Clinicopathologic features and survival outcomes of ocular melanoma: a series of 31 cases from a tertiary university hospital
Article information
Abstract
Background
We aimed to determine the effect of clinicopathologic features on overall survival among Caucasian ocular melanoma patients in the Central Anatolia region of Turkey.
Methods
This single-center study included conjunctival (n = 12) and uveal (n = 19) melanoma patients diagnosed between January 2008 and March 2020. Clinicopathologic features and outcomes were reviewed retrospectively. Five cases were tested for BRAF V600 mutations with real-time polymerase chain reaction, and one case was tested with next-generation sequencing. Survival was calculated using the Kaplan-Meier method.
Results
Thirty-one patients had a mean initial age of 58.32 years (median, 61 years; range 25 to 78 years). There were 13 male and 18 female patients. The median follow-up time was 43.5 months (range, 6 to 155 months) for conjunctival melanoma and 35 months (range, 8 to 151 months) for uveal melanoma. When this study ended, eight of the 12 conjunctival melanoma patients (66.7%) and nine of the 19 uveal melanoma patients (47.4%) had died. The presence of tumor-infiltrating lymphocytes was related to improved overall survival in conjunctival melanoma (p = .014), whereas the presence of ulceration (p = .030), lymphovascular invasion (p = .051), tumor in the left eye (p = .012), tumor thickness of > 2 mm (p = .012), and mitotic count of >1/mm2 (p = .012) reduced the overall survival in conjunctival melanoma. Uveal melanoma tumors with the largest diameter of 9.1–15 mm led to the lowest overall survival among subgroups (p = .035). Involvement of the conjunctiva (p=.005) and lens (p = .003) diminished overall survival in uveal melanoma. BRAF V600 mutation was present in one case of conjunctival melanoma, GNAQ R183Q mutation was present in one case of uveal melanoma. Patients with uveal melanoma presented with an advanced pathological tumor stage compared to those with conjunctival melanoma (p = .019).
Conclusions
This study confirmed the presence of tumor-infiltrating lymphocytes as a favorable factor in conjunctival melanoma and conjunctival and lens involvement as unfavorable prognostic factors in uveal melanoma for overall survival, respectively.
Two types of ocular melanoma exist: uveal melanoma (UM) and conjunctival melanoma (CM) [1]. UM represents 79%–91% of all ocular melanomas [2], whereas CM represents < 10% [3]. The incidence of CM in the white population is 0.2–0.8 cases/million [3]. The incidence of UM in the American and European populations is 5–6 cases/million, while it is 7 cases/million in Australia [4]. UM is most commonly seen in Caucasians, followed by Hispanics, Asians, and Blacks, with decreasing frequency [5]. UM's incidence has been relatively stable over the years [6]; however, CM's incidence has shown a rising trend similar to that of cutaneous melanoma [3,7–11]. Although CM is rare, it has the potential to metastasize not only to the eye, eyelid, orbit, and surrounding lymphatics but also to distant sites like the lungs, skin, liver, and brain [12]. The 10-year mortality rate for UM is 50% in the U.S. and higher than other melanoma subtypes [2,13]. Although ocular melanoma is rare, its morbidity is also high, leading to vision loss even in surviving patients. Thus, this study aimed to evaluate the effects of clinicopathologic features on overall survival (OS) among ocular melanoma patients and discuss the relevant literature to highlight some aspects of these deadly diseases.
MATERIALS AND METHODS
This single-center study included CM (n = 12) and UM (n = 19) patients diagnosed between January 2008 and March 2020. The final follow-up was conducted by April 2021. Clinicopathologic and outcome data from electronic medical records were reviewed retrospectively.
Baseline clinical variables assessed included age, sex, location and laterality of the tumor, primary surgical treatment, known comorbidities. Pathologic features of each tumor were reviewed primarily from archival slides stained with hematoxylin and eosin and pathology reports. Any histochemical, immunohistochemical, and molecular studies performed at the time of diagnosis were recorded. Real-time polymerase chain reaction (RT-PCR) for BRAF V600 mutation was tested using either the AmoyDx BRAF V600 mutations detection kit (Amoy Diagnostics Co., Xiamen, China) or Cobas BRAF V600 mutation kit (Roche, Pleasanton, CA, USA). Next-generation sequencing (NGS) with Qiagen GeneReader workflows (Qiagen, Hilden, Germany) was performed according to the manufacturer’s protocol by isolating DNA from the enucleation specimen for UM. Pathological tumor (pT) staging was performed according to the eighth edition of the American Joint Committee on Cancer (AJCC) staging system for UM and CM [14]. Microscopic satellitosis was evaluated as a separated tumor nest from the primary tumor by a normal stroma as described previously by Esmaeli et al. [15]. Infiltration of the iris, ciliary body, anterior chamber, Schlemm canal, lens, posterior chamber, choroid, retina, vitreous, sclera, optic disc, and conjunctiva was evaluated in UM patients [16]. Growth pattern (solid mass, dome shape, mushroom shape [17], or diffuse [18]) was classified as previously described for UM cases [16]. Tumor diameter [16], histologic type and grade [19], and nucleolar prominence were additionally assessed as histopathologic parameters in UM cases.
OS time was calculated as the time from diagnosis to death from any cause or to last follow-up (for surviving patients) within each group (CM and UM) separately. Kaplan-Meier survival analyses and log-rank tests were conducted to identify statistically significant univariable predictors of OS using the SPSS ver. 23 (IBM Corp., Armonk, NY, USA). The statistically significant difference between groups was determined as p < .05. The frequencies of common categorical variables for UM and CM were compared using the 2-tailed Fisher exact test.
RESULTS
Ocular melanoma patient demographics and clinical history
Thirty-one patients had a mean initial age of 58.32 ± 0.50 years (median, 61 years; range, 25 to 78 years). There were 13 male and 18 female patients. In terms of tumor site, the tumor was located at the uvea for 19 patients (61%) (choroidal, n = 16; ciliary body, n = 2; iris, n = 1), conjunctiva for nine patients (29%) (bulbar, n = 5; palpebral, n = 3; unknown, n = 1), and eyelid for three patients (10%). Primary treatment most commonly included enucleation (n = 3 for CM, n = 17 for UM), followed by local excision (n = 6 for CM, n = 2 for UM), and exenteration (n = 3 for CM).
Conjunctival melanoma
Table 1 represents the main clinicopathologic characteristics of CM cases and their relationship to OS. At diagnosis, the mean age was 56.08 ± 11.21 years (median, 57.5 years; range, 35 to 74 years). The median follow-up time was 43.5 months and ranged from 6–155 months. When this study ended, eight of the 12 patients (66.7%) had died (Fig. 1A). Three patients with left-sided CM had a reduced OS compared to right-sided patients (p = .012) (Fig. 1B).

Univariate analysis of predictors for overall survival time (mo) in conjunctival melanoma, calculated from Kaplan-Meier analysis with comparisons performed with the log-rank test
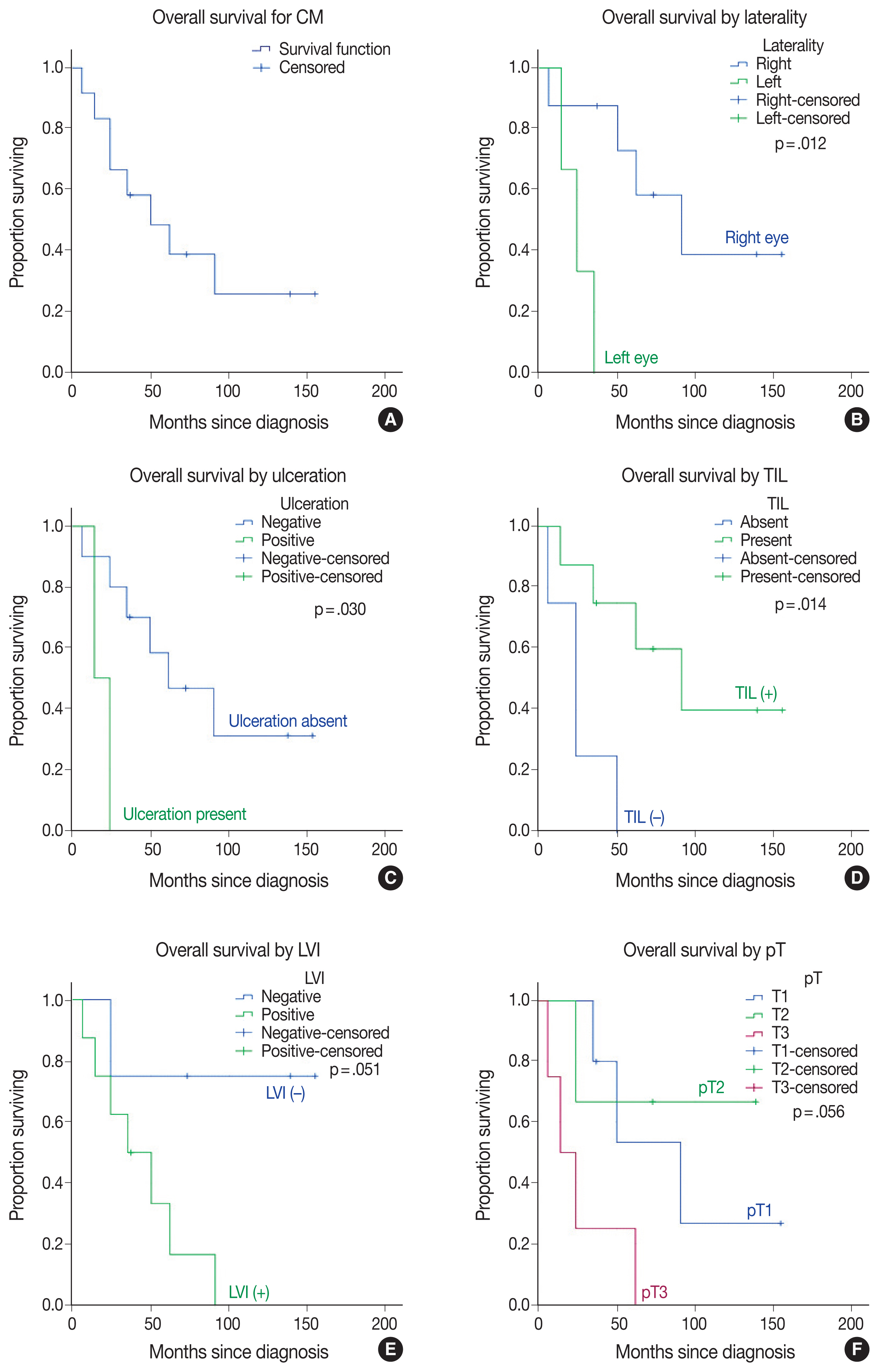
Kaplan-Meier overall survival curves in conjunctival melanoma (CM) (A) patients are compared for tumor variables by laterality (B), ulceration (C), tumor-infiltrating lymphocytes (TILs) (D), lymphovascular invasion (LVI) (E), and pathological tumor (pT) staging (F).
Histopathologically, three cases with CM were identical to superficial spreading melanoma, suggesting a role of cumulative sun damage similar to pathway I of the skin melanoma classification [1]. One CM showed histologically nodular melanoma features (Fig. 2A). The epithelioid type was the most common cell type (n = 9/12, 75%). Ulceration was present in two cases and related to poor outcomes (p = .030) (Fig. 1C). The majority of CM tumors were mildly pigmented. The presence of tumor-infiltrating lymphocytes (TILs) was associated with improved OS (p = .014) in CM (Figs. 1D, 2B, C).

(A) A conjunctival melanoma with nodular and well-circumscribed appearance is located in the left-eye nasal side bulbar conjunctiva in a 73-year-old male patient. (B, C) A subepithelial portion of melanoma nodule shows increased vascularization and a moderate amount of tumor-infiltrating lymphocytes. In addition, junctional involvement of the conjunctival epithelium (C, D) and prominent pagetoid spreading (D) are present at the nodule periphery. (D) Monotonous-appearing melanocytes have invaded the stroma in a nested growth pattern and exhibit slight, scattered pigmentation. (E) Beneath the intraepithelial melanocytic proliferation, the conjunctival stroma is invaded by epithelioid melanocytes with large eosinophilic cytoplasm (F) with rare intranuclear eosinophilic pseudo-inclusion (arrow), and prominent nucleoli dominate in this BRAF V600 mutant conjunctival melanoma.
Necrosis was present in four of the CM cases. No mortality occurred in tumors with a thickness of ≤ 2 mm (p = .012). A mitotic count of ≤ 1/mm2 was significantly related to improved OS (p = .012). A lower mitotic count and tumor thickness were shared between three identical cases; expectedly, their effects on OS were similar. Lymphovascular invasion (LVI) was identified in eight of the CM cases and associated with reduced OS (p = .051) (Fig. 1E). Although microscopic satellitosis and surgical margin positivity reduced the OS, they did not statistically significantly affect it. Based on the eighth edition of AJCC staging for CM [14], the tumors were staged as pT1a in one (8.3%), pT1b in four (33.3%), pT2a in two (16.7%), pT2b in one (8.3%), pT3b in one (8.3%), pT3c in two (16.7%), and pT3d in one (8.3%) case (s), respectively. No case presented with central nervous system involvement (pT4). Tumors of any size with local invasion (pT3) were linked to a decreased OS, although this relationship did not exactly reach statistical significance (p = .056) (Fig. 1F). Regional lymph node metastasis occurred in four (33%) of CM patients; one also had parotid gland metastasis, and another had additional lung metastasis. Three cases underwent BRAF V600 mutation analysis with RT-PCR. One patient with lymph node metastasis had BRAF V600 mutations (Fig. 2); others, including the patient with parotid gland metastasis, were wild-type for BRAF V600 mutation.
Uveal melanoma
Table 2 represents some of the clinicopathologic features of UM cases investigated in this study and their relationship to OS (Fig. 3). More patients were female (n = 12/19, 63.2%). The mean age at diagnosis was 59.74 ± 15.25 years (median, 63 years; range, 25 to 78 years). Follow-up time ranged from 8–151 months with a median of 35 months. When this study ended, nine of the 19 patients (47.4%) had died (Fig. 3A)

Univariate analysis of predictors for overall survival time (mo) in uveal melanoma, calculated from Kaplan-Meier analysis with comparisons performed with the log-rank test
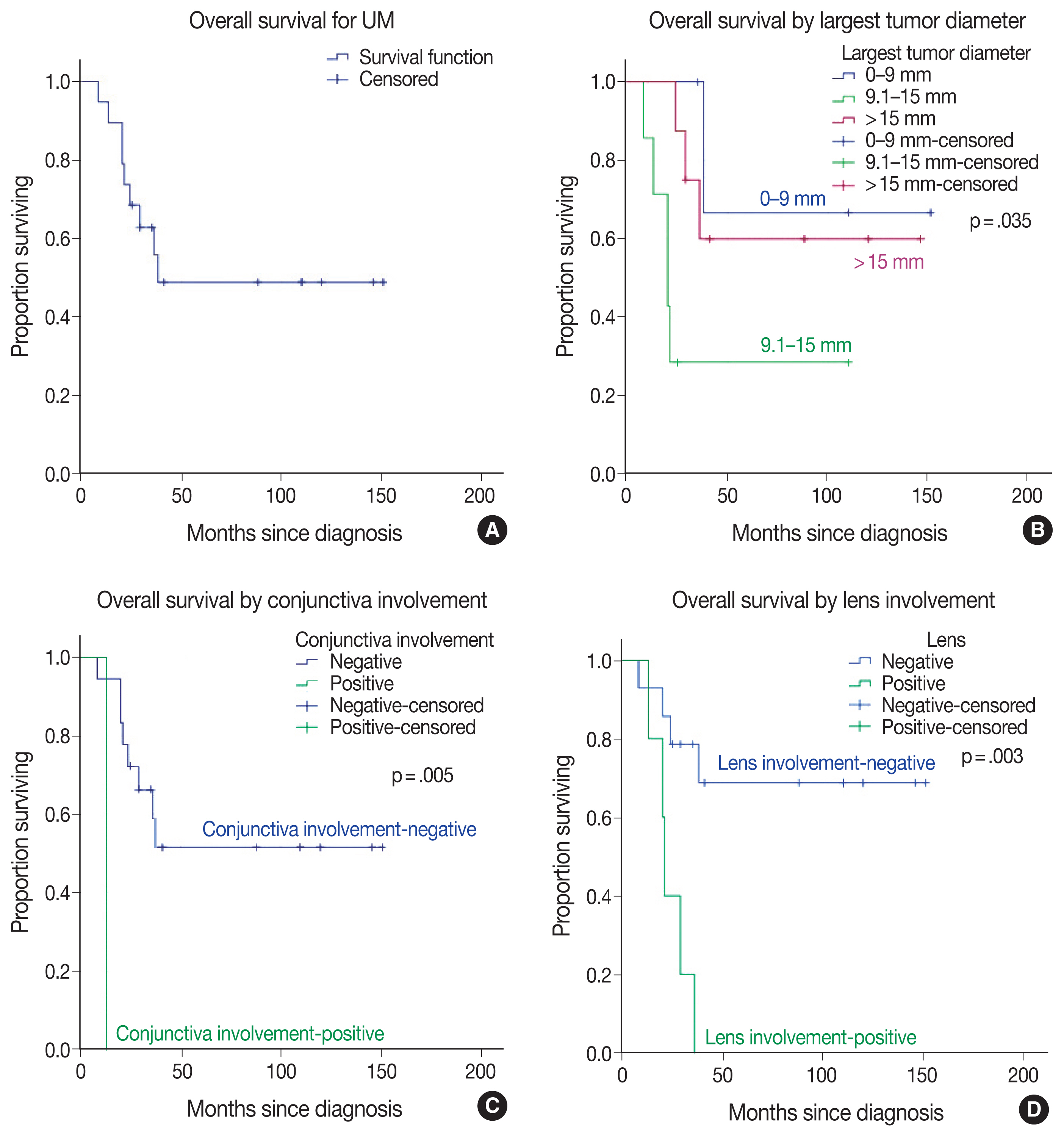
Kaplan-Meier overall survival curves in uveal melanoma (UM) patients (A) compared by largest tumor diameter (B), conjunctival involvement (C), and lens involvement (D).
Two UM tumors arose from the iris and ciliary body and were treated with local excision, whereas the remaining patients (n = 17/19, 89.5%) underwent enucleation. The mean largest tumor diameter was 13.68 mm (median, 14 mm; range, 4 to 21 mm). The UM tumor with the largest diameter interval of 9.1–15 mm led to the lowest OS among subgroups (p = .035) (Fig. 3B). Conjunctiva involvement was present in 1 UM case and inversely associated with OS (p=.005) (Fig. 3C). The other involved ocular structures in UM patients were the sclera in nine (47.4%), anterior chamber in four (21.1%), ciliary body in eight (42.1%), iris in four (21.1%), Schlemm canal in four (21.1%), posterior chamber in seven (36.8%), lens in five (26.3%), vitreous in 18 (94.7%), choroid in 17 (89.5%), retina in five (26.3%), and optic disc in two (10.5%) patients, respectively. Even though the involvement of each ocular structure diminished the OS time and probability in UM patients, lens involvement had particular importance statistically (p = .003) (Fig. 3D). Scleral involvement of tumors was confined to the intrascleral area in five (26.3%) UM cases and extended to < 5 mm of extrascleral area in two (10.5%) UM cases or ≥ 5 mm of the extrascleral area in two (10.5%) UM cases (p = .226).
The most frequent growth pattern was a dome shape (n = 8/19, 42.1%) (Fig. 4A), followed by a mushroom shape (n = 6/19, 31.6%), diffuse shape (n = 3/19, 15.8%), and a solid mass (n = 2/19, 10.5%). Epithelioid cell melanoma (Fig. 4) was present in 12 cases (63.2%). Based on the eighth edition of AJCC staging for iris [14], ciliary body, and choroid [14] melanomas, pathological tumor stages of cases were pT1b in one (5.3%), pT2b in three (15.8%), pT3 in one (5.3%), pT3a in five (26.3%), pT3b in one (5.3%), pT3d in one (5.3%), pT4a in four (21.1%), pT4d in one (5.3%), and pT4e in two (10.5%), respectively. Pigmentation was dense and covered > 75% of the UM (Fig. 4) in eight (42.1%) tumors (p = .296). Necrosis was evident in 10 (55.6%) UM cases (p = .166). TILs were present in six UM cases (33.3%) (p = .233). The mitotic count was > 1/mm2 in seven UM cases (36.8%) (p = .448). Inconspicuous, prominent, and prominent and large nucleoli were present in two (11.1%), eight (44.4%), and eight (44.4%) UM cases, respectively. BRAF V600 mutations were absent in three UM cases tested with RT-PCR (n = 2) and NGS (n = 1). NGS data from the enucleation specimen revealed a mutation in GNAQ (guanine nucleotide–binding protein G(q) subunit alpha) exon 4 codon 183 that resulted in a substitution of arginine with glutamine. Additionally, this case was immunohistochemically intact for mismatch repair proteins (MLH1, PMS2, MSH2, and MSH6).
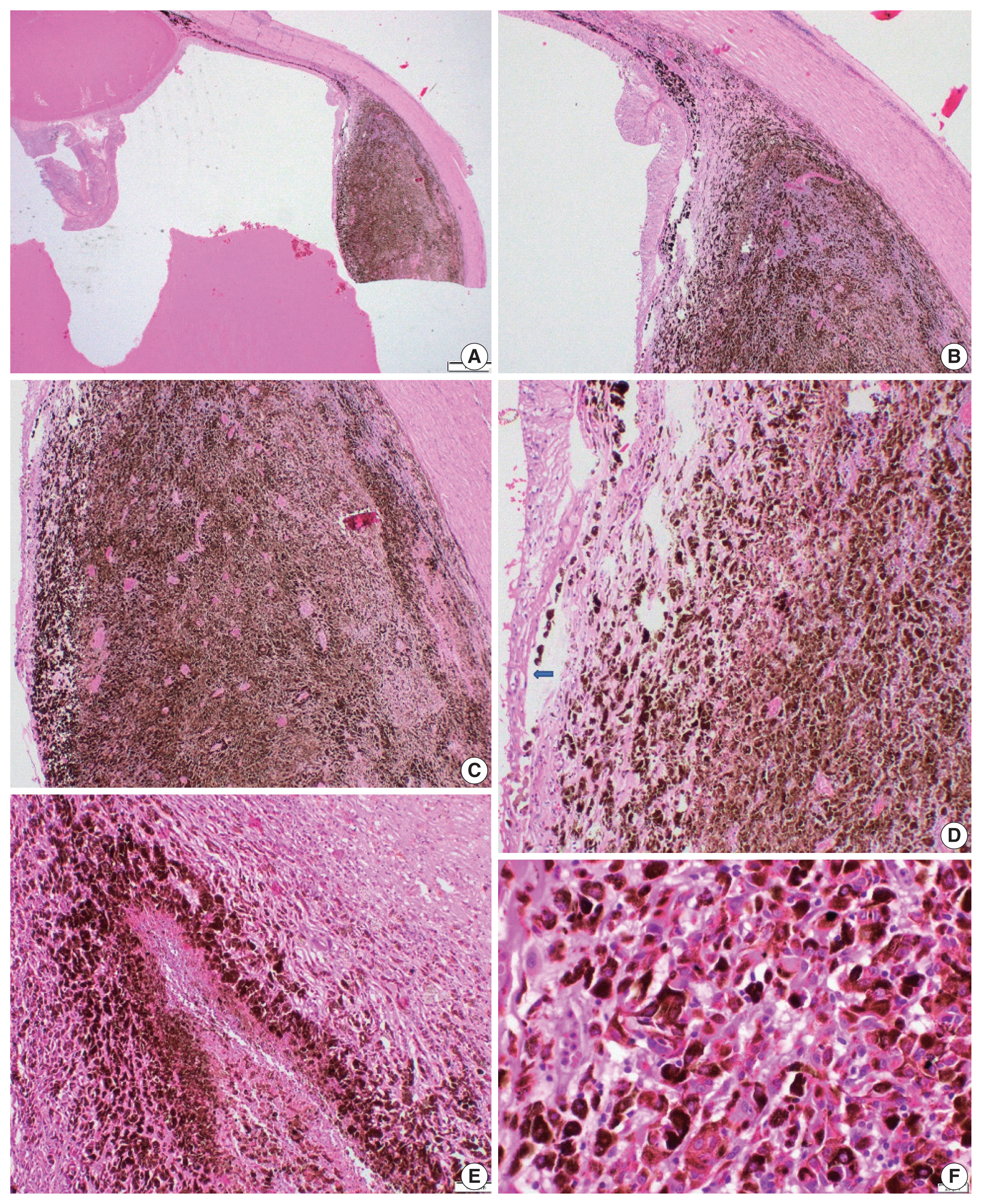
Right enucleation from a 25-year-old female patient revealed a densely pigmented, dome-shaped posterior choroidal melanoma with a basal diameter of 16 mm and a tumor thickness of 3.3 mm (A–C). Effacement of the overlying retinal layer by infiltrating melanocytes (D) (arrow). Densely pigmented atypical melanocytes are arranged around the necrosis reminiscent of pseudo-palisading necrosis (E). A closer look highlights the atypical epithelioid melanocytes with large nuclei and prominent nucleoli (F). This case is wild-type for BRAF V600 mutation.
Comparisons between CM and UM cases revealed that pT staging was the only significant difference between them. The variations resulted specifically from the pT1 and pT4 categories when the comparison were broken down further. UM patients presented with an advanced pT category compared to CM patients (p = .019) (Supplementary Table S1).
DISCUSSION
CM is most common in middle-aged (55–65 years) patients [20], mainly similar to our study. In this study, female and male patients were equal in number. Berta-Antalics et al. [21] reported 80 CM cases among 42 female patients and 38 male patients. Similar to our research, others reported that the most frequently involved site was the bulbar conjunctiva in 60% [21] to 75% [22] of patients.
In this study, UM patients totaled 61.3% of all ocular melanomas, which was a lower percentage than that in previously published reports [2,13]. The 5- and 10-year OS rates for UM were the same and 48.8% (Fig. 3A). Although there was no mortality at 5–10 years, OS probabilities were lower than previously documented [2,23,24]. At the end of this study, all deceased patients (n = 9/19, 47.4%) were within their first five years after diagnosis. In a very long-term (≥ 20 years) follow-up study including 289 UM patients, 239 participants (83%) were deceased at the study’s end, and 145 (61%) of these deaths were due to UM [24]. Their 5- and 10-year audited melanoma-specific Kaplan-Meier survival estimates were 68% and 57%, respectively [24]. The differences between our study and the mentioned article could be due to the small sample size in this study.
In our study, female patients (n = 12, 63%) were dominant for UM, consistent with previously published reports [25]. We did not find statistical significance for gender-related OS differences. Shields et al. [26] also reported no association between gender and metastasis or mortality in a comprehensive study for UM. Kaliki et al. [27] divided 122 UM patients into three groups regarding diagnosis age. They reported that patients who were diagnosed at ≤ 20 years had lower metastasis rates than patients who were diagnosed at middle (21–60 years) or elderly (> 60) ages [27]. In our study, there were no patients aged < 20 years old. Besides, we identified no significant difference in OS between age groups by decade.
This study established that a tumor thickness of > 2 mm (p = .012) and ulceration (p = .030) reduced the OS of CM patients. Esmaeli et al. [15] also determined that a tumor thickness of > 2 mm was significantly correlated with regional lymph node metastasis (p = .033), regional lymph node or distant metastasis (p = .005), and death from disease (p = .004). Additionally, ulceration was related to an increased risk for nodal metastasis, distant metastasis, and death from disease in CM [15,28]. In our study, a mitotic count of > 1/mm2 and LVI diminished the OS probability for CM patients. Tuomaala et al. [29] suggested an increasing mitotic count was related to a shorter time to recurrence (p = .042). Esmaeli et al. [15] reported that mitotic count ≥ 1 mm2 and vascular invasion were significantly correlated with regional lymph node metastasis and death from the disease.
Tumor diameter (largest basal diameter and tumor thickness) is one of the most important prognostic factors for UM [26,30,31]. In this study, when we compared the UM tumors according to the largest tumor diameter, 5-year OS rates were 66.7%, 28.6%, and 60% for UM tumors of small (≤ 9 mm), middle (9.1–15 mm), and large (> 15 mm) sizes, respectively. OS rates differed significantly between middle- and large-sized subgroups (p = .035) (Table 2, Fig. 3B). Middle-sized UM tumors had the worst prognosis in our study. This could be due to features other than tumor size; however, the rarity of cases prevented us from performing a multivariate analysis for further comparisons.
In this study, the presence of TILs had a favorable effect on OS (p = .014) for CM patients. Cao et al. [32] studied programmed cell death 1/programmed cell death-ligand 1 (PD-L1) proteins and TILs in CM. Although TIL density was not directly associated with survival or tumoral/stromal PD-L1 expression (p > .05), these investigators determined that smaller tumors had higher TILs than larger tumors. Correspondingly, thicker tumors had a lower number of CD3+ CD8+ T-cells (p = .030) and tumor-infiltrating M2 macrophages (p = .020) [32]. Another study revealed that tumors with neural cell-adhesion molecule expression had a 6.4-fold higher risk of dying from CM (p = .020) and had no or only weak CD3+ infiltration (p = .030) [33]. In a recent review by Brouwer et al. [34], the earliest report indicating that the presence of TILs, detected by hematoxylin and eosin, led to better survival was published in 1980 by Crawford [35] and contained 19 cases. Two other studies [36,37] with different approaches followed this publication and reported that the presence of TILs was associated with better survival in CM. Therefore, although our study contained a small sample size, the presence of TILs with improved OS in CM might be regarded as a valuable factor to conclude or support the role of TILs for OS in CM in the area of a few research reports.
Vodencarevic et al. [38] published 32 UM cases, and choroidal involvement was the most common site, affecting 27 (84%) patients, followed by ciliary body involvement in four (13%) and iris involvement in one (3%) patient(s), respectively. We also found that the choroid was the most common site of involvement. Ciliary body melanoma can extend to the lens and result in cataract [39]. Various changes in the lens were reported in ciliary body melanoma [40]. This study determined that lens and conjunctival involvement negatively affected OS (Table 2, Fig. 3C, D). To the best of our knowledge, these features have not been reported previously for UM. However, a chance factor could not be excluded for the significance of conjunctival involvement with worse OS since only one patient with UM infiltrating the conjunctiva was present.
Jain et al. [11] stated that their CM patients were diagnosed with early-stage disease, and staging for them was as follows: pTis in 43 (14.9%), pT1 in 169 (58.7%), pT2 in 33 (11.5%), pT3 in 12 (4.2%), and pTx in 31 (10.8%). Wolf et al. [22] also confirmed pT1 disease in 83.3% of CM patients. Pathological tumor staging was reported to be associated with cumulative mortality rates and survival in CM patients [11]. Even though the result did not reach statistical significance, CM patients with pT3 had increased mortality and reduced OS compared to those with pT1 in our study (Table 1, Fig. 1F).
In large-scale studies, the 10-year mortality rate for CM has been reported as approximately 30%–40% [41,42]. However, in this study, OS ratios were lower than previous estimates [10,11, 41,42], and OS rates for CM at 1, 2, 3, 5, and 10 year(s) were 91.7%, 66.7%, 58.3%, 48.6%, and 25.9%, respectively (Fig. 1A). However, the small sample size is the main limitation of the generalizability of this study’s results.
GNAQ/guanine nucleotide-binding protein subunit alpha 11 (GNA11) mutations are the most common (90%) mutations in UM patients [43]. One UM patient had a single-nucleotide variation at codon 183 on exon 4 of the GNAQ gene, which triggered a substitution of arginine with glutamine. This particular mutation is rarely reported in UM [44]. GNAQ/GNA11 mutations induce abnormal activation of the mitogen-activated protein kinase (MAPK) pathway, making MAPK/MEK (MAPK kinase) signaling pathway inhibitors an impressive target for therapy [45].
In conclusion, OS data with a detailed histopathological evaluation of ocular melanoma patients suggested some previously known and not well-identified features for OS. The presence of TILs is currently not well defined and is a controversial prognostic feature for CM. However, this study suggests TILs as a favorable factor for OS in CM. The current study also confirmed the poor prognostic effect on OS for ulceration, a tumor thickness of > 2 mm, and a mitotic count of > 1/mm2; those features were previously determined and suggested to be included in future tumor classification guidelines [15,28]. For UM, conjunctival and lens involvement have not previously been reported as unfavorable prognostic factors for OS to the best of our knowledge. Future studies may verify the relationship between survival and detailed clinicopathologic features of CM and UM. Next, these features may benefit initial patient management, treatment, and surveillance.
Supplementary Information
The Data Supplement is available with this article at https://doi.org/10.4132/jptm.2022.03.10.
Notes
Ethics Statement
Approval from the institutional research ethics board (2020-357) was obtained in accordance with the 1964 Declaration of Helsinki and its later amendments. The need for informed consent was waived due to the retrospective nature of the study.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: SK, FPUG, BÖ. Data curation: SK, FPUG, BÖ, ÖE. Investigation: SK, FPUG. Methodology: SK, FPUG. Supervision: FPUG, ÖE. Writing—original draft: SK. Writing—review & editing: SK, FPUG, BÖ, ÖE. Approval of final manuscript: SK, FPUG, BÖ, ÖE.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
