Renal cell carcinoma concomitant with multiple myeloma
Article information
The concomitant presentation of multiple myeloma (MM) and renal cell carcinoma (RCC) in same patient is rare. The risk of developing a secondary neoplasia in primary malignancies is much higher (~31%) compared to the general population. The most common hematological malignancy associated with RCC is non-Hodgkin lymphoma. An association between MM and RCC has been reported previously, but literature on the topic is sparse. Secondary malignancies concomitant with MM are thought to be mostly due to the use of chemotherapeutic agents (alkylating agents) in addition to genetic and environmental factors. As treatment of RCC does not include alkaylating agent, their role in post RCC, MM can be debated and indicates shared risk factor beyond chemotherapy as aetiology. The fact is supported by SEER (Epidemiology and End Results registry) data of bidirectional occurrence RCC followed by MM and vice versa. These shared risk factors include age, lifestyle, environmental factors and genetic mutations [1-3]. Although many hypotheses have been proposed to establish an association between MM and RCC, none have been proven. Here we report an association of incidentally-detected clear cell RCC in a known case of MM.
Our patient was a 63-year-old male with a baseline diagnosis of MM IgA kappa (International Staging System II/DurieSalmon Staging System III A) in 2011. He received four cycles of RD regimen (lenalidomide-dexamethasone) and exhibited a complete response. He underwent autologous stem cell transplant in 2011 followed by thalidomide maintenance. After treatment, the patient had his first relapse in November 2015, for which he received four cycles VRD (bortezomib-lenalidomide-dexamethasone), to which he exhibited a partial response and was kept on RD maintenance.
In January 2020, the patient was re-evaluated and was found to have 68% plasma cells on bone marrow. M-band was 5.5 g/dL; serum-free light-chain (SFLC) kappa 898 and lambda of 14.6 with SFLC ration 61.5. Bone marrow fluorescent in-situ hybridization analysis demonstrated a deletion in the 16q23 chromosome. Whole-body positron emission tomography computed tomography scan showed multiple lytic lesions in the left parietal, sphenoid, basiocciput, clivus, bilateral mandible, humeri, sternum, ribs, femur, and sacrum along with a lower pole left renal mass. The patient was started on dexamethasone pulse therapy followed by bortezomib, pomalidomide and dexamethasone, meanwhile the renal mass was evaluated. Renal mass biopsy showed features of clear cell RCC. The patient has completed three cycles of the chemotherapy and is under follow-up monitoring (Fig. 1).
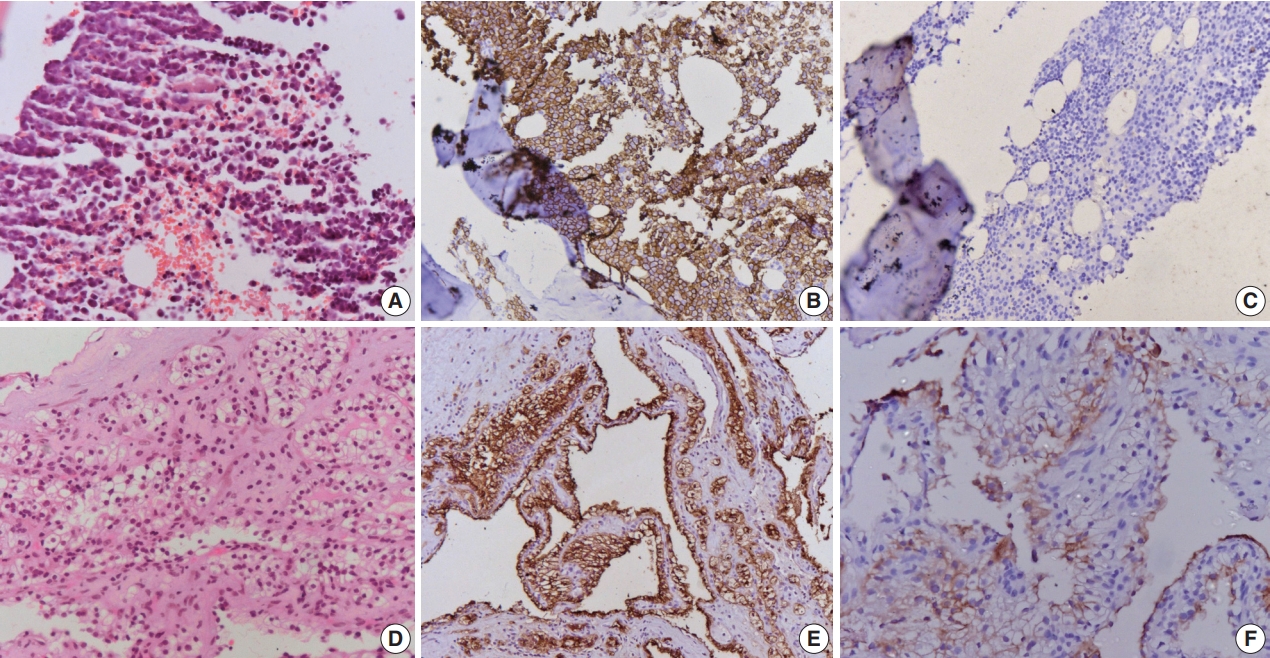
(A) Microphotograph of lytic lesion biopsy shows sheets of mature plasma cells with mild atypia. (B) Cells are immunopositive for CD138, (C) while negative for cytokeratin. (D) Biopsy of the renal mass shows cells arranged in sheets with clear cytoplasm and small nuclei. The cells are immunopositive for cytokeratin (E) and CD10 (F).
Ojha et al. [1] found that the relative risk of RCC is higher (89%) among post MM cases than MM in post RCC (51%) compared to the general population. The index patient is a 63-year-old male with MM and concomitant RCC as a second primary malignancy after a time gap of 120 months [1].
The recently proposed bidirectional association between MM and RCC, may be related to certain genetic risk factors. The roles of c-met receptor (family of MET gene) and its ligand hepatocyte growth factor are well-established in MM and RCC. Hence, MET may be a candidate gene for understanding the bidirectional association between MM and RCC [1,4]. Both synchronous and metachronous occurrence of MM and RCC have been described in literature, with time intervals between the two varying from 1 to 300 months for metachronous cases. There is a male predominance with median age of 50 years. The most common histological type was clear cell-type with occasional cases of chromophobe- and transitional-type RCC. RCC was treated by partial or complete nephrectomy in these cases. MM exhibited morphology ranging from classical to anaplastic variant [5,6].
However, considering the previous literature and the present case, myeloma should be suspected in RCC patients with new lytic bone lesions, and any renal masses should be carefully investigated in MM patients.
Notes
Ethics Statement
Not applicable.
Availability of Data and Material
Availability of Data and Material: Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author contributions
Conceptualization: SM. Data curation: AN. Formal analysis: AN. Investigation: LK. Methodology: PR. Writing—original draft: AN, PR. Writing—review & editing: SM. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
