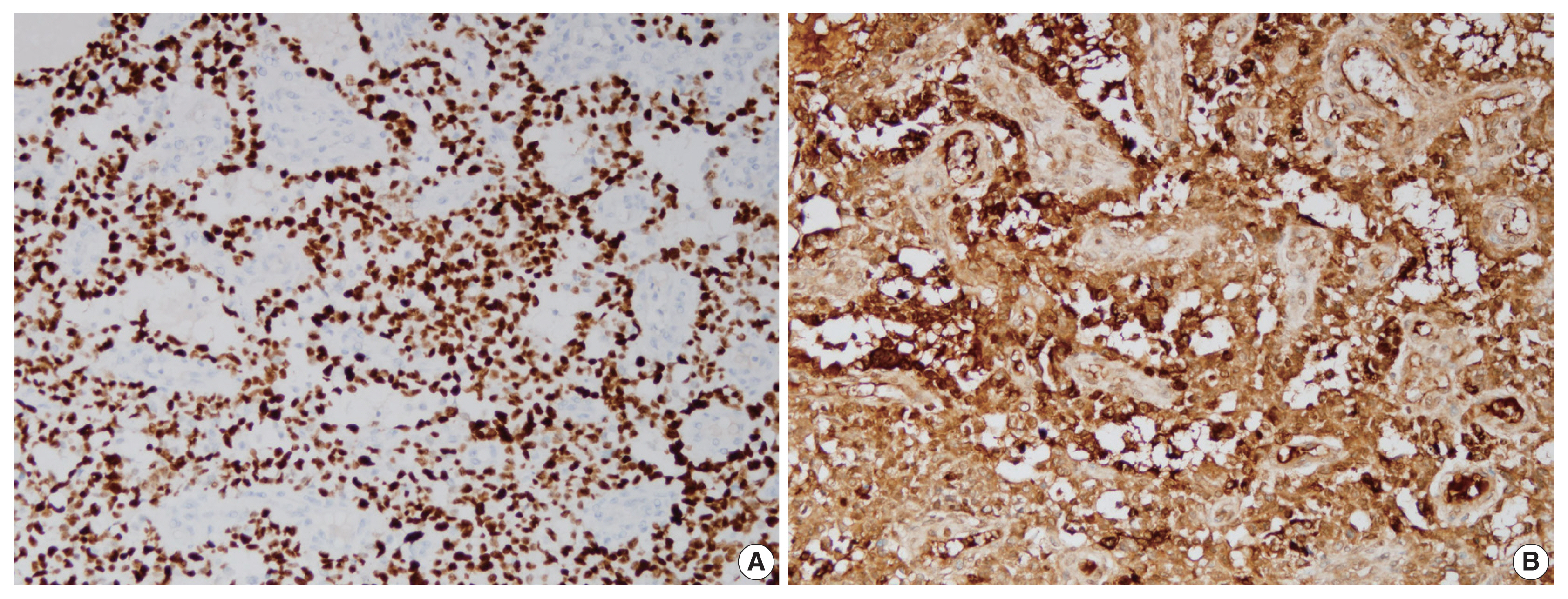
On immunohistochemical staining, the tumor cells show diffuse strong nuclear positivity for Sal-like protein 4 (A) and diffuse strong cytoplasmic positivity for α-fetoprotein (B).
DISCUSSION
YST usually occurs in the gonads and is primarily found in infants and children. Extragonadal sites have included the sacrococcygeal region, retroperitoneum, mediastinum, brain, and very rarely, the head and neck area [2]. In addition, YST is usually accompanied by other germ cell tumor components, like teratoma or embryonal cell carcinoma. For example, the pure form YST of the testis accounts for just 2.4% of the reported adult testicular YSTs [3].
Definite diagnosis of YST can be made by biopsy. Upon microscopy, several histological patterns have been described. The most common is a reticular/microcystic pattern, which is a meshwork of anastomosing spaces and cysts lined by a single layer of tumor cells [4]. Other patterns include endodermal sinus, solid, myxomatous, papillary, glandular, macrocystic, and polyvesicular vitelline [4]. Our case demonstrated reticular, microcystic, and polyvesicular vitelline growth patterns and Schiller-Duval bodies. Shiller-Duval bodies are specific for YST, but they are not essential for diagnosis. The differential diagnoses of this case include sinonasal nonintestinal-type adenocarcinoma, SMARCB1-deficient sinonasal carcinoma with pure yolk sac differentiation, and SMARCA4-deficient teratocarcinosarcoma. Clear cytoplasm, an occasional fibrovascular core, and cytokeratin 7 negativity exclude sinonasal nonintestinal-type adenocarcinoma. SMARCB1-deficient sinonasal carcinoma usually shows high-grade histologic features, including prominent necrosis and mitoses, but relatively uniform cytology [5]. In our case, INI1 positivity excluded the possibility of SMARCB1-deficient sinonasal carcinoma with pure yolk sac differentiation, and BRG1 positivity ruled out the possibility of SMARCA4-deficient teratocarcinosarcoma.
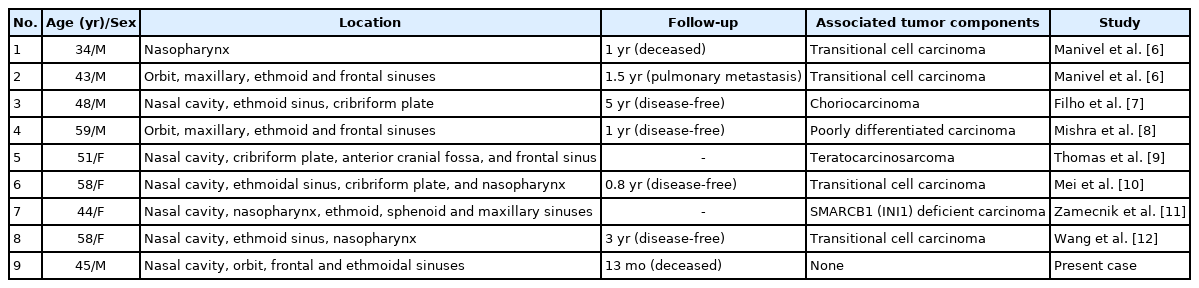
Eight adult-onset YSTs have been reported in the sinonasal area in the English literature (Table 1) [6–12]; four cases were accompanied by a transitional cell carcinoma component. Choriocarcinoma, poorly differentiated carcinoma, teratocarcinosarcoma, and SMARCB1 (INI1)-deficient carcinoma components were present in the remaining four cases, respectively. The reason for the common coexistence of a transitional cell carcinoma component in sinonasal YST remains uncertain. In our case, a transitional cell carcinoma component was not observed, as confirmed by p40 negativity. There are two theories about the origin of extragonadal YST. In the first theory, primordial germ cells are arrested or misplaced during migration from the cranial space to a gonadal site and consequently arrest in the cranial cavity [13]. The second theory holds that extragonadal tumors arise as a result of aberrant somatic differentiation [14]. Previous reports support the second theory. In contrast to previous cases, our case showed histologically pure YST features, which have never been reported. Our case therefore supports the first theory because its histologic features included pure YST not accompanied by other tumor components.
Surgical resection, combined chemotherapy, and radiotherapy are available treatments for YST. Generally, YST is sensitive to chemotherapy. However, the head and neck region, including the sinonasal area as in our case, is very complex and difficult for R0 resection. As a result, the anatomic location seems to affect the prognosis, although the number of sinonasal YST cases is very small [15]. In addition, increasing age is known to negatively affect clinical behavior, and this trend is consistent with our case findings [16].
In conclusion, although adult-onset sinonasal pure YST is extremely rare, differential diagnosis is important. YST can present with many various scenarios in the sinonasal area, so YST should be considered if the clinical findings are histologically suspicious.
Notes
Ethics Statement
This case was deemed exempt by the Asan Medical Center Institutional Review Board (IRB #2019-0598). Patient consent waiver was obtained for this study.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: KJC, KCJ, JS. Data curation: JHK, KCJ, KJC, JS. Methodology: KJC, JS. Writing—original draft: KJC, JS. Writing—review & editing: KJC, JS. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.